Postoperative Bloodstream Infection Is Associated with Early Vascular Complications in Pediatric Liver Transplant Recipients with Biliary Atresia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Surgical Technique
2.3. Postoperative Immunosuppressive Regimen
2.4. Definition and Workup of Bacterial Infection
2.5. Perioperative Antibacterial Protocol
2.6. Perioperative Management of Coagulopathy
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Complications and Operative Outcomes
3.3. Bacterial Infection Episodes
3.4. Risk Factors for Vascular Complications after PLT
3.5. Vascular Characteristics of Doppler Ultrasonography before and after BSI in BT Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davenport, M. Biliary atresia: From australia to the zebrafish. J. Pediatr. Surg. 2016, 51, 200–205. [Google Scholar] [CrossRef]
- Nio, M.; Wada, M.; Sasaki, H.; Kazama, T.; Tanaka, H.; Kudo, H. Technical standardization of kasai portoenterostomy for biliary atresia. J. Pediatr. Surg. 2016, 51, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, A.G.; Kim, H.B.; Vakili, K. Pediatric Liver Transplantation; Seminars in Pediatric Surgery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–223. [Google Scholar]
- Baek, S.H.; Kang, J.-M.; Ihn, K.; Han, S.J.; Koh, H.; Ahn, J.G. The epidemiology and etiology of cholangitis after kasai portoenterostomy in patients with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J. From the classic concepts to modern practice. Clin. Microbiol. Infect. 2014, 20, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Dorschner, P.; McElroy, L.; Ison, M. Nosocomial infections within the first month of solid organ transplantation. Transpl. Infect. Dis. 2014, 16, 171–187. [Google Scholar] [CrossRef]
- Moreno, A.; Cervera, C.; Gavaldá, J.; Rovira, M.; De La Cámara, R.; Jarque, I.; Montejo, M.; De La Torre-Cisneros, J.; Cisneros, J.M.; Fortún, J. Bloodstream infections among transplant recipients: Results of a nationwide surveillance in spain1. Am. J. Transplant. 2007, 7, 2579–2586. [Google Scholar] [CrossRef]
- Gunawan, T.A.; Widiyanto, G.; Yuanita, A.; Mulyani, N.S.; Makhmudi, A. Liver transplant score for prediction of biliary atresia patients’ survival following kasai procedure. BMC Res. Notes 2018, 11, 381. [Google Scholar]
- Piardi, T.; Lhuaire, M.; Bruno, O.; Memeo, R.; Pessaux, P.; Kianmanesh, R.; Sommacale, D. Vascular complications following liver transplantation: A literature review of advances in 2015. World J. Hepatol. 2016, 8, 36. [Google Scholar] [CrossRef]
- Kamran Hejazi Kenari, S.; Mirzakhani, H.; Eslami, M.; Saidi, R.F. Current state of the art in management of vascular complications after pediatric liver transplantation. Pediatr. Transplant. 2015, 19, 18–26. [Google Scholar] [CrossRef]
