Amplatzer™ Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. AVP Delivery Technique
2.3. Outcomes and Follow-Up
2.4. Statistical Analysis
3. Results
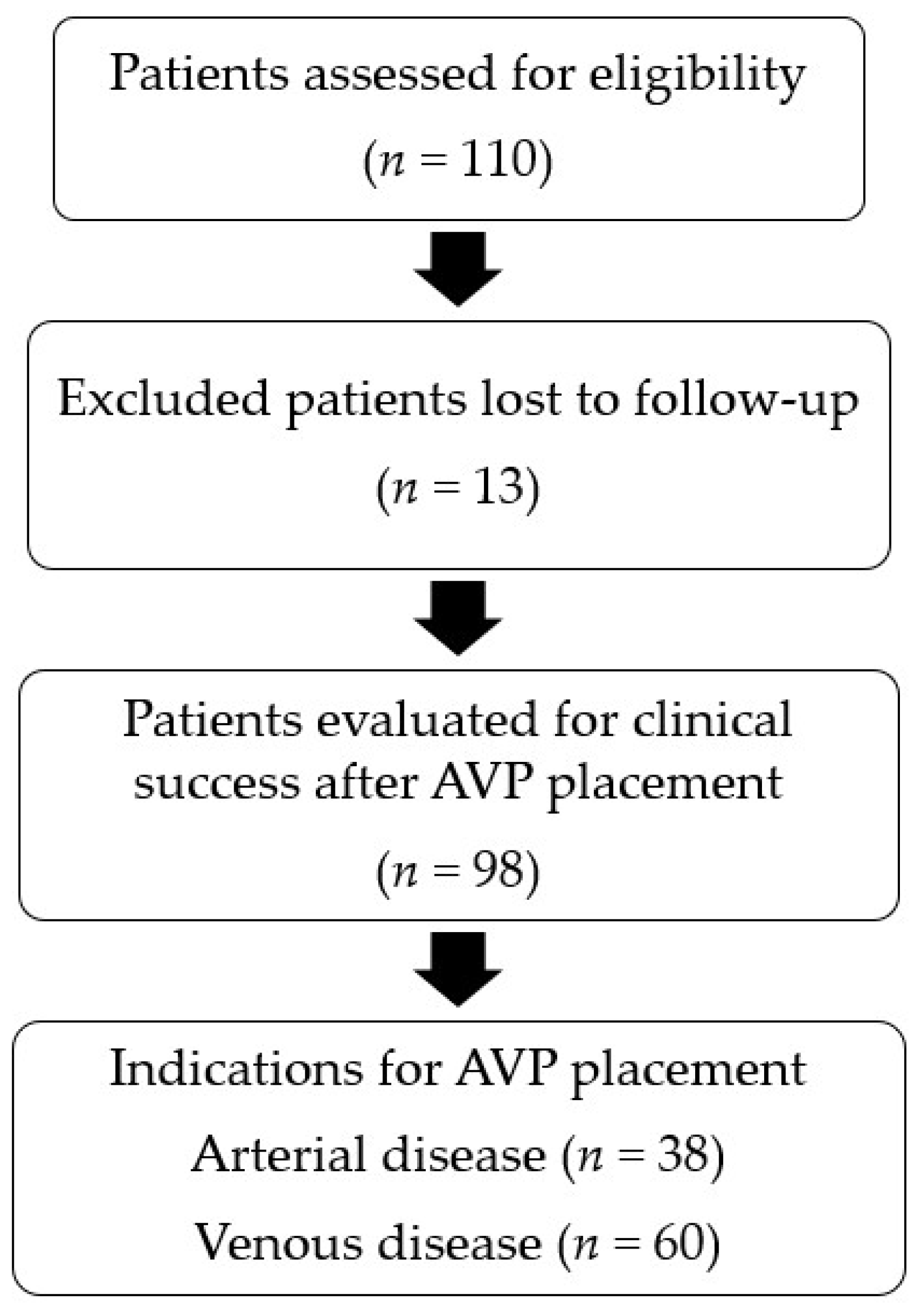
3.1. Patients
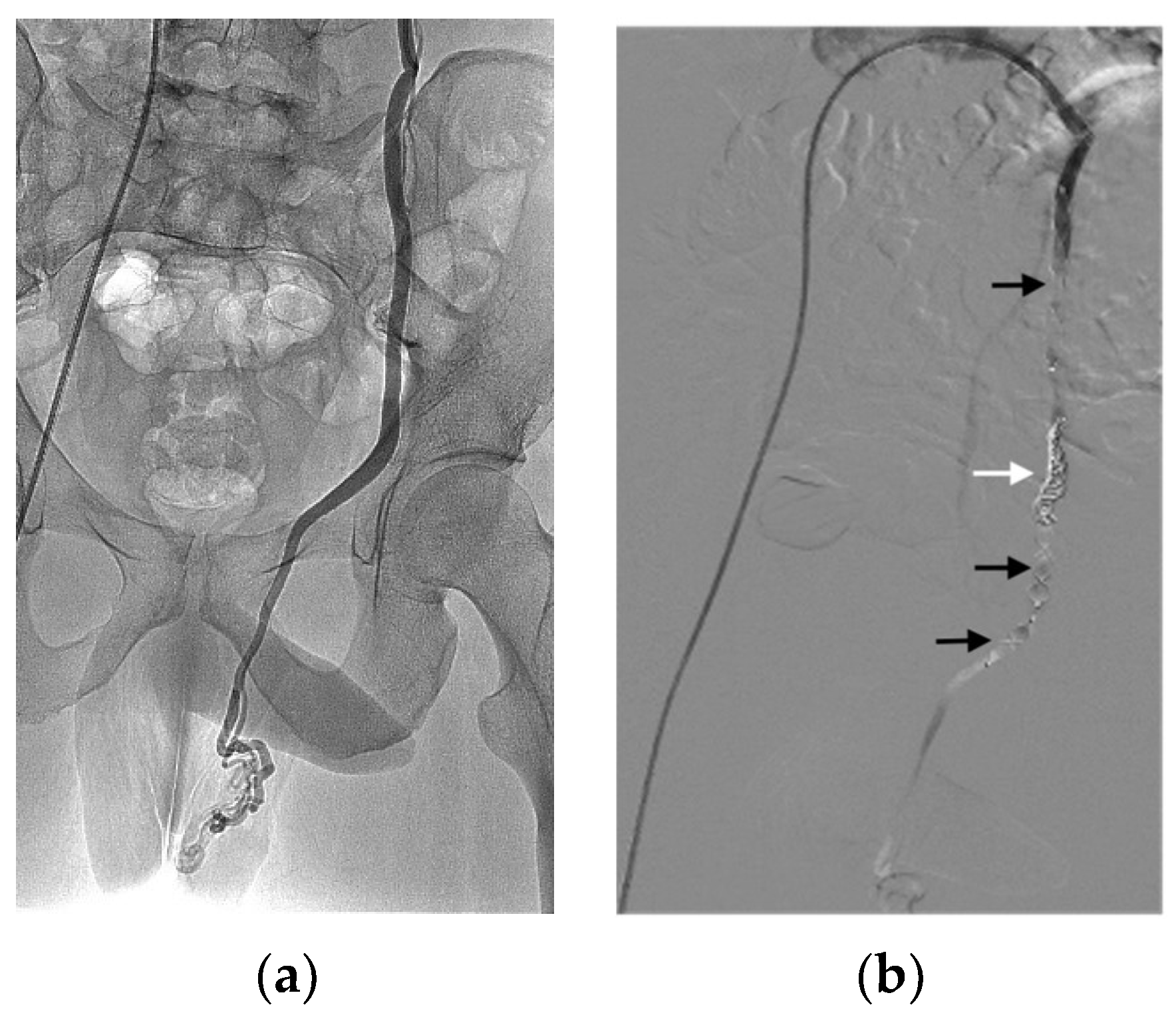
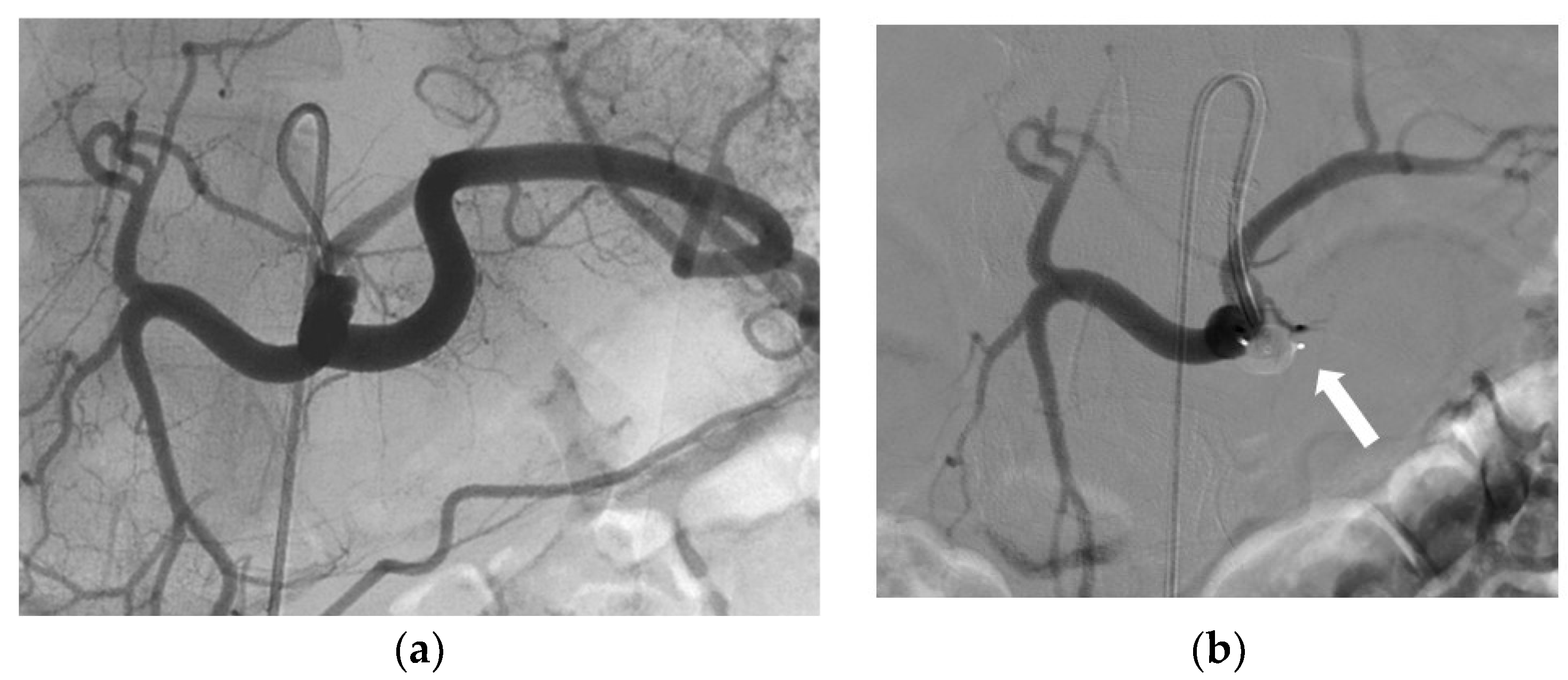
3.2. Indications and Techniques
3.3. Effectiveness
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rösch, J.; Dotter, C.T.; Brown, M.J. Selective arterial embolization: A new method for control of acute gastrointestinal bleeding. Radiology 1972, 102, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, H.; Vernhet-Kovacsik, H.; Mouroz, P.R.; Otal, P.; Meyrignac, O.; Mokrane, F.Z. Future of interventional radiology. Presse Med. 2019, 48, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Medsinge, A.; Zajko, A.; Orons, P.; Amesur, N.; Santos, E. A case-based approach to common embolization agents used in vascular interventional radiology. Am. J. Roentgenol. 2014, 203, 699–708. [Google Scholar] [CrossRef]
- Sharafuddin, M.J.A.; Gu, X.; Urness, M.; Amplatz, K. The nitinol vascular occlusion plug: Preliminary experimental evaluation in peripheral veins. J. Vasc. Interv. Radiol. 1999, 10, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Tuite, D.J.; Kessel, D.O.; Nicholson, A.A.; Patel, J.V.; McPherson, S.J.; Shaw, D.R. Initial clinical experience using the Amplatzer Vascular Plug. Cardiovasc. Interv. Radiol. 2007, 30, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, H.; Tam, M.D.; Zhou, D.; Wang, D.X.; Spain, J. The Amplatzer Vascular Plug: A review of the device and its clinical applications. Cardiovasc. Interv. Radiol. 2012, 35, 725–740. [Google Scholar] [CrossRef]
- Güneyli, S.; Çinar, C.; Bozkaya, H.; Parıldar, M.; Oran, İ. Applications of the Amplatzer Vascular Plug to various vascular lesions. Diagn. Interv. Radiol. Ank. Turk. 2014, 20, 155–159. [Google Scholar] [CrossRef]
