Abstract
The present systematic review aimed to determine the chronic effects of the combination of transcranial direct current stimulation (tDCS) and exercise on motor function and performance outcomes. We performed a systematic literature review in the databases MEDLINE and Web of Science. Only randomized control trials that measured the chronic effect of combining exercise (comprising gross motor tasks) with tDCS during at least five sessions and measured any type of motor function or performance outcome were included. A total of 22 interventions met the inclusion criteria. Only outcomes related to motor function or performance were collected. Studies were divided into three groups: (a) healthy population (n = 4), (b) neurological disorder population (n = 14), and (c) musculoskeletal disorder population (n = 4). The studies exhibited considerable variability in terms of tDCS protocols, exercise programs, and outcome measures. Chronic use of tDCS in combination with strength training does not enhance motor function in healthy adults. In neurological disorders, the results suggest no additive effect if the exercise program includes the movements pretending to be improved (i.e., tested). However, although evidence is scarce, tDCS may enhance exercise-induced adaptations in musculoskeletal conditions characterized by pain as a limiting factor of motor function.
1. Introduction
Noninvasive brain-stimulation techniques have gained attention in neuroscience and clinical research due to their ability to modulate cortical excitability and influence various cognitive and motor functions. Among these techniques, transcranial direct current stimulation (tDCS) involves the application of low-intensity (1–3 mA) constant electrical currents to specific cortical areas through two electrodes on the scalp [1,2]. This safe, painless, and noninvasive technique [3] can induce modulations in cortical excitability lasting up to 90 min with just a 13 min application [4,5]. Furthermore, tDCS polarity, whether anodal (a-tDCS) or cathodal (c-tDCS), can, respectively, increase or decrease the resting membrane potential of the targeted brain area [1].
The versatility of tDCS has sparked research interest across various fields, such as cognitive enhancement [6] or pain management [7]. However, a special focus has been directed towards investigating its immediate (i.e., acute) effects on motor function, including rehabilitation [8] or sports performance [9,10,11,12,13,14,15]. For example, among the healthy population, several systematic reviews and meta-analyses suggest that a single session of tDCS may increase performance in several motor tasks, like endurance time to exhaustion, visuomotor skills, and strength [9,10,11]. Other populations, like adults with neurological disorders, may also benefit from the acute effects of tDCS. For example, Beretta et al. [7] revealed moderate improvements in postural control and balance following a single session of tDCS in adults with neurological disorders.
However, most of the research conducted to date has primarily focused on the acute effects of a single tDCS session [12,13,14,15]. These acute effects may be especially relevant in contexts where immediate performance is crucial, such as competitive sports. Nevertheless, tDCS could be systematically employed to induce or enhance the chronic adaptations derived from other interventions like exercise. The acute effects of tDCS may increase the quality of gross motor-task performance (depending on the task) during training sessions, thus optimizing or accelerating motor-skill acquisition or exercise-induced adaptations [12]. This may be relevant not only in the field of sports performance but also for populations like adults with neurological or musculoskeletal disorders, in which initial motor performance deficits may compromise the quality of life and/or rehabilitation.
However, evidence about the potential benefits of systematically (i.e., chronically) incorporating tDCS into exercise protocols is scarce and controversial. Two studies did not report any effect of tDCS on motor performance after 12 sessions of tDCS combined with exercise in stroke survivors [16,17]. However, Wang et al.’s [18] systematic review revealed that tDCS led to greater improvements in the dynamic postural stability index following a period of 4–6 sessions of postural training and stimulation in healthy subjects. Additionally, the variability in experimental designs, combined with the lack of long-term evidence, poses challenges in synthesizing studies for meta-analyses [19,20].
Therefore, the aim of the present systematic review is to determine the chronic effects of combining tDCS with exercise (comprising gross motor tasks) on motor function and performance outcomes. We hypothesize that the immediate effects of tDCS before or during exercise, when used systematically during a training or rehabilitation period, would chronically enhance motor function and performance to a greater extent than exercise alone. We addressed this aim through a systematic literature search that identified three main different populations where the effects on motor function of chronic tDCS in combination with exercise have been investigated: (a) healthy population, (b) neurological disorder population, and (c) musculoskeletal disorder population.
2. Methods
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21].
2.1. Search Strategy
A systematic literature search was conducted on the US National Library of Medicine (PubMed) and Web of Science databases up to 28 March 2023. The search strategy employed the following terms: (exercise OR “resistance training” OR endurance OR aerobic OR “strength training” OR running OR cycling) AND (a-tDCS OR anodal-tDCS OR c-tDCS OR cathodal-tDCS OR tDCS OR “transcranial direct current stimulation”). The search was performed by D. M.-F. In cases of uncertainty, a second author (G. M.) was involved in the process until a consensus was reached. The authors of the selected articles were contacted to request any relevant missing information.
2.2. Eligibility Criteria and Study Selection
After the removal of duplicates, the titles and abstracts of the remaining articles were screened. Subsequently, the full texts of the obtained reports were evaluated. The following inclusion criteria had to be met in the studies: (a) published in English, (b) adult population (aged over 18), (c) randomized control trials, (d) the intervention protocol combined exercise with tDCS, (e) the protocol mainly comprised gross motor tasks (e.g., gait, dumbbell biceps curl, or cycling), (f) measurement of any type of motor function or performance outcome before and after the intervention (e.g., grip strength, knee extension peak torque, or 10 m walk test time), and (g) at least 5 sessions conducted during the intervention. Studies involving cognitive interventions were excluded.
2.3. Data Collection and Extraction
After study selection, the essential characteristics of the trials were reported in three tables, including the main author and year of publication, sample (size, age, and gender), tDCS protocol, exercise protocol, and outcomes. Only outcomes related to motor function or performance were collected. If any study had more than two intervention groups, only data from the sham stimulation plus exercise group and the real stimulation plus exercise group were collected. The participants’ inclusion criteria were reported in the tables for neurological and musculoskeletal disorder populations.