- Ziaziaris, W.A.; Darani, A.; Holland, A.J.; Alexander, A.; Karpelowsky, J.; Barbaro, P.; Stormon, M.; O’Loughlin, E.; Shun, A.; Thomas, G. Reducing the incidence of hepatic artery thrombosis in pediatric liver transplantation: Effect of microvascular techniques and a customized anticoagulation protocol. Pediatr. Transplant. 2017, 21, e12917. [Google Scholar] [CrossRef]
- Pastacaldi, S.; Teixeira, R.; Montalto, P.; Rolles, K.; Burroughs, A.K. Hepatic artery thrombosis after orthotopic liver transplantation: A review of nonsurgical causes. Liver Transplant. 2001, 7, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.P.; Yuen, P.S.; Star, R.A. Microparticles: Markers and mediators of sepsis-induced microvascular dysfunction, immunosuppression, and aki. Kidney Int. 2015, 87, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Kim, H.K.; Wang, Y.; Bubeck Wardenburg, J. Adam10 mediates vascular injury induced by staphylococcus aureus α-hemolysin. J. Infect. Dis. 2012, 206, 352–356. [Google Scholar] [CrossRef]
- Lubkin, A.; Torres, V.J. Bacteria and endothelial cells: A toxic relationship. Curr. Opin. Microbiol. 2017, 35, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lemichez, E.; Lecuit, M.; Nassif, X.; Bourdoulous, S. Breaking the wall: Targeting of the endothelium by pathogenic bacteria. Nat. Rev. Microbiol. 2010, 8, 93–104. [Google Scholar] [CrossRef]
- Bray, M.A.; Sartain, S.E.; Gollamudi, J.; Rumbaut, R.E. Microvascular thrombosis: Experimental and clinical implications. Transl. Res. 2020, 225, 105–130. [Google Scholar] [CrossRef]
- Shepherd, R.; Turmelle, Y.; Nadler, M.; Lowell, J.; Narkewicz, M.; McDiarmid, S.; Anand, R.; Song, C.; Group, S.R. Risk factors for rejection and infection in pediatric liver transplantation. Am. J. Transplant. 2008, 8, 396–403. [Google Scholar] [CrossRef]
- Spada, M.; Riva, S.; Maggiore, G.; Cintorino, D.; Gridelli, B. Pediatric liver transplantation. World J. Gastroenterol. WJG 2009, 15, 648. [Google Scholar] [CrossRef]
- Lee, E.J.; Vakili, K. Pediatric liver transplantation. In Pediatric Solid Organ Transplantation: A Practical Handbook; Springer: Singapore, 2023; pp. 415–427. [Google Scholar]
- Alcamo, A.M.; Alessi, L.J.; Vehovic, S.N.; Bansal, N.; Bond, G.J.; Carcillo, J.A.; Green, M.; Michaels, M.G.; Aneja, R.K. Severe sepsis in pediatric liver transplant patients: The emergence of multidrug-resistant organisms. Pediatr. Crit. Care Med. A J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2019, 20, e326. [Google Scholar] [CrossRef]
- Møller, D.L.; Sørensen, S.S.; Wareham, N.E.; Rezahosseini, O.; Knudsen, A.D.; Knudsen, J.D.; Rasmussen, A.; Nielsen, S.D. Bacterial and fungal bloodstream infections in pediatric liver and kidney transplant recipients. BMC Infect. Dis. 2021, 21, 541. [Google Scholar] [CrossRef]
