Medical Rehabilitation of Patients with Post-COVID-19 Syndrome—A Comparison of Aerobic Interval and Continuous Training
Abstract
:1. Introduction
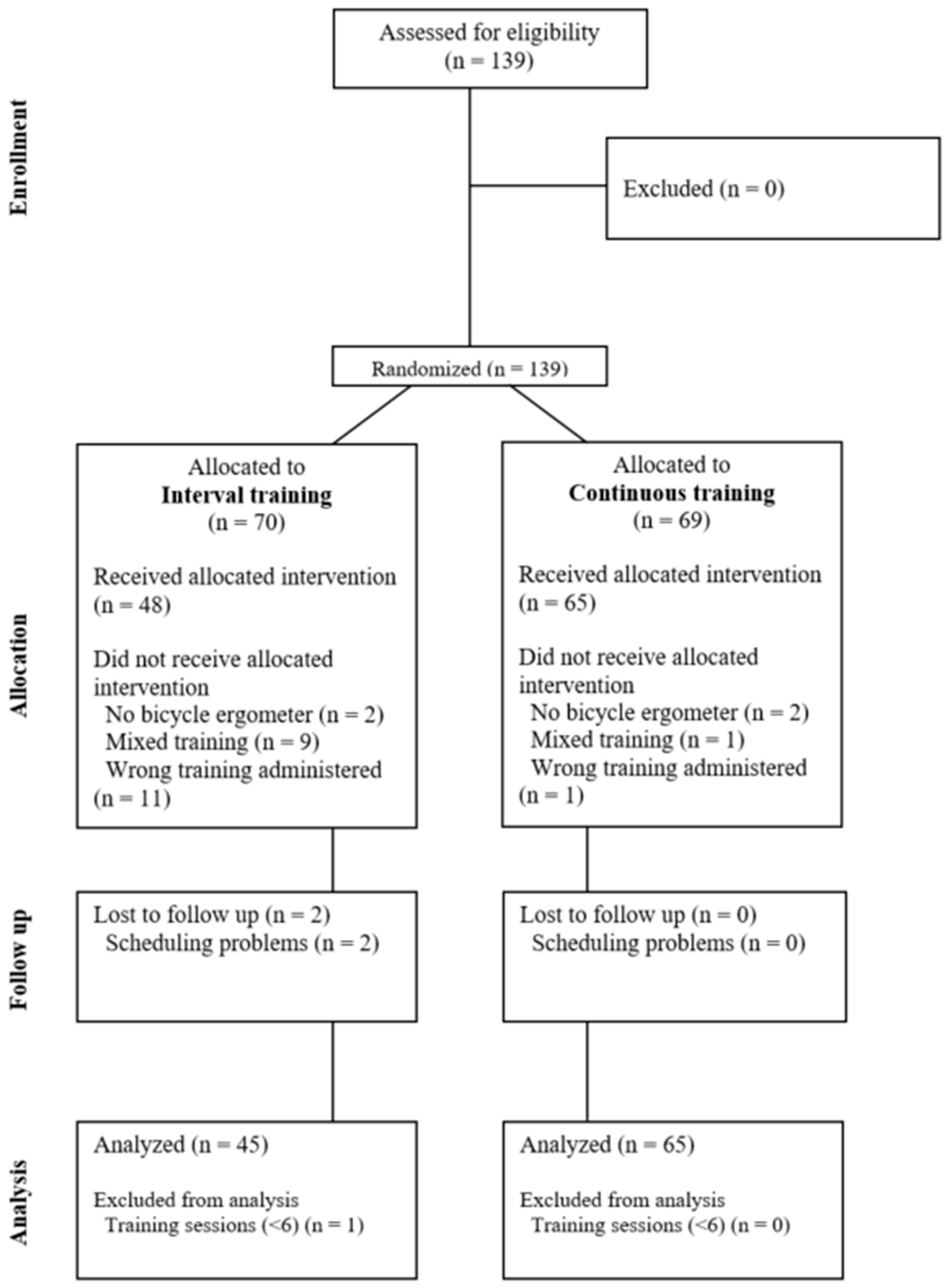
2. Materials and Methods
2.1. Study Design
2.2. Study Populations
2.2.1. PCS Patients
2.2.2. CAD Patients
2.2.3. Ethical Approval
2.2.4. Exercise Training
2.2.5. Assessment of Perceived Disease Burden, Functional Status, and Fatigue
2.2.6. Cardiopulmonary Exercise Testing (CPET)
2.2.7. Laboratory Parameters
2.2.8. Statistical Analysis
3. Results
3.1. Disease Perception
3.2. Baseline Performance and Physical Exercise Training
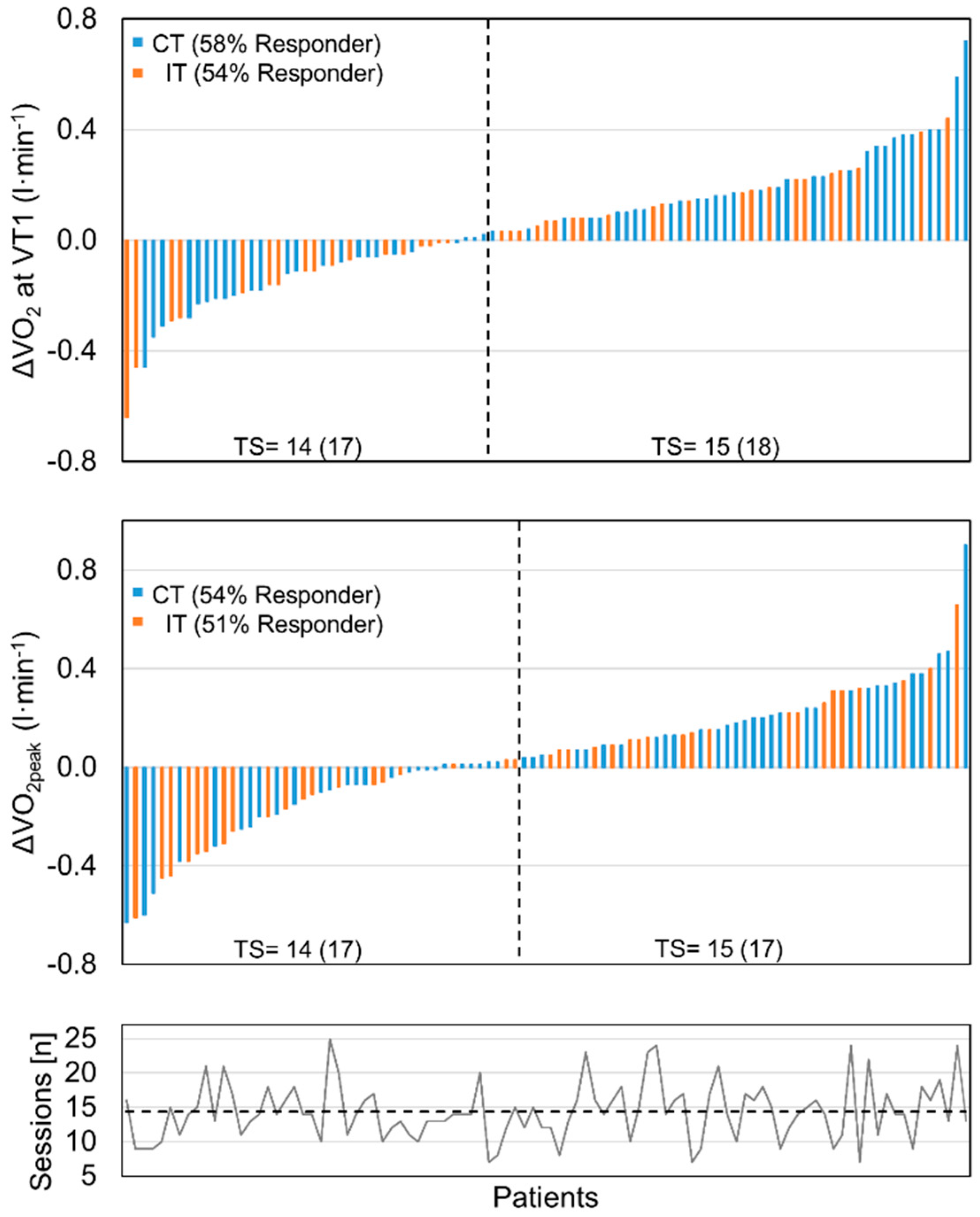
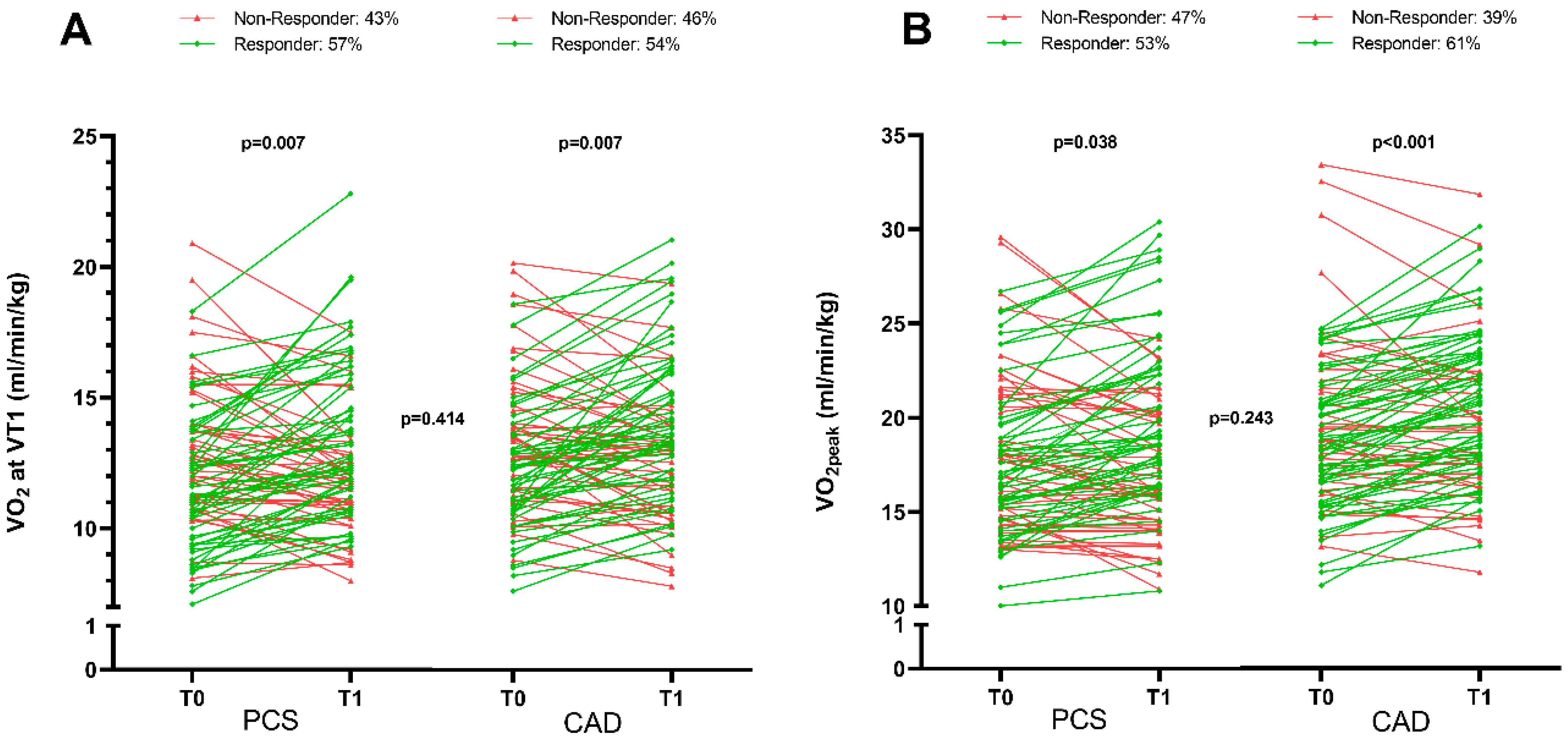
3.3. Effect of Training Modalities on Physical Exercise Capacity
3.4. Comparison of Medical Rehabilitation Effectiveness on Physical Performance Improvement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Böing, S.; Brinkmann, F.; Franke, C.; Glöckl, R.; Gogoll, C.; Hummel, T.; et al. S1-Leitlinie Post-COVID/Long-COVID [S1 Guideline Post-COVID/Long-COVID]. Pneumologie 2021, 75, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Rabady, S.; Altenberger, J.; Brose, M.; Denk-Linnert, D.-M.; Fertl, E.; Götzinger, F.; de la Cruz Gomez Pellin, M.; Hofbaur, B.; Hoffmann, K.; Hoffmann-Dorninger, R.; et al. Leitlinie S1: Long COVID: Differenzialdiagnostik und Behandlungsstrategien [Guideline S1: Long COVID: Diagnostics and treatment strategies]. Wien. Klin. Wochenschr. 2021, 133 (Suppl. S7), 237–278. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Serviente, C.; Decker, S.T.; Layec, G. From heart to muscle: Pathophysiological mechanisms underlying long-term physical sequelae from SARS-CoV-2 infection. J. Appl. Physiol. 2022, 132, 581–592. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Nittas, V.; Gao, M.; West, E.A.; Ballouz, T.; Menges, D.; Wulf Hanson, S.; Puhan, M.A. Long COVID Through a Public Health Lens: An Umbrella Review. Public Health Rev. 2022, 43, 1604501. [Google Scholar] [CrossRef]
- Garvey, C.; Bayles, M.P.; Hamm, L.F.; Hill, K.; Holland, A.; Limberg, T.M.; Spruit, M.A. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: An Official Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 75–83. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Barbara, C.; Clavario, P.; De Marzo, V.; Lotti, R.; Guglielmi, G.; Porcile, A.; Russo, C.; Griffo, R.; Mäkikallio, T.; Hautala, A.J.; et al. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur. J. Prev. Cardiol. 2022, 29, e258–e260. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Martínez, B.J.S.-A.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.; Cavalheri, V.; Hill, K. Effects of high intensity interval training on exercise capacity in people with chronic pulmonary conditions: A narrative review. BMC Sports Sci. Med. Rehabil. 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Myers, J.; Forman, D.E.; Lavie, C.J.; Guazzi, M. Should high-intensity-aerobic interval training become the clinical standard in heart failure? Heart Fail. Rev. 2013, 18, 95–105. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Timmons, M.P.; Kruger, C.; McNeely, M.; Taylor, D.A.; Clark, A.M. Meta-Analysis of Aerobic Interval Training on Exercise Capacity and Systolic Function in Patients with Heart Failure and Reduced Ejection Fractions. Am. J. Cardiol. 2013, 111, 1466–1469. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients with Heart Failure with Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA 2021, 325, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Adolfo, J.R.; Dhein, W.; Sbruzzi, G. Intensity of physical exercise and its effect on functional capacity in COPD: Systematic review and meta-analysis. J. Bras. Pneumol. 2019, 45, e20180011. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Nonoyama, M.; Goldstein, R.S.; Hill, K.; Dolmage, T.E.; Mathur, S.; Brooks, D. Interval versus continuous training in individuals with chronic obstructive pulmonary disease—A systematic review. Thorax 2010, 65, 157–164. [Google Scholar] [CrossRef]
- Keech, A.; Way, K.; Holgate, K.; Fildes, J.; Indraratna, P.; Yu, J. HIIT for post-COVID patients within cardiac rehabilitation: Response to letter to the editor. Int. J. Cardiol. 2021, 322, 291–292. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med. Sci. Sport. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Topp, C.W.; Østergaard, S.D.; Søndergaard, S.; Bech, P. The WHO-5 Well-Being Index: A systematic review of the literature. Psychother. Psychosom. 2015, 84, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, J.; Tempel, J. Erhaltung, Förderung und Entwicklung der Arbeitsfähigkeit: Konzepte und Forschungsergebnisse aus Finnland. In Fehlzeiten—Report 2002, Demographischer Wandel: Herausforderung für die Betriebliche Personal- und Gesundheitspolitik; Badura, B., Schellschmidt, H., Vetter, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Westenberger, A.; Nöhre, M.; Brähler, E.; Morfeld, M.; de Zwaan, M. Psychometric properties, factor structure, and German population norms of the multidimensional fatigue inventory (MFI-20). Front. Psychiatry 2022, 13, 1062426. [Google Scholar] [CrossRef]
- Herrmann, C.; Buss, U. Vorstellung und Validierung einer deutschen Version der “Hospital Anxiety and Depression Scale” (HAD-Skala). Ein Fragebogen zur Erfassung des psychischen Befindens bei Patienten mit körperlichen Beschwerden. [Description and validation of a German version of the Hospital Anxiety and Depression Scale (HADS): A questionnaire for identifying emotional disorders in physically ill patients]. Diagnostica 1994, 40, 143–154. [Google Scholar]
- Bullinger, M.; Kirchberger, I.; Ware, J. Der deutsche SF-36 Health Survey Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität [The German SF-36 Health Survey Translation and psychometric testing of a cross-disease instrument for assessing health-related quality of life]. J. Public Health 1995, 3, 21–36. [Google Scholar] [CrossRef]