For significant results, the corresponding p-value was recorded. Whenever possible, the level of significance from the magnitude of change comparison or effect x time interaction was extracted to compare the tDCS effect with the sham group.
2.4. Risk of Bias and Quality of Evidence Assessment
The risk of bias and the methodological quality of the included studies were evaluated using the Physiotherapy Evidence Database (PEDro) scale [22]. Studies with a score of ≥6/10 were considered “high quality”, while those with lower scores were categorized as “low quality”. The methodological quality of each study was assessed by D. M.-F. In case of uncertainty, a second author (G. M.) participated in the rating process until a consensus was reached.
3. Results
3.1. Search Results
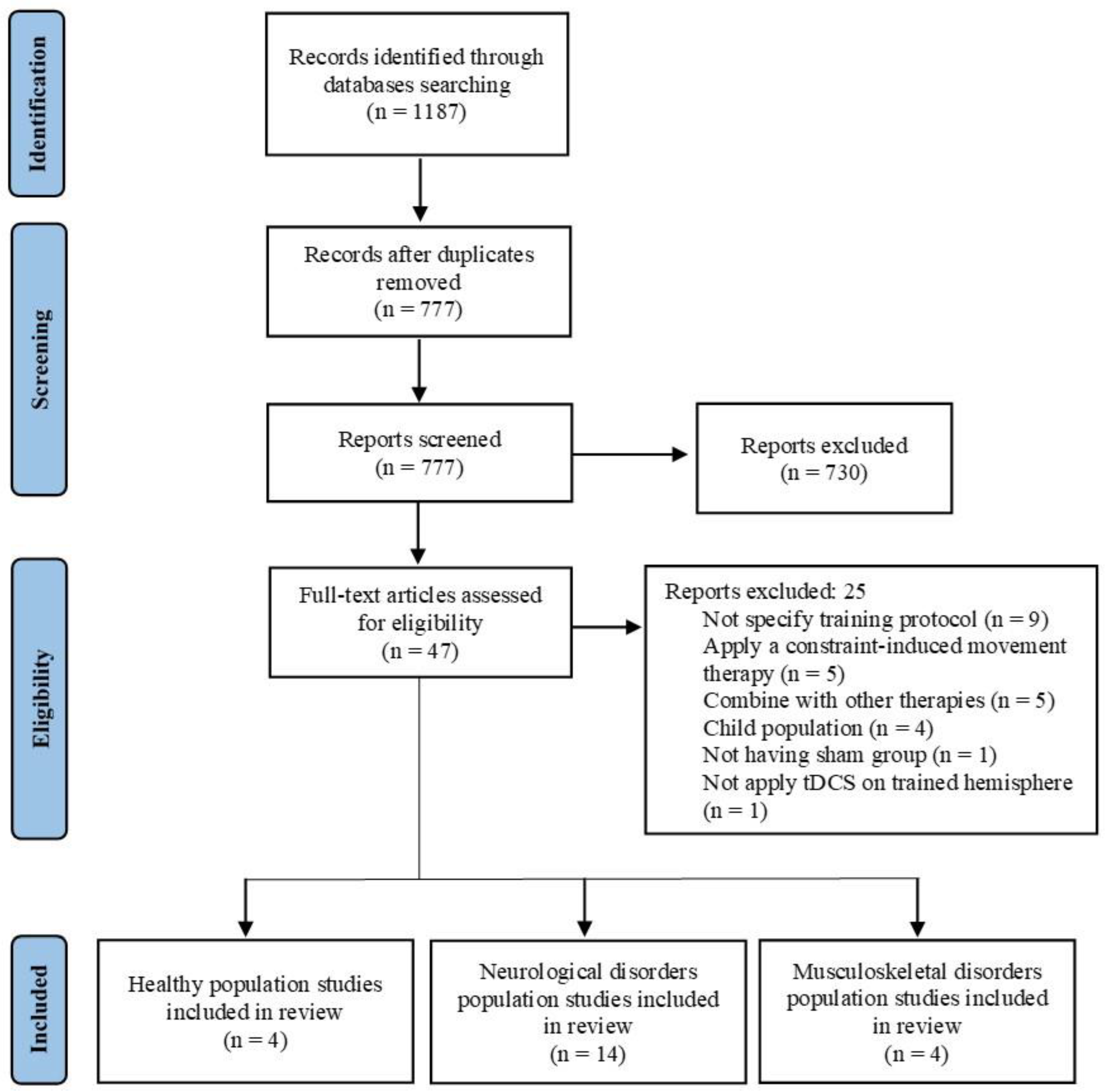
Figure 1 shows the flow diagrams for the entire search process. Initially, 1187 studies were identified (575 in PubMed and 612 in Web of Science). After removing duplicates, 777 studies remained. A screening of titles and abstracts resulted in 47 studies for full-text screening. Ultimately, 22 studies that met the inclusion criteria were selected. After performing a qualitative analysis, studies were divided into three groups based on population characteristics: (a) healthy population (n = 4), (b) neurological disorder population (n = 14), and (c) musculoskeletal disorder population (n = 4).
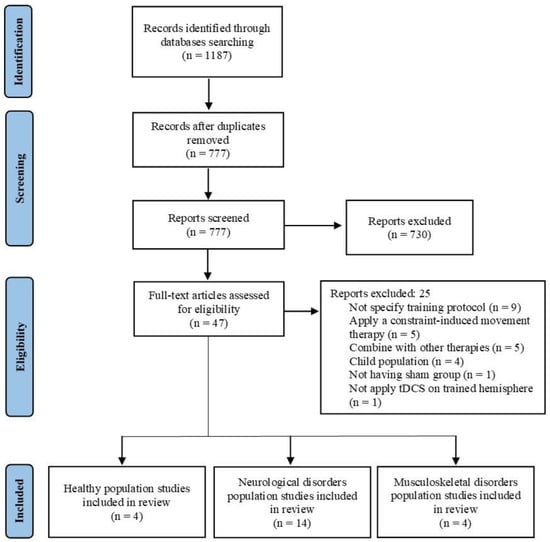
Figure 1.