- Levi, M.; Keller, T.T.; van Gorp, E.; ten Cate, H. Infection and inflammation and the coagulation system. Cardiovasc. Res. 2003, 60, 26–39. [Google Scholar] [CrossRef]
- Fani, F.; Regolisti, G.; Delsante, M.; Cantaluppi, V.; Castellano, G.; Gesualdo, L.; Villa, G.; Fiaccadori, E. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J. Nephrol. 2018, 31, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Boehme, A.K.; Smith, C.J.; Meisel, A.; Buckwalter, M.S. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke 2020, 51, 3156–3168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Su, S.; Xie, Y.; Shen, J.; Zhu, W.; Xiang, M. Murine models of vascular endothelial injury: Techniques and pathophysiology. Thromb. Res. 2018, 169, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Ohta, K.; Ohta, K.; Nakayama, Y.; Hashida, Y.; Toma, T.; Saito, T.; Maruhashi, K.; Yachie, A. Effects of antithrombin iii treatment in vascular injury model of mice. Pediatr. Int. 2011, 53, 747–753. [Google Scholar] [CrossRef]
- Kishi, Y.; Sugawara, Y.; Matsui, Y.; Akamatsu, N.; Makuuchi, M. Late onset portal vein thrombosis and its risk factors. Hepato Gastroenterol. 2008, 55, 1008–1009. [Google Scholar]
- Lladó, L.; Fabregat, J.; Castellote, J.; Ramos, E.; Torras, J.; Jorba, R.; Garcia-Borobia, F.; Busquets, J.; Figueras, J.; Rafecas, A. Management of portal vein thrombosis in liver transplantation: Influence on morbidity and mortality. Clin. Transplant. 2007, 21, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Charco, R.; Fuster, J.; Fondevila, C.; Ferrer, J.; Mans, E.; Garcıa-Valdecasas, J. Portal Vein Thrombosis in Liver Transplantation; Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3904–3905. [Google Scholar]
- Shin, J.H.; Chang, E.Y.; Chang, H.K.; Kim, S.M.; Han, S.J. Home intravenous antibiotic treatment for intractable cholangitis in patients with biliary atresia following kasai portoenterostomies. J. Korean Surg. Soc. 2011, 80, 355–361. [Google Scholar] [CrossRef]
- Mahajan, S.; Lal, B.B.; Kumar, P.; Upadhyay, P.; Mukund, A.; Sood, V.; Khanna, R.; Alam, S. Treatment of intractable cholangitis in children with biliary atresia: Impact on outcome. Indian J. Gastroenterol. 2023, 42, 209–218. [Google Scholar] [CrossRef]
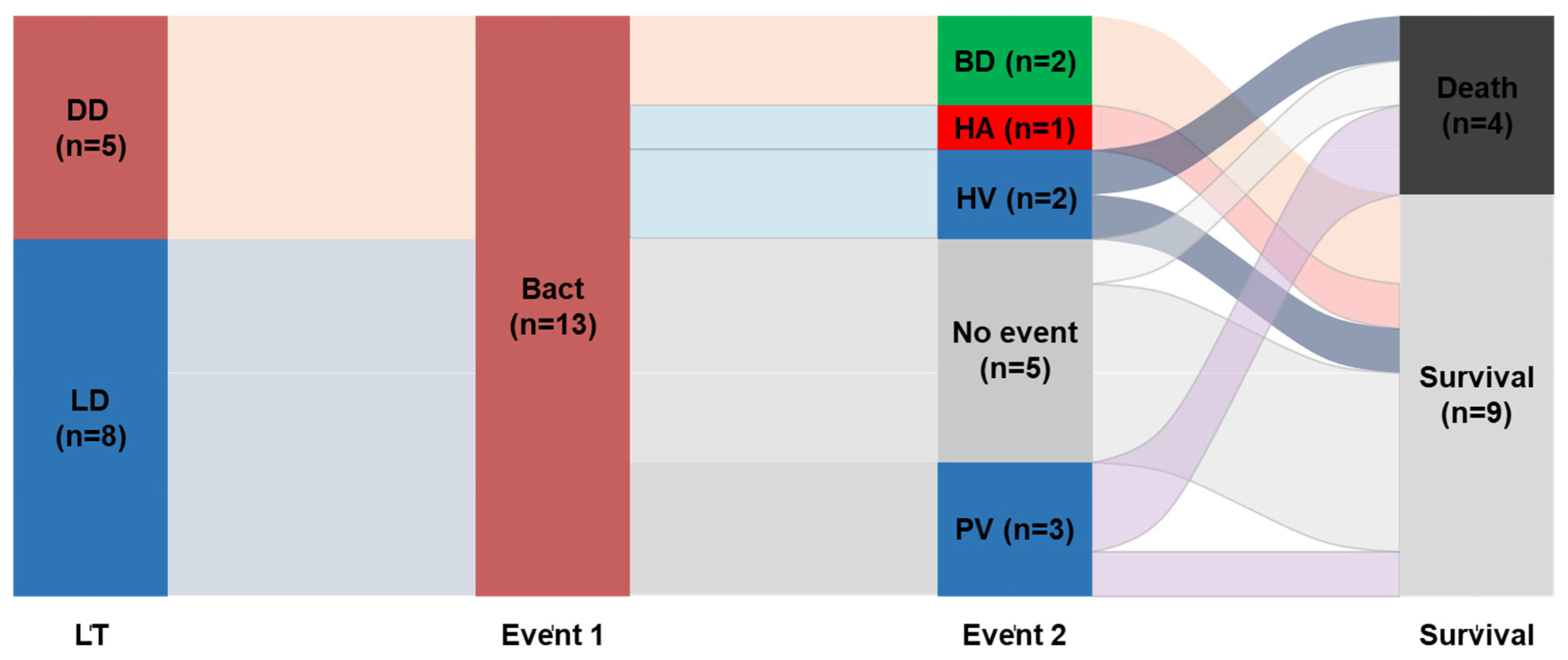
| All PLTs (n = 67) | BT (n = 13) | NBT (n = 54) | p-Value | |
|---|---|---|---|---|
| Age, years | 1.08 (0.67–5.33) | 0.75 (0.5–1.79) | 1.17 (0.67–7.00) | 0.098 |
| Female | 40 (59.7%) | 8 (61.5%) | 32 (59.3%) | 0.880 |
| Weight, kg | 10.1 (8.0–17.5) | 8.6 (7.0–13.2) | 10.8 (8.0–22.8) | 0.086 |
| Presence of recurrent cholangitis before PLT (>2 admissions/year) | 24 (35.8%) | 7 (53.8%) | 17 (31.5%) | 0.197 |
| History of bacteremia before PLT within 1 month | 6 (9.0%) | 3 (23.1%) | 3 (5.6%) | 0.082 |
| Presence of bacteremia at the time of PLT | 2 (3.0%) | 2 (15.4%) | 0 (0%) | 0.035 * |