- Brähler, E.; Mühlan, H.; Albani, C.; Schmidt, S. Teststatistische Prüfung und Normierung der deutschen Versionen des EUROHIS-QOL Lebensqualität-Index und des WHO-5 Wohlbefindens-Index [Test statistical testing and standardization of the German versions of the EUROHIS-QOL quality of life index and the WHO-5 well-being index]. Diagnostica 2007, 53, 83–96. [Google Scholar] [CrossRef]
- Dowman, L.M.; May, A.K.; Hill, C.J.; Bondarenko, J.; Spencer, L.; Morris, N.R.; Alison, J.A.; Walsh, J.; Goh, N.S.L.; Corte, T.; et al. High intensity interval training versus moderate intensity continuous training for people with interstitial lung disease: Protocol for a randomised controlled trial. BMC Pulm. Med. 2021, 21, 361. [Google Scholar] [CrossRef]
- Schmitz, B.; Niehues, H.; Thorwesten, L.; Klose, A.; Krüger, M.; Brand, S.-M. Sex Differences in High-Intensity Interval Training–Are HIIT Protocols Interchangeable Between Females and Males? Front. Physiol. 2020, 11, 38. [Google Scholar] [CrossRef]
- Sainani, K.L. Dealing with Missing Data. PM R 2015, 7, 990–994. [Google Scholar] [CrossRef]
- Schäfer, H.; Teschler, M.; Mooren, F.C.; Schmitz, B. Altered tissue oxygenation in patients with post COVID-19 syndrome. Microvasc. Res. 2023, 148, 104551. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent Exertional Intolerance after COVID-19: Insights from Invasive Cardiopulmonary Exercise Testing. Chest 2022, 161, 54–63. [Google Scholar] [CrossRef]
- Clavario, P.; De Marzo, V.; Lotti, R.; Barbara, C.; Porcile, A.; Russo, C.; Beccaria, F.; Bonavia, M.; Bottaro, L.C.; Caltabellotta, M.; et al. Cardiopulmonary exercise testing in COVID-19 patients at 3 months follow-up. Int. J. Cardiol. 2021, 340, 113–118. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Franco-López, F.; Buendía-Romero, Á.; Martínez-Cava, A.; Sánchez-Agar, J.A.; Martínez, B.J.S.; Courel-Ibáñez, J.; Pallarés, J.G. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: A randomized controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, M.; Rzepka-Cholasińska, A.; Pietrzykowski, Ł.; Michalski, P.; Kosobucka-Ozdoba, A.; Jasiewicz, M.; Kasprzak, M.; Kryś, J.; Kubica, A. Effects of Multidisciplinary Rehabilitation Program in Patients with Long COVID-19: Post-COVID-19 Rehabilitation (PCR SIRIO 8) Study. J. Clin. Med. 2023, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.; Frederix, I.; Dendale, P.; Janssen, A.; Doherty, P.; Piepoli, M.F.; Völler, H.; Davos, C.H.; the Secondary Prevention and Rehabilitation Section of EAPC Reviewers. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: The avenue towards EAPC accreditation programme: A position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 2020, 28, 496–509. [Google Scholar] [CrossRef]
- Hanakam, F.; Ferrauti, A. Ausdauertraining. In Trainingswissenschaft für die Sportpraxis, Lehrbuch für Studium, Ausbildung und Unterricht im Sport; Ferrauti, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 345–404. [Google Scholar]