Flow diagram of the studies that underwent the review process.
3.2. Risk of Bias and Methodological Quality of Studies
All the included studies obtained a PEDro score between 6 and 9 points, indicating a “high methodological quality” (mean score: 7.59 ± 0.91). The most frequently omitted items were the “intention to treat” analysis (16 studies), blinding of therapists (12 studies), blinding of assessors (7 studies), and allocation was concealed (6 studies) (Table 1). Notably, several studies were double blinded without specifying the second blind (i.e., therapist or assessor). In such cases, it was assumed that the assessors were blinded and the therapists were not.

Table 1.
Description of methodological quality assessment with PEDRo scale.
3.3. Healthy Population
3.3.1. Participants and Study Characteristics
Table 2 presents the participants and study characteristics of the healthy population. The total number of participants was 129 (49 M, 50 W, and 30 unspecified [26]). The mean age of the subjects ranged from 20 to 26 years, except for the study by Jung et al. [19], where the mean age ranged from 39 to 40 years.

Table 2.
Main characteristics of the subjects, protocols and main results related to the motor function or performance in a healthy population.
Most studies applied conventional a-tDCS or high-definition tDCS (HD-tDCS) [26]. The target electrode was placed over the primary motor area (M1) for a-tDCS (intensity: 2 mA; surface electrode: 25–28 cm2) or at Cz for HD-tDCS (intensity: 2 mA; surface electrode: 3.14 cm2). All studies, except one [24], applied tDCS online.
The training protocols varied significantly across the studies (session range: 7–21). The study by Jung et al. [19] implemented a “strength-endurance” circuit training. The other studies involved different strengthening exercises, such as dumbbell wrist extension at 70% 1 RM [23], knee flexion and extension with a maximum intention on an isokinetic machine at 30°/s [25], or various foot-core exercises [26].
3.3.2. Primary Outcomes
No study reported greater improvements in strength [23,24,25,26], “strength-endurance”, Sargent jump height [24], or balance [26] in the real tDCS group compared to the sham group. Only Xiao et al. [21] reported greater improvement in toe flexor strength (31 ± 19% vs. 9 ± 17%) (Table 2).
3.4. Neurological Disorder Population
3.4.1. Participants and Study Characteristics
Table 3 summarizes the participants and study characteristics of the neurological disorder population. The total number of participants was 409 (237 M, 210 W) with a mean age ranging between 40 and 73 years. Among the participants, 258 were stroke patients, 109 had multiple sclerosis, 22 were Parkinson’s disease patients, and 20 were old people with mild cognitive impairment.

Table 3.
Main characteristics of the subjects, protocols, and main results related to motor function or performance in a neurological disorder population.
The tDCS was applied over M1 (intensity: 1–2.5 mA; surface electrode: 12.5–35 cm2) [16,17,27,29,30,31,33,35,36,38], 3 cm lateral to the inion (intensity: 2 mA; surface electrode: 25–35 cm2) [32,34], supplementary motor area (intensity: 1 mA; surface electrode: 25 cm2) [28], or 2 cm anterior to the vertex (intensity: 2 mA; surface electrode: 35 cm2] [37]. Wong et al. [26] applied a-tDCS on ipsilesional M1 or c-tDCS on contralesional M1 in stroke patients. Five studies applied online stimulation, while nine studies were offline (see Table 3).
The exercise programs varied across the studies (session range 6–36). The main components of the protocols involved gait [27,28,31,34,37], strength training alone [16,17,30] or combined with treadmill walking [29,33], circuit training combined with treadmill walking [32], cycling [36], elliptical ergometer [35], or Tai Chi [38].
3.4.2. Primary Outcomes
The combination of tDCS with exercise in patients with neurological disorders shows varying effects across different outcome measures. In stroke patients, no significant additional effects were observed in dexterity [16,17], spasticity [16], range of motion [17], or balance [27]. However, tDCS showed additional effects over just exercise in the Fugl-Meyer Assessment in three studies [29,30,31], but not in the other four [16,17,27,28]. Similarly, tDCS plus exercise had no additional benefits for the Wolf Motor-Function Test in one study [16] but led to greater improvements in the functional ability score in another one [30]. tDCS did not add further benefits for the Trunk Impairment Scale and Performance-Oriented Mobility Assessment than just exercise [28].
Regarding strength in stroke patients, tDCS enhanced the effect of exercise on knee flexion and extension peak torque of the paretic limb but not on the nonparetic limb, further reducing extension but not the flexion bilateral deficit [29]. However, grip strength [16,17,30], the strength of different joint actions, and performance in the Five Times Sit-to-Stand Test [30] did not benefit from adding tDCS to exercise.
Adding tDCS to exercise improved the gait speed of stroke patients in two studies [28,29] but not in the other three [27,30,31]. Similarly, the Timed Up-and-Go Test performance benefited from adding tDCS to exercise in one study [28] but not in two other studies [27,30]. No additive effects were found when gait speed was measured while patients performed other motor tasks (i.e., motor dual-task gait) [31].
Cardiovascular fitness, measured as external work performed at maximum oxygen consumption or at the gas-exchange threshold, showed greater improvements in the tDCS group in one study [29]. However, there were no significant differences in maximum oxygen consumption or oxygen consumption at the gas-exchange threshold in the same study.
In multiple sclerosis patients, balance improved more in the tDCS group in one study [33]. Regarding functional tests, the Timed Up-and-Go Test improved more in the tDCS group in one study [36] and did not differ in two studies [32,34], and there was no comparison between groups in one study [33]. The Figure-of-Eight Walk Test and Dynamic Gait Index did not differ between groups in one study [32]. tDCS had a significant effect on gait speed in one study [35] and did not have an effect in another study [34], and the significance differed on the method of assessment in two studies (i.e., gait speed vs. distance covered in a 10 m Walk Test and a 2 min Walk Test vs. 5 m Walk Test, respectively) [35,36].