| WBC (μL) | 6330 (4650–10,530) | 10,530 (6435–15,025) | 5955 (4543–8933) | 0.006 * |
| Hemoglobin (g/dL) | 10.2 (8.9–11.2) | 10.1 (9.2–10.8) | 10.2 (8.8–11.3) | 1.000 |
| Platelet (103/μL) | 132 (95–181) | 167 (80–215) | 130 (95–181) | 0.570 |
| AST (IU/L) | 147 (66–224) | 185 (89–387) | 145 (66–211) | 0.186 |
| ALT (IU/L) | 46 (27–122) | 46 (22–140) | 45 (27–123) | 0.842 |
| Total bilirubin (mg/dL) | 7.2 (3.1–13.1) | 10.9 (6.1–18.8) | 6.1 (2.7–11.8) | 0.046 * |
| Protein (g/dL) | 5.9 (5.0–6.5) | 6.0 (4.8–6.5) | 5.9 (5.1–6.5) | 0.585 |
| Albumin (g/dL) | 3.3 (3.0–3.7) | 3.3 (3.0–3.9) | 3.3 (3.0–3.7) | 0.553 |
| Creatinine (mg/dL) | 0.20 (0.20–0.30) | 0.20 (0.20–0.47) | 0.20 (0.20–0.29) | 0.568 |
| C-reactive protein (mg/L) | 9.05 (2.62–19.53) | 12.0 (3.2–76.3) | 8.1 (2.1–17.3) | 0.174 |
| INR | 1.27 (1.12–1.52) | 1.50 (1.21–2.00) | 1.26 (1.11–1.37) | 0.039 * |
| MELD/PELD score | 10.4 (1.3–16.0) | 15.4 (9.9–23.2) | 9.0 (1.2–15.4) | 0.037 * |
| Admission status at the time of PLT | 0.052 | |||
| Outpatient | 27 (40.3%) | 3 (23.1%) | 24 (44.4%) | |
| Inpatient | 32 (47.8%) | 6 (46.2%) | 26 (48.1%) | |
| Intensive care unit | 8 (11.9%) | 4 (30.8%) | 4 (7.4%) | |
| Follow-up period, months | 45 (21–76) | 46 (15–96) | 45 (21–77) | 0.885 |
| All PLTs (n = 67) | BT (n = 13) | NBT (n = 54) | p-Value | |
|---|---|---|---|---|
| Donor age | 31 (25–37) | 26 (23–33) | 32 (27–38) | 0.039 * |
| Donor graft weight (g) | 283 (222–345) | 283 (207–337) | 283 (224–351) | 0.587 |
| GRWR | 2.40 (1.69–3.12) | 3.04 (2.38–3.77) | 2.22 (1.48–3.01) | 0.047 * |
| Graft type | 0.508 | |||
| Lateral segment | 52 (77.6%) | 12 (92.3%) | 40 (74.1%) | |
| Left lobe | 8 (11.9%) | 0 | 8 (14.8%) | |
| Right lobe | 3 (4.5%) | 0 | 3 (5.6%) | |
| Whole liver | 3 (4.5%) | 1 (7.7%) | 2 (3.7%) | |
| Reduced or monosegment | 1 (1.5%) | 0 | 1 (1.9%) | |
| Deceased donor | 21 (31.3%) | 5 (38.5%) | 16 (29.6%) | 0.74 |
| Complex vascular reconstruction | 11 (16.4%) | 1 (7.7%) | 10 (18.5%) | 0.677 |
| All PLTs (n = 67) | BT (n = 13) | NBT (n = 54) | p-Value | |
|---|---|---|---|---|
| Portal vein stenosis | 4 (6.0%) | 2 (15.4%) | 2 (3.7%) | 0.167 |
| Portal vein thrombosis | 6 (9.0%) | 2 (15.4%) | 4 (7.4%) | 0.329 |
| Hepatic artery stenosis | 4 (6.0%) | 1 (7.7%) | 3 (5.6%) | 1 |
| Hepatic artery thrombosis | 2 (3.0%) | 1 (7.7%) | 1 (1.9%) | 0.353 |
| Hepatic vein stenosis | 3 (4.5%) | 1 (7.7%) | 2 (3.7%) | 0.482 |
| Any type of vascular complications | 16 (23.9%) | 6 (46.2%) | 10 (18.5%) | 0.046 * |
| Biliary complications | 9 (13.4%) | 2 (15.4%) | 7 (13.0%) | 1 |
| All types of complication | 20 (29.9%) | 6 (46.2%) | 14 (25.9%) | 0.185 |
| Ventilator dependence, days | 2 (1–5) | 3 (1–9) | 2 (1–4) | 0.607 |
| ICU stay, days | 4 (3–8) | 5 (4–18) | 4 (3–6) | 0.063 |
| Hospital stay, days | 25 (20–33) | 31 (25–61) | 24 (19–32) | 0.107 |