| Overall (n = 110) | IT (n = 45) | CT (n = 65) | p-Value | |
|---|---|---|---|---|
| Anthropometric data | ||||
| Age, years | 49.3 ± 11.8 | 50.5 ± 10.1 | 48.4 ± 12.8 | 0.359 |
| Sex, n (%) | 0.693 | |||
| female | 42 (38.2) | 16 (35.6) | 26 (40.0) | |
| male | 68 (61.8) | 29 (64.4) | 39 (60.0) | |
| Height, cm | 174.6 ± 9.5 | 174.5 ± 9.2 | 174.7 ± 9.7 | 0.924 |
| Weight, kg | 92.5 ± 21.0 | 93.3 ± 18.2 | 91.9 ± 22.8 | 0.372 |
| BMI, kg·m−2 | 30.2 ± 6.0 | 30.6 ± 5.7 | 29.9 ± 6.2 | 0.270 |
| Clinical data | ||||
| Diseases of the circulatory system, n (%) | 72 (65.6) | 29 (64.4) | 43 (66.2) | 0.855 |
| Arterial hypertension | 55 (50) | 22 (48.9) | 33 (50.8) | 0.848 |
| Pulmonary embolism | 10 (9.1) | 4 (8.9) | 6 (9.2) | 0.952 |
| Paroxysmal tachycardia | 15 (13.6) | 4 (8.9) | 11 (16.9) | 0.231 |
| Coronary artery disease | 3 (2.7) | 2 (4.4) | 1 (1.5) | 0.405 |
| Other | 23 (20.9) | 8 (17.8) | 15 (23.1) | 0.506 |
| Endocrine, nutritional, or metabolic diseases, n (%) | 73 (66.4) | 31 (68.9) | 42 (64.6) | 0.645 |
| Obesity | 57 (51.8) | 26 (57.8) | 31 (47.7) | 0.302 |
| Type 2 diabetes mellitus | 12 (10.9) | 3 (6.7) | 9 (13.8) | 0.239 |
| Hypothyroidism, n (%) | 11 (10.0) | 3 (6.7) | 8 (12.3) | 0.337 |
| Other | 25 (22.7) | 8 (17.8) | 17 (26.2) | 0.307 |
| Diseases of the musculoskeletal system and connective tissue, n (%) | 43 (39.1) | 12 (26.7) | 31 (47.7) | 0.026 |
| Diseases of the nervous system, n (%) | 28 (25.5) | 13 (28.9) | 15 (23.1) | 0.502 |
| Migraine/headache | 3 (2.7) | 1 (2.2) | 2 (3.1) | 0.789 |
| Other | 25 (22.7) | 12 (26.7) | 13 (20.0) | 0.426 |
| Mental and behavioral disorders, n (%) | 28 (25.5) | 13 (28.9) | 15 (23.1) | 0.502 |
| Depressive/adjustment disorders | 19 (17.3) | 9 (20.0) | 10 (15.4) | 0.542 |
| Other | 10 (9.1) | 4 (8.9) | 6 (9.2) | 0.952 |
| Diseases of the respiratory system, n (%) | 15 (13.6) | 8 (17.8) | 7 (10.8) | 0.316 |
| Diseases of the digestive system, n (%) | 14 (12.7) | 5 (11.1) | 9 (13.8) | 0.676 |
| Neoplasms, n (%) | 7 (6.4) | 2 (4.4) | 5 (7.7) | 0.497 |
| Medication | ||||
| ACE inhibitor | 19 (17.3) | 8 (17.8) | 11 (16.9) | 0.909 |
| Statin | 20 (18.2) | 8 (17.8) | 12 (18.5) | 0.928 |
| Beta blocker | 40 (36.4) | 16 (35.6) | 24 (36.9) | 0.885 |
| AT-II receptor blocker | 24 (21.8) | 11 (24.4) | 13 (20.0) | 0.588 |
| Calcium channel blocker | 21 (19.1) | 11 (24.4) | 10 (15.4) | 0.254 |
| Anticoagulant | 22 (20.0) | 8 (17.8) | 14 (21.5) | 0.632 |
| Antiarrhythmic | 1 (0.9) | 0 (0) | 1 (1.5) | 0.408 |
| Diuretic | 22 (20.0) | 12 (26.7) | 10 (15.4) | 0.165 |
| Glucocorticoid | 18 (16.4) | 7 (15.6) | 11 (16.9) | 0.851 |
| Analgesic | 26 (23.6) | 10 (22.2) | 16 (24.6) | 0.774 |
| Antidepressant | 15 (13.6) | 7 (15.6) | 8 (12.3) | 0.636 |
| Diabetes medication | 6 (5.5) | 1 (2.2) | 5 (7.7) | 0.218 |
| Blood Parameters | ||||
| Leukocytes, n/nL | 6.9 ± 1.8 | 7.0 ± 1.8 | 6.8 ± 1.9 | 0.674 |
| Erythrocytes, M/µL | 4.9 ± 0.4 | 4.9 ± 0.4 | 4.9 ± 0.5 | 0.847 |