In Parkinson’s disease patients, the only study included did not report significant improvements in bradykinesia, Timed Up-and-Go Test, gait speed, or balance in the real tDCS group compared to the sham group [37].
In old people with mild cognitive impairment, the only study included did not report significant improvements in normal gait speed or in motor dual-task walk speed [38].
3.5. Musculoskeletal Disorder Population
3.5.1. Participants and Study Characteristics
Table 4 summarizes the participants and study characteristics of the musculoskeletal disorder population. The total number of participants was 112 (32 M, 80 W). The mean age of the participants ranged from 20 to 25 years, except for the study by Chang et al. [36], where the age range was 60–64 years. Among the participants, 54 had chronic ankle instability, 30 had knee osteoarthritis, and 28 were women experiencing patellofemoral pain.

Table 4.
Main characteristics of the subjects, protocols, and main results related to motor function or performance in the musculoskeletal disorder population.
Most studies applied conventional a-tDCS or HD-tDCS [40]. The target electrode was allocated to M1 (intensity: 1–2 mA; surface electrode: 15–35 cm2) [39,41] or Cz (intensity: 2 mA; surface electrode: 0.79 cm2) [40,42]. Two studies employed online tDCS, while the other two offline tDCS (see Table 4). All studies implemented a strength-training program (session range 10–16).
3.5.2. Primary Outcomes
In individuals with chronic ankle instability, balance improved more in the tDCS group in one study [40], but not in the other one [39]. However, there were no significant differences between groups in ankle proprioception [40], strength, and Side Hop Test [39].
For individuals with knee osteoarthritis, the only included study reported a greater improvement in the tDCS group for the Western Ontario and McMaster Universities Osteoarthritis Index Physical Function Subscale [41].
In the only study that included women with patellofemoral pain, the tDCS group exhibited a significant improvement in strength compared to the sham group [42].
4. Discussion
The purpose of this systematic review was to examine the chronic effects of combining tDCS with exercise on motor function and performance. We hypothesized that systematic application of tDCS before or during exercise over a training or rehabilitation period would chronically enhance motor function and performance to a greater extent than just exercise alone. Our findings suggest that combining tDCS with exercise, compared to just exercise, (a) did not demonstrate greater chronic effects on performance in healthy individuals; (b) the effects on function and performance in neurological disorder populations varied depending on the task tested, but overall results suggest modest or null additive effects when exercise is specific enough to the motor function that wants to be improved; and (c) enhanced the effects of exercise over function in musculoskeletal conditions characterized by pain as a limiting factor of motor function (i.e., knee osteoarthritis and patellofemoral pain).
To the best of our knowledge, this is the first systematic review focused on the chronic effects of combining exercise with tDCS in healthy adults. Previous acute studies have demonstrated the positive effects of tDCS on various aspects of motor function, such as strength or endurance [9,10,11]. This acute enhancement in motor function can improve sports performance during competition and may also increase performance during training, influencing exercise-induced adaptations. For example, an acute session of tDCS has been shown to increase not only the total amount of training volume but also to enable a higher concentric movement velocity during a strength-training session [43]. Since training volume and concentric movement velocity during training influence strength training chronic adaptations [44], modulating both variables through tDCS could influence chronic adaptations. However, our systematic review suggests that, in healthy populations, tDCS does not provide additional chronic benefits when combined with exercise in strength, “strength-endurance”, jump height, or balance [23,24,25,26]. Therefore, although tDCS might be considered as an adjuvant method to enhance short-term performance, it may not provide further benefits when used chronically during training sessions. However, it is important to note that only four studies met the inclusion criteria for this review, highlighting the need for further research to establish reliable conclusions.
Stroke patients have lower corticospinal excitability in the affected hemisphere, which usually correlates with chronic poor motor function [45]. Given the potential of tDCS to increase cortical excitability [4,5], this technique could help to reduce symptoms in this population. This hypothesis has been tested by proving the effects of tDCS in combination with walking- [27,28,29,31] and strength-training-based programs [16,17,29,30] on several motor-function tests. When specific tests have been used to determine the effect over a particular domain of motor function, the results do not support the additive effect of tDCS over upper limb dexterity, spasticity, range of motion, or balance [16,17,27]. Although some studies found additive effects from using tDCS during strength training over knee flexion and extension peak torque of the paretic limb [29], no study has found additive effects over the strength of several joint actions nor over multiple-joint lower limb strength tested by the Five Times Sit-to-Stand Test [16,17,30]. When studies have used functional scales that assess multiple domains of motor function, the results are contradictory, with some studies showing positive or no effects of tDCS over tests like the Fugl-Meyer Assessment [16,17,27,28,29,30,31], Wolf Motor-Function Test [16,30], or Performance-Oriented Mobility Assessment [28]. Also, the effects over gait are mixed, with studies showing improved gait speed [28,29] or no additive effects [27,30,31]. Although the high controversy in the results may be related to high heterogeneity in training programs and/or the tests used, overall, the results suggest that tDCS does not enhance the effects of exercise on motor function in this population. These findings align with other systematic reviews and meta-analyses investigating the effects of combining tDCS with other therapies (e.g., virtual reality, physical therapy, or constraint-induced movement therapy) on upper limb spasticity, which report mixed results [19,20,46].