| Re-laparotomy | 6 (9.0%) | 4 (30.8%) | 2 (3.7%) | 0.011 * |
| Total bilirubin within 1 year (mg/dL) | 0.50 (0.30–0.68) | 0.40 (0.20–0.60) | 0.50 (0.30–0.70) | 0.316 |
| Albumin within 1 year (g/dL) | 4.1 (3.8–4.3) | 3.9 (2.9–4.1) | 4.1 (3.9–4.3) | 0.036 * |
| AST within 1 year (IU/L) | 32 (26–38) | 35 (23–48) | 38 (25) | 0.622 |
| ALT within 1 year (IU/L) | 20 (14–27) | 23 (11–41) | 23 (14) | 0.489 |
| INR within 1 year | 1.14 (1.07–1.23) | 1.15 (1.08–1.25) | 1.14 (1.06–1.22) | 0.532 |
| 3-year graft survival | 79.80% | 76.9% | 80.5% | 0.495 |
| 3-year overall survival | 81.30% | 84.6% | 80.5% | 0.789 |
| Pathogens | Number of Episodes (%) | Source of Infection |
|---|---|---|
| Gram-positive | ||
| Staphylococcus aureus | 3 (17.6) | Intra-abdominal |
| Staphylococcus epidermidis | 3 (17.6) | Catheter |
| Enterococcus faecium | 4 (23.5) | Intra-abdominal |
| Gram-negative | ||
| Acinetobacter bereziniae | 1 (5.9) | Catheter |
| Herbaspirillum aquaticum | 1 (5.9) | Catheter |
| Enterobacter cloacae | 1 (5.9) | Biliary tract |
| Klebsiella pneumoniae | 1 (5.9) | Intra-abdominal |
| Escherichia coli | 1 (5.9) | Biliary tract |
| Burkholderia cepacia | 1 (5.9) | Gastrointestinal tract |
| Pseudomonas aeruginosa | 1 (5.9) | Respiratory tract |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Weight < 10 kg | 2.381 (0.750–7.562) | 0.141 | 0.560 (0.114–2.751) | 0.475 |
| History of bacteremia before LT within 1 month | 0.613 (0.066–5.674) | 0.667 | ||
| Bacteremia after LT within 1 month | 3.771 (1.040–13.682) | 0.043 | 5.691 (1.071–30.228) | 0.041 * |
| GRWR > 4 | 15.600 (1.566–155.410) | 0.019 | 27.214 (1.984–373.252) | 0.013 * |
| DDLT vs. LDLT (reference: LDLT) | 0.994 (0.296–3.339) | 0.993 | ||
| Complex vascular reconstruction | 2.095 (0.525–8.365) | 0.295 | ||
| MELD/PELD > 20 | 1.731 (0.380–7.891) | 0.479 | ||
| ABO-incompatible (reference: ABO-compatible) | 1.314 (0.229–7.530) | 0.759 | ||
| Patient Number | Post-PLT Days When BSI Occurred | Doppler Days before BSI | Doppler Days after BSI | HA RI Value before BSI | HA RI Value after BSI | HV Phase before BSI | HV Phase after BSI | Perfusion Status | Pathogens of BSI | Resistance for Antibiotics | Resolved by Antibiotics | Antibiotics | Vascular Complications | Treatment by Intervention or Operation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 4 | −1 | 3 | 0.71 | NA | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Staphylococcus epidermidis | No | Yes | Teicoplanin | No | No |
| P2 | 5 | −2 | 2 | 0.84 | 0.62 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Enterococcus faecium | No | Yes | Teicoplanin | PV stenosis PV thrombosis | Yes |
| P3 | 2 | −1 | 1 | 0.63 | NA | Triphasic | Triphasic | Parenchymal ischemic change after BSI | Staphylococcus aureus | No | No | Vancomycin | PV thrombosis | Yes |