| Red cell distribution width (RDW), % | 13.1 ± 0.9 | 13.1 ± 0.7 | 13.2 ± 1.1 | 0.568 |
| Hemoglobin, g/dL | 14.5 ± 1.4 | 14.7 ± 1.2 | 14.4 ± 1.5 | 0.314 |
| Hematocrit, % | 43.6 ± 3.8 | 44.0 ± 3.3 | 43.3 ± 4.1 | 0.368 |
| Mean corpuscular volume (MCV), fl | 90.0 ± 5.3 | 90.6 ± 3.2 | 89.7 ± 6.3 | 0.407 |
| Mean corpuscular hemoglobin (MCH), pg | 30.0 ± 2.1 | 30.2 ± 1.1 | 29.8 ± 2.5 | 0.330 |
| Mean cellular hemoglobin concentration (MCHC), g/dL | 33.3 ± 1.1 | 33.4 ± 0.8 | 33.2 ± 1.2 | 0.474 |
| Thrombocytes, n/nL | 254.6 ± 67.1 | 261.1 ± 54.0 | 250.2 ± 74.8 | 0.402 |
| HbA1c, % | 6.1 ± 0.8 | 6.1 ± 0.7 | 6.0 ± 0.9 | 0.891 |
| C-reactive protein, mg/dL | 0.4 ± 0.6 | 0.3 ± 0.3 | 0.4 ± 0.7 | 0.424 |
| Sodium, mmol/L | 141.0 ± 2.1 | 141.5 ± 2.2 | 140.6 ± 2.1 | 0.023 |
| Potassium, mmol/L | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.4 | 0.401 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.438 |
| Urea, mg/dL | 30.5 ± 8.0 | 30.4 ± 7.2 | 30.6 ± 8.5 | 0.882 |
| eGFR, mL/min/1.73qm | 95.5 ± 15.0 | 93.7 ± 15.0 | 96.8 ± 15.1 | 0.305 |
| Uric acid, mg/dL | 5.9 ± 1.5 | 6.1 ± 1.4 | 5.7 ± 1.5 | 0.100 |
| Triglycerides, mg/dL | 166.7 ± 112.0 | 169.0 ± 89.6 | 165.1 ± 125.8 | 0.858 |
| Cholesterol, mg/dL | 207.5 ± 49.4 | 207.6 ± 60.0 | 207.5 ± 41.0 | 0.989 |
| HDL cholesterol, mg/dL | 55.1 ± 16.7 | 55.3 ± 17.8 | 55.1 ± 16.0 | 0.947 |
| LDL cholesterol, mg/dL | 134.0 ± 43.5 | 136.5 ± 52.7 | 132.2 ± 36.1 | 0.635 |
| LDL/HDL ratio | 2.6 ± 1.0 | 2.6 ± 0.9 | 2.6 ± 1.1 | 0.890 |
| Creatin kinase, U/L | 159.2 ± 125.7 | 163.2 ± 110.6 | 156.6 ± 135.7 | 0.789 |
| Glutamate oxalacetate transaminase (GOT), U/L | 32.6 ± 13.1 | 34.2 ± 15.7 | 31.6 ± 12.0 | 0.322 |
| Glutamate pyruvate transaminase (GPT), U/L | 39.7 ± 23.1 | 42.1 ± 19.9 | 38.0 ± 25.1 | 0.370 |
| Gamma glutamyl transferase (gamma GT), U/L | 38.9 ± 26.5 | 45.0 ± 32.8 | 34.8 ± 20.5 | 0.071 |
| Alkaline phosphatase, U/L | 79.4 ± 25.5 | 77.8 ± 23.1 | 80.4 ± 27.1 | 0.599 |
| Thyroid stimulating hormone (TSH), mlU/L | 1.4 ± 0.8 | 1.5 ± 0.9 | 1.2 ± 0.6 | 0.164 |
| Overall | IT | CT | p-Value | |
|---|---|---|---|---|
| (n = 110) | (n = 45) | (n = 65) | ||
| Multidimensional fatigue inventory (MFI-20) | ||||
| Overall Score | ||||
| T0 | 69.8 ± 13.2 | 68.2 ± 14.9 | 70.9 ± 11.9 | 0.339 |
| T1 | 58.6 ± 15.9 | 54.3 ± 14.6 | 61.4 ± 16.1 | 0.029 # |
| Δ | −11.3 ± 15.0 *** | −14.2 ± 12.6 | −9.4 ± 16.3 | 0.124 |
| Physical Fatigue | ||||
| T0 | 77.2 ± 15.5 | 76.3 ± 16.8 | 77.8 ± 14.1 | 0.663 |
| T1 | 62.8 ± 19.2 | 57.7 ± 17.7 | 66.1 ± 19.6 | 0.033 # |
| Δ | −14.3 ± 20.5 *** | −19.1 ± 18.9 | −11.3 ± 21.0 | 0.062 |
| Mental Fatigue | ||||
| T0 | 65.5 ± 20.0 | 64.3 ± 18.9 | 66.3 ± 20.8 | 0.621 |
| T1 | 57.0 ± 19.9 | 53.1 ± 18.8 | 59.6 ± 20.2 | 0.112 |
| Δ | −9.1 ± 16.2 *** | −11.7 ± 14.9 | −7.5 ± 16.9 | 0.212 |
| SF-36 Health-related quality of life | ||||
| Physical Component Score (PCS) | ||||
| T0 | 30.4 ± 7.8 | 31.3 ± 7.0 | 29.9 ± 8.3 | 0.366 |
| T1 | 35.2 ± 9.1 | 38.0 ± 8.8 | 33.4 ± 8.9 | 0.015 # |