Regarding multiple sclerosis patients, a sense of fatigue is one of the most commonly reported symptoms and is known to interfere with daily activities [47]. Because tDCS has shown promising effects on fatigue reduction [48], its application before or during exercise may increase the overall quality of the rehabilitation session and/or increase exercise-program adherence in patients [34,35]. However, the combination of tDCS with exercise yielded mixed results across different outcome measures. The effects of combining tDCS with exercise appear to enhance balance in patients with multiple sclerosis; however, this outcome has only been measured in one study [33]. Regarding gait, some studies have reported additional benefits of tDCS in combination with exercise, such as improved gait speed or performance on the Timed Up-and-Go test [35,36]. Yet, other studies have not found benefits from including tDCS over gait speed, Timed Up-and-Go Test, Figure-of-Eight Walk Test, or Dynamic Gait Index [32,34]. It is worth noting that the studies reporting additive effects of tDCS during exercise for gait performance did not include walking as a part of the rehabilitation protocol. Therefore, it appears that if any positive effects of tDCS are present, they may be limited when the actual motor task (such as walking) is practised during rehabilitation. These findings are consistent with a systematic review highlighting the positive effects of tDCS on gait speed when applied alone or in combination with cycling, but no significant changes were observed in the Multiple Sclerosis Walking Scale [49].
In Parkinson’s disease, patients experienced a reduction in motor cortical excitability, decreased excitatory signalling from the thalamus to cortical areas, degeneration of dopaminergic neurons, and abnormalities in motor cortical region connectivity [50]. Given the potential of tDCS to modulate these factors, it could help reduce Parkinson’s disease symptoms [50]. However, the effects of tDCS in combination with exercise [gait] have only been tested in one study [37]. The results from this study show no additive effects on bradykinesia, Timed Up-and-Go Test, gait speed, or balance compared to the sham group [37]. Findings from a meta-analysis suggest that tDCS may have positive effects on upper limb motor function, speed, and strength, while more complex tasks may be less affected by tDCS [51]. Another meta-analysis indicated that tDCS interventions can provide benefits for functional locomotion, although the effect sizes were relatively small, and the treatment effects may be enhanced when multiple regions of the motor and prefrontal cortices are targeted [52]. Nevertheless, both meta-analyses synthetize the effects of not only acute and chronic studies but also the tDCS application alone or combined with other protocols (e.g., cognitive training, gait, physical therapy, etc.). From the included studies in these meta-analyses and in our review, it seems that, although tDCS could have a positive effect when applied alone, the additional benefits on motor function are overshadowed by the benefits acquired from gait training without amplifying them.
Age-related changes in motor cortical properties include a decreased corticospinal excitability that may affect motor function [53]. Although tDCS has the potential to modulate this excitability, the only study that met the inclusion criteria did not report a tDCS effect combined with Tai Chi during 12 weeks on gait speed in older adults with mild cognitive impairment [38]. Similarly, another study did not report additional benefits on gait speed when tDCS was applied alone over older adults with mild cognitive and function impairments, although it had an additional effect on balance [54]. However, findings from Rostami et al. [55] indicate the potential benefits of applying tDCS alone for five consecutive days in improving gait, balance, and lower extremity functional performance in healthy older adults. Therefore, although evidence is based only on one study, the possible benefits on gait speed from using tDCS in older people may be limited to a healthy population without relevant cognitive or function impairment.
Following a ligament injury, the individual experiences cortical abnormalities in the somatosensory, motor, and frontal cortex, such as increased motor thresholds and smaller motor-evoked potential amplitudes in the musculature surrounding the injured joint [56]. Considering this, tDCS may be beneficial for people experiencing chronic ankle instability. Only balance benefited from including tDCS plus strength training in one study [40], but not in the other one [39]. Also, no additional benefits were observed in the proprioception, strength, and Side Hop Test in any of the two studies that met the inclusion criteria. Therefore, evidence for the effectiveness of tDCS to increase the magnitude of exercise-induced adaptations is low. Additionally, only two studies were included and both used tDCS during exercise (i.e., online). Further research is needed to determine if priming M1 before exercise can enhance adaptations in this population.
As tDCS could modulate pain [7], combining tDCS with exercise for knee osteoarthritis or patellofemoral pain holds promise for increasing the responsiveness of the brain to the corticomotor benefits of exercise and/or providing additive effects on pain-system function [41,42]. The two studies that investigated the additive effects of combining tDCS with exercise (strength training in both cases) on knee osteoarthritis [41] and patellofemoral pain [42] found additive effects of combining tDCS with exercise on motor function [41] and knee-extensors strength [42]. These findings align with the findings from the Rahimi et al. [57] study, which showed that combining tDCS with a physiotherapy program can improve function in patients with knee osteoarthritis. However, other studies did not find the benefits of applying tDCS alone on knee function [58,59] or gait [59]. Therefore, although evidence is scarce, these results suggest that tDCS could potentially enhance exercise-induced adaptations in those conditions where pain is the limiting factor of motor function, such as knee osteoarthritis or patellofemoral pain.
5. Conclusions
The results of the present systematic review suggest that the effectiveness of tDCS to enhance exercise-induced adaptations may depend on the specific combination of treatment modalities and individual patient characteristics. Specifically, chronic use of tDCS in combination with strength training does not seem to enhance motor function in healthy adults. In neurological disorders, the results are contradictory, but, overall, the results suggest that the additive effects may be null if the actual exercise program already includes the functional movements pretending to be improved. However, although evidence is scarce, tDCS may enhance exercise-induced adaptations in those musculoskeletal conditions characterized by pain as a limiting factor of motor function.
Notwithstanding, these conclusions should be interpreted with caution due to several limitations derived from the included manuscripts. The studies included in this systematic review exhibited considerable variability in terms of tDCS protocols, exercise programs, and outcome measures. Other studies which combined tDCS with exercise were not included in this review due to a lack of specific exercise protocol descriptions (e.g., exercise description, number of repetitions and sets, intensity, etc.), although their results were mixed, as found in this review. Also, many populations include a few or even a single study or had relatively small sample sizes, limiting the statistical power and the generalizability of the findings.
Therefore, the chronic clinical application of this technique needs further investigation to determine if the presumed acute effects on several motor-function capacities have any priming effect over chronic exercise-induced adaptations. This review highlights the need for future investigations with standardized and detailed protocols and larger sample sizes, together with long-term follow-up assessments to enhance the quality of evidence and provide more robust conclusions regarding the chronic effects of tDCS combined with exercise on motor function and performance outcomes.