| P4 | 6 | −2 | 3 | 0.73 | 0.7 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Staphylococcus aureus, Staphylococcus epidermidis | No | Yes | Teicoplanin | No | No |
| P5 | 5 | −1 | 2 | 0.59 | 0.77 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Enterococcus faecium | No | Yes | Teicoplanin | No | No |
| P6 | 26 | −1 | 6 | NA | NA | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Staphylococcus epidermidis | No | Yes | Teicoplanin | PV stenosis | Yes |
| P7 | 6 | −2 | 1 | 0.7 | 0.56 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Enterococcus faecium | No | Yes | Teicoplanin | No | No |
| P8 | 5 | −2 | 1 | 0.67 | 0.69 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Staphylococcus aureus | No | No | Teicoplanin | No | No |
| P9 | 15 | −3 | 4 | 0.75 | 0.78 | Triphasic | Triphasic | No abnormal perfusion before and after BSI | Acinetobacter bereziniae, Herb spirillum aquaticum | Yes | Yes | Colistin | No | No |
| P10 | 0 | No | 1 | NA | 0.78 | NA | Biphasic | No abnormal perfusion after BSI | Enterobacter cloacae | Yes | Yes | Amikacin, Teicoplanin | No | No |
| P11 | 5 | −3 | 4 | 0.6 | 0.8 | Triphasic | Triphasic | Parenchymal ischemic change after BSI | Klebsiella pneumoniae (ESBL positive) | Yes | Yes | Amikacin | HA thrombosis | No |
| P12 | 0 | No | 1 | NA | 0.75 | NA | Triphasic | No abnormal perfusion after BSI | Escherichia coli, Burkholderia cepacia | Yes | Yes | Tigecycline | HA stenosis | Yes |
| P13 | 6 | −2 | 1 | 0.71 | 0.72 | Triphasic | Monophasic | No abnormal perfusion before and after BSI | Pseudomonas aeruginosa | No | Yes | Amikacin | HV stenosis | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, H.J.; Kang, J.-M.; Koh, H.; Kim, M.S.; Ihn, K. Postoperative Bloodstream Infection Is Associated with Early Vascular Complications in Pediatric Liver Transplant Recipients with Biliary Atresia. J. Clin. Med. 2023, 12, 6760. https://doi.org/10.3390/jcm12216760
Jeon HJ, Kang J-M, Koh H, Kim MS, Ihn K. Postoperative Bloodstream Infection Is Associated with Early Vascular Complications in Pediatric Liver Transplant Recipients with Biliary Atresia. Journal of Clinical Medicine. 2023; 12(21):6760. https://doi.org/10.3390/jcm12216760
Chicago/Turabian StyleJeon, Ho Jong, Ji-Man Kang, Hong Koh, Myoung Soo Kim, and Kyong Ihn. 2023. "Postoperative Bloodstream Infection Is Associated with Early Vascular Complications in Pediatric Liver Transplant Recipients with Biliary Atresia" Journal of Clinical Medicine 12, no. 21: 6760. https://doi.org/10.3390/jcm12216760
APA StyleJeon, H. J., Kang, J.-M., Koh, H., Kim, M. S., & Ihn, K. (2023). Postoperative Bloodstream Infection Is Associated with Early Vascular Complications in Pediatric Liver Transplant Recipients with Biliary Atresia. Journal of Clinical Medicine, 12(21), 6760. https://doi.org/10.3390/jcm12216760