| Δ | 4.6 ± 7.2 *** | 6.9 ± 7.3 | 3.1 ± 6.7 | 0.013 # |
| Mental Component Score (MCS) | ||||
| T0 | 35.7 ± 12.2 | 36.5 ± 11.2 | 35.1 ± 12.9 | 0.573 |
| T1 | 41.7 ± 11.5 | 41.5 ± 11.6 | 41.9 ± 11.6 | 0.852 |
| Δ | 6.0 ± 10.2 *** | 6.0 ± 8.5 | 6.0 ± 11.2 | 0.984 |
| Wellbeing (WHO-5) | ||||
| T0 | 8 (22) | 8 (20) | 8 (18) | 0.356 |
| T1 | 14 (24) | 15 (23) | 12.5 (22) | 0.126 |
| Δ | 4 (26) *** | 5 (22) | 4 (26) | 0.301 |
| Hospital anxiety and depression scale (HADS) | ||||
| Anxiety | ||||
| T0 | 7 (16) | 8 (15) | 6.5 (16) | 0.19 |
| T1 | 6 (18) | 6 (18) | 5.5 (15) | 0.935 |
| Δ | 0 (18) ** | 0 (17) | 0 (18) | 0.628 |
| Depression | ||||
| T0 | 7 (19) | 8 (16) | 7 (19) | 0.64 |
| T1 | 5 (20) | 5 (20) | 5 (20) | 0.681 |
| Δ | 0 (22) * | 0 (22) | 0 (17) | 0.71 |
| Workability Index (WAI) $ | 22.1 ± 7.4 | 23.7 ± 8.0 | 21.1 ± 6.8 | 0.104 |
| max. incapacity for work last 12 months § | 99 (365) | 99 (365) | 99 (365) | 0.859 |
| Overall | IT | CT | p-Value | |
|---|---|---|---|---|
| (n = 110) | (n = 45) | (n = 65) | ||
| Resting | ||||
| Heart rate, beat·min−1 | ||||
| T0 | 89.1 ± 11.5 | 88.3 ± 11.2 | 89.7 ± 11.7 | 0.545 |
| T1 | 83.3 ± 11.6 | 83.6 ± 11.1 | 83.1 ± 12.0 | 0.82 |
| Δ | −6.0 ± 10.1 *** | −4.0 ± 9.7 | −7.2 ± 10.2 | 0.126 |
| O2 pulse, mL·beat−1 | ||||
| T0 | 6.8 ± 1.9 | 6.8 ± 1.8 | 6.8 ± 1.9 | 0.915 |
| T1 | 6.8 ± 1.6 | 7.0 ± 1.4 | 6.6 ± 1.6 | 0.169 |
| Δ | −0.0 ± 1.5 | 0.2 ± 1.4 | −0.2 ± 1.5 | 0.313 |
| Ventilatory equivalent O2 (VE/VO2) | ||||
| T0 | 28.0 ± 5.9 | 27.0 ± 6.3 | 28.5 ± 5.6 | 0.21 |
| T1 | 28.2 ± 6.9 | 27.2 ± 4.9 | 29.0 ± 7.9 | 0.218 |
| Δ | 0.2 ± 7.3 | 0.2 ± 6.7 | 0.2 ± 7.7 | 0.982 |
| Ventilatory equivalent CO2 (VE/VCO2) | ||||
| T0 | 33.9 ± 8.8 | 33.8 ± 12.5 | 34.1 ± 5.0 | 0.88 |
| T1 | 35.1 ± 9.7 | 33.4 ± 3.8 | 36.2 ± 12.0 | 0.162 |
| Δ | 1.0 ± 12.6 | −0.5 ± 13.3 | 1.9 ± 12.2 | 0.367 |
| Ventilatory threshold 1 (VT1) | ||||
| Workload, W (% predicted) | ||||
| T0 | 73.7 ± 27.1 (42.5 ± 14.9) | 73.5 ± 26.9 (42.3 ± 14.2) | 73.8 ± 27.4 (42.7 ± 15.5) | 0.887 |
| T1 | 85.1 ± 25.0 (50.2 ± 15.7) | 84.9 ± 22.3 (50.6 ± 14.1) | 85.2 ± 27.0 (49.9 ± 16.9) | 0.821 |
| Δ | 10.5 ± 20.1 (6.8 ± 12.1) *** | 10.4 ± 22.5 (6.8 ± 12.9) | 10.6 ± 18.3 (6.8 ± 11.5) | 0.998 |
| Heart rate, beat·min−1 (% predicted) | ||||
| T0 | 109.3 ± 13.7 (65.4 ± 7.7) | 107.4 ± 11.9 (64.7 ± 7.3) | 110.6 ± 14.7 (65.9 ± 7.9) | 0.429 |
| T1 | 106.6 ± 20.3 (64.2 ± 11.3) | 105.5 ± 14.5 (64.3 ± 8.4) | 107.3 ± 23.7 (64.1 ± 13.1) | 0.945 |
| Δ | −3.8 ± 16.9 (−2.3 ± 10.6) | −1.5 ± 12.7 (−1.0 ± 7.7) | −5.4 ± 19.3 (−3.2 ± 12.2) | 0.318 |
| O2 pulse, mL·beat−1 (% predicted) | ||||
| T0 | 10.6 ± 2.9 (77.2 ± 16.2) | 11.0 ± 2.8 (79.7 ± 17.3) | 10.3 ± 2.9 (75.5 ± 15.2) | 0.197 |
| T1 | 11.3 ± 2.8 (83.5 ± 17.1) | 11.5 ± 2.4 (85.3 ± 16.0) | 11.2 ± 3.1 (82.2 ± 17.9) | 0.381 |
| Δ | 0.7 ± 1.8 (5.5 ± 14.7) *** | 0.4 ± 1.3 (3.2 ± 8.9) | 1.0 ± 2.1 (7.2 ± 17.7) | 0.196 |
| VO2, mL·min−1·kg−1 (% predicted) | ||||
| T0 | 12.4 ± 3.0 (50.5 ± 11.7) | 12.5 ± 2.9 (51.4 ± 12.1) | 12.4 ± 3.1 (50.0 ± 11.5) | 0.534 |