- Johnson, D.; Sandlow, J. Treatment of varicoceles: Techniques and outcomes. Fertil. Steril. 2017, 108, 378–384. [Google Scholar] [CrossRef]
- Masson, P.; Brannigan, R.E. The varicocele. Urol. Clin. N. Am. 2014, 41, 129–144. [Google Scholar] [CrossRef]
- Halpern, J.; Mittal, S.; Pereira, K.; Bhatia, S.; Ramasamy, R. Percutaneous embolization of varicocele: Technique, indications, relative contraindications, and complications. Asian J. Androl. 2016, 18, 234–238. [Google Scholar]
- Sheehan, M.; Briody, H.; O’Neill, D.C.; Bowden, D.; Davis, N.F.; Given, M.; Mohan, P.; Lee, M.J. Pain relief after varicocele embolization: The patient’s perspective. J. Med. Imaging Radiat. Oncol. 2020, 64, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tam, M.D.B.S.; Pierce, G.; McLennan, G.; Sands, M.J.; Lieber, M.S.; Wang, W. Utility of the Amplatzer Vascular Plug in splenic artery embolization: A comparison study with conventional coil technique. Cardiovasc. Interv. Radiol. 2011, 34, 522–531. [Google Scholar] [CrossRef]
- Ahuja, C.; Farsad, K.; Chadha, M. An overview of splenic embolization. Am. J. Roentgenol. 2015, 205, 720–725. [Google Scholar] [CrossRef]
- Jambon, E.; Hocquelet, A.; Petitpierre, F.; Le Bras, Y.; Marcelin, C.; Dubuisson, V.; Grenier, N.; Cornelis, F. Proximal embolization of splenic artery in acute trauma: Comparison between Penumbra occlusion device versus coils or Amplatzer Vascular Plug. Diagn. Interv. Imaging 2018, 99, 801–808. [Google Scholar] [CrossRef]
- Wang, W.; Tam, M.D.; Spain, J.; Quintini, C. Gelfoam-assisted Amplatzer Vascular Plug technique for rapid occlusion in proximal splenic artery embolization. AJR Am. J. Roentgenol. 2013, 200, 677–681. [Google Scholar] [CrossRef]
- Widlus, D.M.; Moeslein, F.M.; Richard, H.M. Evaluation of the Amplatzer Vascular Plug for proximal splenic artery embolization. J. Vasc. Interv. Radiol. JVIR 2008, 19, 652–656. [Google Scholar] [CrossRef]