Author Contributions
V.L.-A., J.A.S.-M., I.M.-G., D.C.-P. and G.M. devised the study. D.M.-F., D.C.-P. and G.M. developed the methodology. D.M.-F. performed database search, executed screening process, data extraction, and risk of bias assessment. In case of doubt, D.C.-P. and G.M. participated in the processes. D.M.-F. wrote the initial draft of the manuscript. D.C.-P. and G.M. critically revised and corrected the manuscript’s initial draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Spanish Ministry of Science, Innovation, and Universities under Grant (PID2021-128204OA-I00-AEI/FEDER, UE); Xunta de Galicia, Universidade da Coruna/CISUG (ED431B 2021/28). JASM and VLA acquired the funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, G.; Cao, Y.; Duan, Y.Y. A Novel Highly Durable Carbon/Silver/Silver Chloride Composite Electrode for High-Definition Transcranial Direct Current Stimulation. Nanomaterials. 2021, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Coffman, B.A.; Clark, V.P.; Parasuraman, R. Battery powered thought: Enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage 2014, 85, 895–908. [Google Scholar] [CrossRef]
- Cardenas-Rojas, A.; Pacheco-Barrios, K.; Giannoni-Luza, S.; Rivera-Torrejon, O.; Fregni, F. Noninvasive brain stimulation combined with exercise in chronic pain: A systematic review and meta-analysis. Expert Rev. Neurother. 2020, 20, 401–412. [Google Scholar] [CrossRef]
- Beretta, V.S.; Santos, P.C.R.; Orcioli-Silva, D.; Zampier, V.C.; Vitório, R.; Gobbi, L.T.B. Transcranial direct current stimulation for balance rehabilitation in neurological disorders: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101736. [Google Scholar] [CrossRef]
- Shyamali Kaushalya, F.; Romero-Arenas, S.; García-Ramos, A.; Colomer-Poveda, D.; Marquez, G. Acute effects of transcranial direct current stimulation on cycling and running performance. A systematic review and meta-analysis. Eur. J. Sport Sci. 2022, 22, 113–125. [Google Scholar] [CrossRef]
- Alix-Fages, C.; Romero-Arenas, S.; Castro-Alonso, M.; Colomer-Poveda, D.; Río-Rodriguez, D.; Jerez-Martínez, A.; Fernandez-Del-Olmo, M.; Márquez, G. Short-term effects of anodal transcranial direct current stimulation on endurance and maximal force production. A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 536. [Google Scholar] [CrossRef]
- Chinzara, T.T.; Buckingham, G.; Harris, D.J. Transcranial direct current stimulation and sporting performance: A systematic review and meta-analysis of transcranial direct current stimulation effects on physical endurance, muscular strength and visuomotor skills. Eur. J. Neurosci. 2022, 55, 468–486. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; Oliveira, B.R.R.; Márquez, G. Acute Effects of Anodal Transcranial Direct Current Stimulation in Resistance and Power Exercises: A Brief Review for Coaches and Practitioners. Strength Cond. J. 2022, 44, 57–68. [Google Scholar] [CrossRef]
- Lattari, E.; Filho, B.J.R.; Junior, S.J.F.; Murillo-Rodriguez, E.; Rocha, N.; Machado, S.; Neto, G.A.M. Effects on Volume Load and Ratings of Perceived Exertion in Individuals’ Advanced Weight Training After Transcranial Direct Current Stimulation. J. Strength Cond. Res. 2020, 34, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Marinus, N.; Van Hoornweder, S.; Aarts, M.; Vanbilsen, J.; Hansen, D.; Meesen, R. The influence of a single transcranial direct current stimulation session on physical fitness in healthy subjects: A systematic review. Exp. Brain Res. 2023, 241, 31–47. [Google Scholar] [CrossRef]
- Vieira, L.A.F.; Lattari, E.; de Jesus Abreu, M.A.; Rodrigues, G.M.; Viana, B.; Machado, S.; Oliveira, B.R.R.; Neto, G.d.A. Transcranial Direct Current Stimulation (tDCS) Improves Back-Squat Performance in Intermediate Resistance-Training Men. Res. Q. Exerc. Sport 2022, 93, 210–218. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Blanchette, A.K.; Mercier, C.; Bernard-Larocque, V.; Milot, M.H. Efficacy, safety, and tolerability of bilateral transcranial direct current stimulation combined to a resistance training program in chronic stroke survivors: A double-blind, randomized, placebo-controlled pilot study. Restor. Neurol. Neurosci. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Palimeris, S.; Ansari, Y.; Remaud, A.; Tremblay, F.; Corriveau, H.; Boudrias, M.H.; Milot, M.H. Effect of a tailored upper extremity strength training intervention combined with direct current stimulation in chronic stroke survivors: A Randomized Controlled Trial. Front. Rehabil. Sci. 2022, 3, 978257. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, S.; Yu, C.; Zhou, J.; Fu, W. Effects of Transcranial Direct Current Stimulation Combined With Physical Training on the Excitability of the Motor Cortex, Physical Performance, and Motor Learning: A Systematic Review. Front. Neurosci. 2021, 15, 648354. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Aburub, A.; Raju, M.; Annino, G. Transcranial direct current stimulation for upper extremity spasticity rehabilitation in stroke survivors: A systematic review of randomized controlled trials. PM&R 2023, 15, 222–234. [Google Scholar]