| T1 | 13.2 ± 3.4 (54.6 ± 11.7) | 12.8 ± 2.9 (54.7 ± 11.9) | 13.5 ± 3.7 (54.6 ± 11.6) | 0.941 |
| Δ | 0.8 ± 2.5 (3.1 ± 10.0) * | 0.3 ± 2.3 (1.4 ± 8.9) | 1.2 ± 2.7 (4.3 ± 10.6) | 0.164 |
| Ventilatory equivalent O2 (VE/VO2) (% predicted) | ||||
| T0 | 27.4 ± 4.7 (78.3 ± 13.4) | 27.1 ± 3.8 (77.3 ± 10.9) | 27.6 ± 5.2 (79.0 ± 14.9) | 0.541 |
| T1 | 26.6 ± 3.9 (75.9 ± 11.2) | 26.7 ± 4.1 (76.3 ± 11.7) | 26.5 ± 3.8 (75.7 ± 10.9) | 0.771 |
| Δ | −0.8 ± 4.4 (−2.1 ± 12.5) | −0.4 ± 3.5 (−1.0 ± 10.1) | −1.0 ± 4.9 (−2.9 ± 14.0) | 0.486 |
| Ventilatory equivalent CO2 (VE/VCO2) | ||||
| T0 | 30.6 ± 4.4 | 30.0 ± 3.4 | 31.0 ± 4.9 | 0.223 |
| T1 | 29.6 ± 3.8 | 29.8 ± 3.6 | 29.5 ± 3.9 | 0.687 |
| Δ | −0.9 ± 3.3 * | −0.3 ± 2.6 | −1.3 ± 3.7 | 0.121 |
| Peak exercise | ||||
| Respiratory exchange rate (RER) (% predicted) | ||||
| T0 | 1.05 ± 0.1 (87.1 ± 7.7) | 1.06 ± 0.1 (87.3 ± 6.7) | 1.05 ± 0.1 (87.0 ± 8.3) | 0.815 |
| T1 | 1.05 ± 0.1 (87.1 ± 6.4) | 1.06 ± 0.1 (87.4 ± 6.2) | 1.05 ± 0.1 (86.8 ± 6.6) | 0.634 |
| Δ | 0.00 ± 0.1 (0.2 ± 6.3) | 0.00 ± 0.1 (0.0 ± 5.4) | −0.0 ± 0.1 (−0.4 ± 6.8) | 0.758 |
| Workload, W (% predicted) | ||||
| T0 | 127.8 ± 38.5 (73.4 ± 21.1) | 130.2 ± 38.0 (74.1 ± 20.7) | 126.2 ± 39.0 (72.9 ± 21.5) | 0.775 |
| T1 | 140.1 ± 39.2 (81.0 ± 24.6) | 142.5 ± 37.1 (84.2 ± 19.5) | 138.3 ± 40.9 (78.7 ± 27.8) | 0.283 |
| Δ | 12.6 ± 18.3 (7.5 ± 15.0) *** | 11.6 ± 18.5 (8.7 ± 14.7) | 13.4 ± 18.4 (6.6 ± 15.3) | 0.507 |
| Heart rate, beat·min−1 (% predicted) | ||||
| T0 | 135.3 ± 21.2 (80.9 ± 11.5) | 134.2 ± 18.3 (80.7 ± 10.4) | 136.1 ± 23.0 (81.0 ± 12.3) | 0.89 |
| T1 | 132.2 ± 20.2 (79.6 ± 10.9) | 129.7 ± 17.0 (79.0 ± 9.4) | 134.0 ± 22.2 (80.1 ± 11.9) | 0.645 |
| Δ | −2.6 ± 13.9 (−1.4 ± 8.5) | −3.3 ± 12.4 (−1.9 ± 7.6) | −2.2 ± 15.1 (−1.1 ± 9.1) | 0.642 |
| O2 pulse, ml·beat−1 (% predicted) | ||||
| T0 | 12.7 ± 3.3 (92.6 ± 17.4) | 13.1 ± 3.3 (94.5 ± 18.4) | 12.4 ± 3.2 (91.4 ± 16.7) | 0.37 |
| T1 | 13.3 ± 3.0 (97.9 ± 16.0) | 13.6 ± 2.8 (100.1 ± 17.2) | 13.1 ± 3.1 (96.3 ± 15.0) | 0.266 |
| Δ | 0.6 ± 1.8 (4.6 ± 12.4) *** | 0.3 ± 1.6 (2.8 ± 11.5) | 0.8 ± 1.9 (5.9 ± 12.9) | 0.233 |
| VO2, mL·min−1·kg−1 (% predicted) | ||||
| T0 | 18.3 ± 4.4 (74.4 ± 15.3) | 18.5 ± 4.3 (75.7 ± 14.8) | 18.2 ± 4.5 (73.6 ± 15.7) | 0.482 |
| T1 | 18.9 ± 4.8 (77.8 ± 15.6) | 18.7 ± 4.4 (78.8 ± 14.8) | 19.1 ± 5.2 (77.1 ± 16.3) | 0.592 |
| Δ | 0.8 ± 2.9 (2.7 ± 11.0) * | 0.2 ± 2.9 (0.8 ± 11.2) | 1.3 ± 2.9 (4.1 ± 10.8) | 0.157 |
| Ventilatory equivalent O2 (VE/VO2) (% predicted) | ||||
| T0 | 33.8 ± 6.1 (96.5 ± 17.5) | 33.6 ± 5.5 (96.0 ± 15.7) | 33.9 ± 6.6 (96.8 ± 18.7) | 0.826 |
| T1 | 33.1 ± 5.1 (94.4 ± 14.5) | 33.3 ± 5.8 (95.1 ± 16.5) | 32.9 ± 4.5 (93.9 ± 12.9) | 0.699 |
| Δ | −0.6 ± 4.6 (−1.7 ± 13.1) | −0.3 ± 3.3 (−1.0 ± 9.5) | −0.8 ± 5.4 (−2.2 ± 15.3) | 0.658 |
| Ventilatory equivalent CO2 (VE/VCO2) | ||||
| T0 | 32.1 ± 5.1 | 31.8 ± 4.3 | 32.3 ± 5.7 | 0.601 |
| T1 | 31.4 ± 4.3 | 31.4 ± 4.5 | 31.4 ± 4.1 | 0.942 |
| Δ | −0.5 ± 3.6 | −0.4 ± 2.5 | −0.6 ± 4.2 | 0.733 |