- Coccolini, F.; Montori, G.; Catena, F.; Kluger, Y.; Biffl, W.; Moore, E.E.; Reva, V.; Bing, C.; Bala, M.; Fugazzola, P.; et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J. Emerg. Surg. WJES 2017, 12, 40. [Google Scholar] [CrossRef]
- Ng, E.H.; Comin, J.; David, E.; Pugash, R.; Annamalai, G. Amplatzer Vascular Plug 4 for proximal splenic artery embolization in blunt trauma. J. Vasc. Interv. Radiol. JVIR 2012, 23, 976–979. [Google Scholar] [CrossRef]
- Gheju, I.; Venter, M.D.; Beuran, M.; Gulie, L.; Racoveanu, I.; Carstea, P.; Iftimie Nastase, I.; Venter, D.P. Grade IV blunt splenic injury--the role of proximal angioembolization. A case report and review of literature. J. Med. Life 2013, 6, 369–375. [Google Scholar]
- Brinkman, D.J.; Troquay, S.; de Jonge, W.J.; Irwin, E.D.; Vervoordeldonk, M.J.; Luyer, M.D.P.; Nederend, J. Morphometric analysis of the splenic artery using contrast-enhanced computed tomography (CT). Surg. Radiol. Anat. 2021, 43, 377–384. [Google Scholar] [CrossRef]
- Dorenberg, E.J.; Hafsahl, G.; Andersen, R.; Krohg-Sørensen, K. Recurrent rupture of a hypogastric aneurysm caused by spontaneous recanalization of an Amplatzer Vascular Plug. J. Vasc. Interv. Radiol. 2006, 17, 1037–1041. [Google Scholar] [CrossRef]
- Gómez-Martínez, P.; Ciampi Dopazo, J.J.; González Fejás, A.; Lanciego, C. Recanalización espontánea tras la embolización de una arteria renal con un tapón vascular Amplatzer tipo 4. Radiología 2014, 56, 357–360. (In Spanish) [Google Scholar] [CrossRef]
- Fidelman, N.; Gordon, R.L.; Bloom, A.I.; LaBerge, J.M.; Kerlan, R.K. Reperfusion of pulmonary arteriovenous malformations after successful embolotherapy with vascular plugs. J. Vasc. Interv. Radiol. JVIR 2008, 19, 1246–1250. [Google Scholar] [CrossRef]