- Huang, J.; Qu, Y.; Liu, L.; Zhao, K.; Zhao, Z. Efficacy and safety of transcranial direct current stimulation for post-stroke spasticity: A meta-analysis of randomised controlled trials. Clin. Rehabil. 2022, 36, 158–171. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.M.; Kidgell, D.J. Anodal tDCS applied during strength training enhances motor cortical plasticity. Med. Sci. Sports Exerc. 2013, 45, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Salazar Fajardo, J.C.; Kim, S.; Kim, B.; Oh, S.; Yoon, B. Effect of tDCS Combined With Physical Training on Physical Performance in a Healthy Population. In Research Quarterly for Exercise and Sport; Taylor Francis Group: Abingdon, UK, 2023; pp. 1–8. [Google Scholar]
- Maeda, K.; Yamaguchi, T.; Tatemoto, T.; Kondo, K.; Otaka, Y.; Tanaka, S. Transcranial direct current stimulation does not affect lower extremity muscle strength training in healthy individuals: A triple-blind, sham-controlled study. Front. Neurosci. 2017, 11, 179. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, B.; Yu, C.; Shen, B.; Zhang, X.; Ye, D.; Deng, L.; Xu, Y.; Zhou, J.; Fu, W. Effects of intervention combining transcranial direct current stimulation and foot core exercise on sensorimotor function in foot and static balance. J. Neuroeng. Rehabil. 2022, 19, 98. [Google Scholar] [CrossRef]
- Madhavan, S.; Cleland, B.T.; Sivaramakrishnan, A.; Freels, S.; Lim, H.; Testai, F.D.; Corcos, D.M. Cortical priming strategies for gait training after stroke: A controlled, stratified trial. J. Neuroeng. Rehabil. 2020, 17, 111. [Google Scholar] [CrossRef]
- Manji, A.; Amimoto, K.; Matsuda, T.; Wada, Y.; Inaba, A.; Ko, S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci. Lett. 2018, 662, 302–305. [Google Scholar] [CrossRef]
- Massaferri, R.; Montenegro, R.; de Freitas Fonseca, G.; Bernardes, W.; Cunha, F.A.; Farinatti, P. Multimodal physical training combined with tDCS improves physical fitness components in people after stroke: A double-blind randomized controlled trial. Top. Stroke Rehabil. 2023, 30, 635–648. [Google Scholar] [CrossRef]
- Prathum, T.; Piriyaprasarth, P.; Aneksan, B.; Hiengkaew, V.; Pankhaew, T.; Vachalathiti, R.; Klomjai, W. Effects of home-based dual-hemispheric transcranial direct current stimulation combined with exercise on upper and lower limb motor performance in patients with chronic stroke. Disabil. Rehabil. 2022, 44, 3868–3879. [Google Scholar] [CrossRef]
- Wong, P.L.; Yang, Y.R.; Huang, S.F.; Wang, R.Y. Effects of Transcranial Direct Current Stimulation Followed by Treadmill Training on Dual-Task Walking and Cortical Activity in Chronic Stroke: A Double-Blinded Randomized Controlled Trial. J. Rehabil. Med. 2023, 55, 5258. [Google Scholar] [CrossRef]
- Baroni, A.; Magro, G.; Martinuzzi, C.; Brondi, L.; Masiero, S.; Milani, G.; Zani, G.; Bergonzoni, A.; Basaglia, N.; Straudi, S. Combined effects of cerebellar tDCS and task-oriented circuit training in people with multiple sclerosis: A pilot randomized control trial. Restor. Neurol. Neurosci. 2022, 40, 85–95. [Google Scholar] [CrossRef]
- Marotta, N.; de Sire, A.; Marinaro, C.; Moggio, L.; Inzitari, M.T.; Russo, I.; Tasselli, A.; Paolucci, T.; Valentino, P.; Ammendolia, A. Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach. J. Clin. Med. 2022, 11, 3505. [Google Scholar] [CrossRef]
- Nguemeni, C.; Hiew, S.; Kögler, S.; Homola, G.A.; Volkmann, J.; Zeller, D. Split-Belt Training but Not Cerebellar Anodal tDCS Improves Stability Control and Reduces Risk of Fall in Patients with Multiple Sclerosis. Brain Sci. 2022, 12, 63. [Google Scholar] [CrossRef]
- Pilloni, G.; Choi, C.; Shaw, M.T.; Coghe, G.; Krupp, L.; Moffat, M.; Cocco, E.; Pau, M.; Charvet, L. Walking in multiple sclerosis improves with tDCS: A randomized, double-blind, sham-controlled study. Ann. Clin. Transl. Neurol. 2020, 7, 2310–2319. [Google Scholar] [CrossRef] [PubMed]
- Rahimibarghani, S.; Azadvari, M.; Emami-Razavi, S.Z.; Harirchian, M.H.; Rahimi-Dehgolan, S.; Fateh, H.R. Effects of Nonconsecutive Sessions of Transcranial Direct Current Stimulation and Stationary Cycling on Walking Capacity in Individuals With Multiple Sclerosis. Int. J. MS Care 2022, 24, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Costa-Ribeiro, A.; Maux, A.; Bosford, T.; Aoki, Y.; Castro, R.; Baltar, A.; Shirahige, L.; Filho, A.M.; Nitsche, M.A.; Monte-Silva, K. Transcranial direct current stimulation associated with gait training in Parkinson’s disease: A pilot randomized clinical trial. Dev. Neurorehabil. 2017, 20, 121–128. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Liu, M.N.; Wang, H.C.; Walsh, V.; Lau, C.I. Combining Transcranial Direct Current Stimulation With Tai Chi to Improve Dual-Task Gait Performance in Older Adults With Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Aging Neurosci. 2021, 13, 766649. [Google Scholar] [CrossRef]
- Bruce, A.S.; Howard, J.S.; Van Werkhoven, H.; McBride, J.M.; Needle, A.R. The Effects of Transcranial Direct Current Stimulation on Chronic Ankle Instability. Med. Sci. Sports Exerc. 2020, 52, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yin, K.; Zhuang, W.; Zhang, C.; Jiang, Y.; Huang, J.; Manor, B.; Zhou, J.; Liu, Y. Effects of combining high-definition transcranial direct current stimulation with short-foot exercise on chronic ankle instability: A pilot randomized and double-blinded study. Brain Sci. 2020, 10, 749. [Google Scholar] [CrossRef]