| Respiratory minute ventilation (VE), L·min−1 (% predicted) | ||||
| T0 | 60.3 ± 15.0 (58.0 ± 13.3) | 61.3 ± 14.1 (58.4 ± 12.2) | 59.6 ± 15.7 (57.8 ± 14.1) | 0.807 |
| T1 | 60.9 ± 15.8 (59.0 ± 13.4) | 61.5 ± 14.8 (59.7 ± 13.0) | 60.4 ± 16.7 (58.4 ± 13.8) | 0.643 |
| Δ | 0.9 ± 11.0 (1.3 ± 10.6) | −0.3 ± 10.4 (0.2 ± 9.7) | 1.7 ± 11.4 (2.0 ± 11.2) | 0.417 |
| Tidal volume (Vt), L (% predicted) | ||||
| T0 | 2.1 ± 0.6 (72.4 ± 23.6) | 2.1 ± 0.5 (73.1 ± 21.1) | 2.1 ± 0.6 (71.9 ± 25.3) | 0.792 |
| T1 | 2.2 ± 0.6 (74.4 ± 20.6) | 2.2 ± 0.6 (74.1 ± 19.2) | 2.2 ± 0.6 (74.7 ± 21.8) | 0.887 |
| Δ | 0.1 ± 0.3 (2.1 ± 23.3) | 0.1 ± 0.3 (1.6 ± 21.9) | 0.1 ± 0.3 (2.4 ± 24.4) | 0.882 |
| Breathing frequency (Bf), breaths·min−1 (% predicted) | ||||
| T0 | 30.1 ± 8.0 (54.7 ± 14.5) | 29.9 ± 6.2 (54.4 ± 11.3) | 30.2 ± 9.1 (54.9 ± 16.4) | 0.874 |
| T1 | 29.1 ± 6.8 (53.0 ± 12.4) | 29.4 ± 6.4 (53.5 ± 11.5) | 28.9 ± 7.2 (52.6 ± 13.0) | 0.729 |
| Δ | −0.6 ± 4.9 (−1.1 ± 8.8) | −1.1 ± 4.1 (−2.0 ± 7.2) | −0.3 ± 5.4 (−0.4 ± 9.7) | 0.386 |
| Breathing reserve (BR), % | ||||
| T0 | 37.9 ± 19.5 | 36.1 ± 19.7 | 39.1 ± 19.4 | 0.44 |
| T1 | 37.6 ± 18.0 | 37.2 ± 18.0 | 37.9 ± 18.1 | 0.853 |
| Δ | 0.6 ± 15.9 | 3.5 ± 13.9 | −1.5 ± 17.0 | 0.129 |
| Overall (n = 110) | IT (n = 45) | CT (n = 65) | p-Value | |
|---|---|---|---|---|
| Sessions prescribed, n | 15 (20) | 16 (16) | 14 (20) | 0.125 |
| Sessions performed, % | 92.7 ± 10.7 | 89.5 ± 11.3 | 94.9 ± 9.6 | 0.012 |
| Workload Increase, % | 10.1 ± 10.5 | 9.6 ± 10.1 | 10.4 ± 10.9 | 0.695 |
| Exercise heart rate, beat·min−1 | 112.2 ± 14.8 | 113.9 ± 14.4 | 111.1 ± 15.0 | 0.317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mooren, J.M.; Garbsch, R.; Schäfer, H.; Kotewitsch, M.; Waranski, M.; Teschler, M.; Schmitz, B.; Mooren, F.C. Medical Rehabilitation of Patients with Post-COVID-19 Syndrome—A Comparison of Aerobic Interval and Continuous Training. J. Clin. Med. 2023, 12, 6739. https://doi.org/10.3390/jcm12216739
Mooren JM, Garbsch R, Schäfer H, Kotewitsch M, Waranski M, Teschler M, Schmitz B, Mooren FC. Medical Rehabilitation of Patients with Post-COVID-19 Syndrome—A Comparison of Aerobic Interval and Continuous Training. Journal of Clinical Medicine. 2023; 12(21):6739. https://doi.org/10.3390/jcm12216739
Chicago/Turabian StyleMooren, Johanna M., René Garbsch, Hendrik Schäfer, Mona Kotewitsch, Melina Waranski, Marc Teschler, Boris Schmitz, and Frank C. Mooren. 2023. "Medical Rehabilitation of Patients with Post-COVID-19 Syndrome—A Comparison of Aerobic Interval and Continuous Training" Journal of Clinical Medicine 12, no. 21: 6739. https://doi.org/10.3390/jcm12216739
APA StyleMooren, J. M., Garbsch, R., Schäfer, H., Kotewitsch, M., Waranski, M., Teschler, M., Schmitz, B., & Mooren, F. C. (2023). Medical Rehabilitation of Patients with Post-COVID-19 Syndrome—A Comparison of Aerobic Interval and Continuous Training. Journal of Clinical Medicine, 12(21), 6739. https://doi.org/10.3390/jcm12216739