- Koganemaru, M.; Tanoue, S.; Kuhara, A.; Kugiyama, T.; Abe, T. Internal coil packing method for the Amplatzer vascular plug 4. Diagn. Interv. Radiol. 2019, 25, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, S.; Ichihashi, S.; Yamamoto, H.; Bolstad, F.; Kichikawa, K. Coil-in-plug technique using the Amplatzer Vascular Plug II to occlude a portosystemic shunt. Vasc. Endovasc. Surg. 2021, 56, 121–125. [Google Scholar] [CrossRef]
- Chegai, F.; Gandini, R. Intraplug coils delivery for fast closure of giant arteriovenous fistulas (AVFs) aneurysm in dialyzed patient. Radiol. Case Rep. 2019, 15, 163–166. [Google Scholar] [CrossRef]
- Trerotola, S.O.; Pyeritz, R.E. Does use of coils in addition to Amplatzer Vascular Plugs prevent recanalization? AJR Am. J. Roentgenol. 2010, 195, 766–771. [Google Scholar] [CrossRef]
- Maldonado Fernández, N.; López Espada, C.; Linares Palomino, J.P.; Pérez Vallecillos, P.; García Róspide, V. Migration and surgical retrieval of an Amplatzer Septal Occluder into abdominal aorta. Ann. Vasc. Surg. 2020, 69, 449.e11–449.e16. [Google Scholar] [CrossRef]
- Maleux, G.; Rega, F.; Heye, S.; Troost, E.; Budts, W. Asymptomatic migration of a first-generation Amplatzer Vascular Plug into the abdominal aorta: Conservative management may be an option. J. Vasc. Interv. Radiol. 2011, 22, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Ozyer, U.; Aytekin, C.; Yildirim, U.M.; Harman, A.; Karakayali, F.; Boyvat, F. Use of the Amplatzer® Vascular Plug II in endovascular occlusion of dialysis shunts with tributary veins. J. Vasc. Access 2011, 12, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Patel, M.; Ginsburg, M.; Jilani, D.; Funaki, B. Effectiveness of collateral vein embolization for salvage of immature native arteriovenous fistulas. J. Vasc. Interv. Radiol. JVIR 2014, 25, 1890–1894. [Google Scholar] [CrossRef] [PubMed]
- Zangan, S.M.; Falk, A. Optimizing arteriovenous fistula maturation. Semin. Interv. Radiol. 2009, 26, 144–150. [Google Scholar] [CrossRef][Green Version]