- Chang, W.-J.; Bennell, K.L.; Hodges, P.W.; Hinman, R.S.; Young, C.L.; Buscemi, V.; Liston, M.B.; Schabrun, S.M. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS ONE 2017, 12, e0180328. [Google Scholar] [CrossRef]
- Rodrigues, G.M.; Paixão, A.; Arruda, T.; de Oliveira, B.R.R.; Neto, G.A.M.; Neto, S.R.M.; Lattari, E.; Machado, S. Anodal Transcranial Direct Current Stimulation Increases Muscular Strength and Reduces Pain Perception in Women With Patellofemoral Pain. J. Strength Cond. Res. 2022, 36, 371–378. [Google Scholar] [CrossRef]
- Alix-Fages, C.; García-Ramos, A.; Calderón-Nadal, G.; Colomer-Poveda, D.; Romero-Arenas, S.; Fernández-Del-Olmo, M.; Márquez, G. Anodal transcranial direct current stimulation enhances strength training volume but not the force–velocity profile. Eur. J. Appl. Physiol. 2020, 120, 1881–1891. [Google Scholar] [CrossRef]
- Weakley, J.; Mann, B.; Banyard, H.; McLaren, S.; Scott, T.; Garcia-Ramos, A. Velocity-Based Training: From Theory to Application. Strength Cond. J. 2021, 43, 31–49. [Google Scholar] [CrossRef]
- Buetefisch, C.M.; Revill, K.P.; Haut, M.W.; Kowalski, G.M.; Wischnewski, M.; Pifer, M.; Belagaje, S.R.; Nahab, F.; Cobia, D.J.; Hu, X.; et al. Abnormally reduced primary motor cortex output is related to impaired hand function in chronic stroke. J. Neurophysiol. 2018, 120, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial direct current stimulation for improving spasticity after stroke: A systematic review with meta-analysis. J. Rehabil. Med. 2016, 48, 565–570. [Google Scholar] [CrossRef]
- Multiple Sclerosis Council for Clinical Practice. Fatigue and Multiple Sclerosis: Evidence Based Management Strategies for Fatigue in Multiple Sclerosis; Paralyzed Veterans of America: Washington, DC, USA, 1998. [Google Scholar]
- Ayache, S.S.; Serratrice, N.; Abi Lahoud, G.N.; Chalah, M.A. Fatigue in Multiple Sclerosis: A Review of the Exploratory and Therapeutic Potential of Non-Invasive Brain Stimulation. Front. Neurol. 2022, 13, 813965. [Google Scholar] [CrossRef] [PubMed]
- Kan, R.L.D.; Xu, G.X.J.; Shu, K.T.; Lai, F.H.Y.; Kranz, G.; Kranz, G.S. Effects of non-invasive brain stimulation in multiple sclerosis: Systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2022, 13, 20406223211069198. [Google Scholar] [CrossRef] [PubMed]
- Pol, F.; Salehinejad, M.A.; Baharlouei, H.; Nitsche, M.A. The effects of transcranial direct current stimulation on gait in patients with Parkinson’s disease: A systematic review. Transl. Neurodegener. 2021, 10, 22. [Google Scholar] [CrossRef]
- Simpson, M.W.; Mak, M. The effect of transcranial direct current stimulation on upper limb motor performance in Parkinson’s disease: A systematic review. J. Neurol. 2020, 267, 3479–3488. [Google Scholar] [CrossRef]
- Lee, H.K.; Ahn, S.J.; Shin, Y.M.; Kang, N.; Cauraugh, J.H. Does transcranial direct current stimulation improve functional locomotion in people with Parkinson’s disease? A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2019, 16, 84. [Google Scholar] [CrossRef]
- Clark, B.C.; Taylor, J.L. Age-Related Changes in Motor Cortical Properties and Voluntary Activation of Skeletal Muscle. Curr. Aging Sci. 2011, 4, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Manor, B.; Zhou, J.; Harrison, R.; Lo, O.-Y.; Travison, T.G.; Hausdorff, J.M.; Pascual-Leone, A.; Lipsitz, L. Transcranial Direct Current Stimulation May Improve Cognitive-Motor Function in Functionally Limited Older Adults. Neurorehabil Neural Repair. 2018, 32, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mosallanezhad, Z.; Ansari, S.; Ehsani, F.; Kidgell, D.; Nourbakhsh, M.R.; Bakhshi, E.; Jaberzadeh, S. Multi-session anodal transcranial direct current stimulation enhances lower extremity functional performance in healthy older adults. Exp. Brain Res. 2020, 238, 1925–1936. [Google Scholar] [CrossRef]
- Needle, A.R.; Lepley, A.S.; Grooms, D.R. Central Nervous System Adaptation After Ligamentous Injury: A Summary of Theories, Evidence, and Clinical Interpretation. Sports Med. 2017, 47, 1271–1288. [Google Scholar] [CrossRef]
- Rahimi, F.; Nejati, V.; Nassadj, G.; Ziaei, B.; Mohammadi, H.K. The effect of transcranial direct stimulation as an add-on treatment to conventional physical therapy on pain intensity and functional ability in individuals with knee osteoarthritis: A randomized controlled trial. Neurophysiol. Clin. 2021, 51, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Rezasoltani, Z.; Najafi, S.; Mohebi, B.; Tabatabaee, S.M.; Dadarkhah, A. Transcranial direct current stimulation for knee osteoarthritis: A single-blind randomized sham-controlled trial. Neurophysiol. Clin. 2021, 51, 329–338. [Google Scholar] [CrossRef]
- Ahn, H.; Woods, A.J.; Kunik, M.E.; Bhattacharjee, A.; Chen, Z.; Choi, E.; Fillingim, R.B. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: An experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul. 2017, 10, 902–909. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).