- Hoit, D.A.; Schirmer, C.M.; Malek, A.M. Use of the Amplatzer Vascular Plug as an anchoring scaffold for coil-mediated parent vessel occlusion: Technical case report. Neurosurgery 2006, 59 (Suppl. S1), ONS-E171–ONS-E172. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.; Kim, Y.H.; Kang, U.R.; Cha, J.G.; Lee, J.; Cha, S.I.; Kim, C.H. Efficacy and safety of Amplatzer Vascular Plug Type IV for embolization of pulmonary arteriovenous malformations. J. Vasc. Interv. Radiol. JVIR 2019, 30, 1082–1088. [Google Scholar] [CrossRef]
- Tapping, C.R.; Ettles, D.F.; Robinson, G.J. Long-term follow-up of treatment of pulmonary arteriovenous malformations with Amplatzer Vascular Plug and Amplatzer Vascular Plug II devices. J. Vasc. Interv. Radiol. JVIR 2011, 22, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Vandy, F.; Criado, E.; Upchurch, G.R.; Williams, D.M.; Rectenwald, J.; Eliason, J. Transluminal hypogastric artery occlusion with an Amplatzer Vascular Plug during endovascular aortic aneurysm repair. J. Vasc. Surg. 2008, 48, 1121–1124. [Google Scholar] [CrossRef]
| AVP I | AVP II | AVP III | AVP 4 | |
|---|---|---|---|---|
| Configuration | One cylindrical module | Three cylindrical modules | Oblong with two enlarged ends | Two profiled modules |
| Advantages and disadvantages | Short anchoring area, high radial force Low thrombogenic capacity at high flow rates | Rapid occlusion, lower risk of migration and recanalisation Long anchoring area | Faster occlusion, especially in high-flow vessels. | Can be used in tortuous anatomy and in medium calibre vessels. Delivered with diagnostic probes |
| Diameter (mm) | 4–16 | 3–22 | 4–14 (long axis) | 4–8 |
| Introducer sheath | 4 Fr: 4/6/8 mm 5 Fr: 10/12 mm 6 Fr: 14/16 mm | 4 Fr: 3/4/6/8 mm 5 Fr: 10/12 mm 6 Fr: 14/16 mm 7 Fr: 18/20/22 mm | 4 Fr: 4/6 mm 5 Fr: 8/10 mm 7 Fr: 12/14 mm | Diagnostic probe 0.038 |
| Guiding catheter | 5 Fr: 4/6/8 mm 6 Fr: 10/12 mm 8 Fr: 14/16 mm | 5 Fr: 3/4/6/8 mm 6 Fr: 10/12 mm 8 Fr: 14/16 mm 9 Fr: 18/20/22 mm | 6 Fr: 4/6 mm 7 Fr: 8/10 mm 9 Fr: 12/14 mm | 4 or 5 Fr depending on the probe model |
| Unconstrained length (mm) | 7–8 | 6–18 | 6.5 | 10–13.5 |
| Maximum length to be delivered (cm) | 100 | 100 | 120 | 100 |
| Features | Median (Range) or No. (%) |
|---|---|
| Age, years | 43 (30–66) |
| Males/Females | 88 (80%)/22 (20%) |
| Coagulation disorders | 5 (6.8%) |
| aPTT > 1.5 | 2 (1.8%) |
| INR > 3 | 2 (1.8%) |
| aPTT > 1.5 and INR > 3 | 1 (0.9%) |
| Model and Size | Number of AVPs (%) |
|---|---|
| AVP IV | 101 (50) |
| 5 mm | 7 (3.5) |
| 6 mm | 10 (4.9) |
| 7 mm | 23 (11.4) |
| 8 mm | 61 (30.2) |
| AVP II | 101 (50) |
| 10 mm | 31 (15.3) |
| 12 mm | 24 (11.9) |
| 14 mm | 12 (5.9) |
| 16 mm | 15 (7.4) |
| 18 mm | 8 (4.0) |
| 20 mm | 4 (2.0) |
| 22 mm | 7 (3.5) |
| Arteries | n = 39 (33%) | Veins | n = 79 (67%) |
|---|---|---|---|
| Splenic | 18 (15.3) | Gonadal | 44 (37.3) |
| Renal | 4 (3.4) | Internal iliac | 8 (6.8) |
| Gastro-duodenal | 3 (2.5) | TIPS 1 | 7 (5.9) |
| Gluteal | 3 (2.5) | Cephalic | 5 (4.2) |
| Carotid | 2 (1.7) | Porto-hepatic fistula | 2 (1.7) |
| Deep femoral | 2 (1.7) | Basilic | 2 (1.7) |
| Main hepatic | 2 (1.7) | Humeral | 2 (1.7) |
| Other | 5 (4.2) | Other | 9 (7.6) |
| Approach | N (%) |
|---|---|
| Arterial | 38 (34) |
| Venous | 73 (66) |
| Degree of urgency | |
| Emergent a | 19 (17) |
| High-priority b | 18 (16) |
| Scheduled c | 74 (67) |
| Indications | |
| Varicocele | 33 (29.8) |
| Haemostasis | 19 (17.1) |
| Pelvic varicose veins | 12 (10.8) |
| Collaterals of arteriovenous dialysis fistulas | 12 (10.8) |
| Arterial aneurysm | 8 (7.2) |
| Occlusion/recalibration TIPS 1 | 7(6.3) |
| Arterio-venous fistula | 6 (5.4) |
| Hepatic arterial infusion | 5 (4.5) |
| Arterio-venous malformation | 4 (3.6) |
| Varicose veins due to portal hypertension | 3 (2.7) |
| Other | 2 (1.8) |
| Utilisation | N (%) |
|---|---|
| One AVP | 24 (20%) |
| One AVP combined with | 94 (80%) |
| Coils | 19 |
| 1 other AVPs + coils | 16 |
| 1 other AVP | 13 |
| 1 other AVP + Polidocanol | 11 |
| Polidocanol | 6 |
| Cyanoacrylate | 6 |
| 1 other AVP + Polidocanol + coils | 5 |
| Polidocanol + coils | 5 |
| Coils + cyanoacrylate | 4 |
| Stent | 4 |
| 1 other AVP + cyanoacrylate | 3 |
| 1 other AVP + stent | 1 |
| Particles | 1 |
| N of materials used in combination with AVPs (n = 94) | |
| AVP | 49 |
| Coils a | 49 |
| Polidocanol b | 27 |
| Cyanoacrylate c | 13 |
| Stent | 5 |
| Particles d | 1 |
| Complications | N (%) |
|---|---|
| Total | 8 (8.2) |
| Minor | 2 (2) |
| Puncture-site hematoma | 1 (1) |
| Patient-reported discomfort | 1 (1) |
| Major | 6 (2.5) |
| Unrelated to AVP use | 5 (5.1) |
| False aneurysm at the puncture site | 3 (3.1) |
| Extensive porto-renal varicose vein thrombosis | 1 (1) |
| Cardiorespiratory failure | 1 (1) |
| Related to AVP use | 1 (1) |
| AVP migration into the IVC requiring removal | 1 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loffroy, R.; Chevallier, O.; Mazit, A.; Malakhia, A.; Midulla, M. Amplatzer™ Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study. J. Clin. Med. 2023, 12, 6790. https://doi.org/10.3390/jcm12216790
Loffroy R, Chevallier O, Mazit A, Malakhia A, Midulla M. Amplatzer™ Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study. Journal of Clinical Medicine. 2023; 12(21):6790. https://doi.org/10.3390/jcm12216790
Chicago/Turabian StyleLoffroy, Romaric, Olivier Chevallier, Amin Mazit, Alexandre Malakhia, and Marco Midulla. 2023. "Amplatzer™ Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study" Journal of Clinical Medicine 12, no. 21: 6790. https://doi.org/10.3390/jcm12216790
APA StyleLoffroy, R., Chevallier, O., Mazit, A., Malakhia, A., & Midulla, M. (2023). Amplatzer™ Vascular Plugs for Embolisation: A 10-Year Single-Centre Retrospective Study. Journal of Clinical Medicine, 12(21), 6790. https://doi.org/10.3390/jcm12216790






