Anterolateral Thigh Chimeric Flap: An Alternative Reconstructive Option to Free Flaps for Large Soft Tissue Defects
Abstract
1. Introduction
2. Patients and Methods
Operative Technique
3. Results
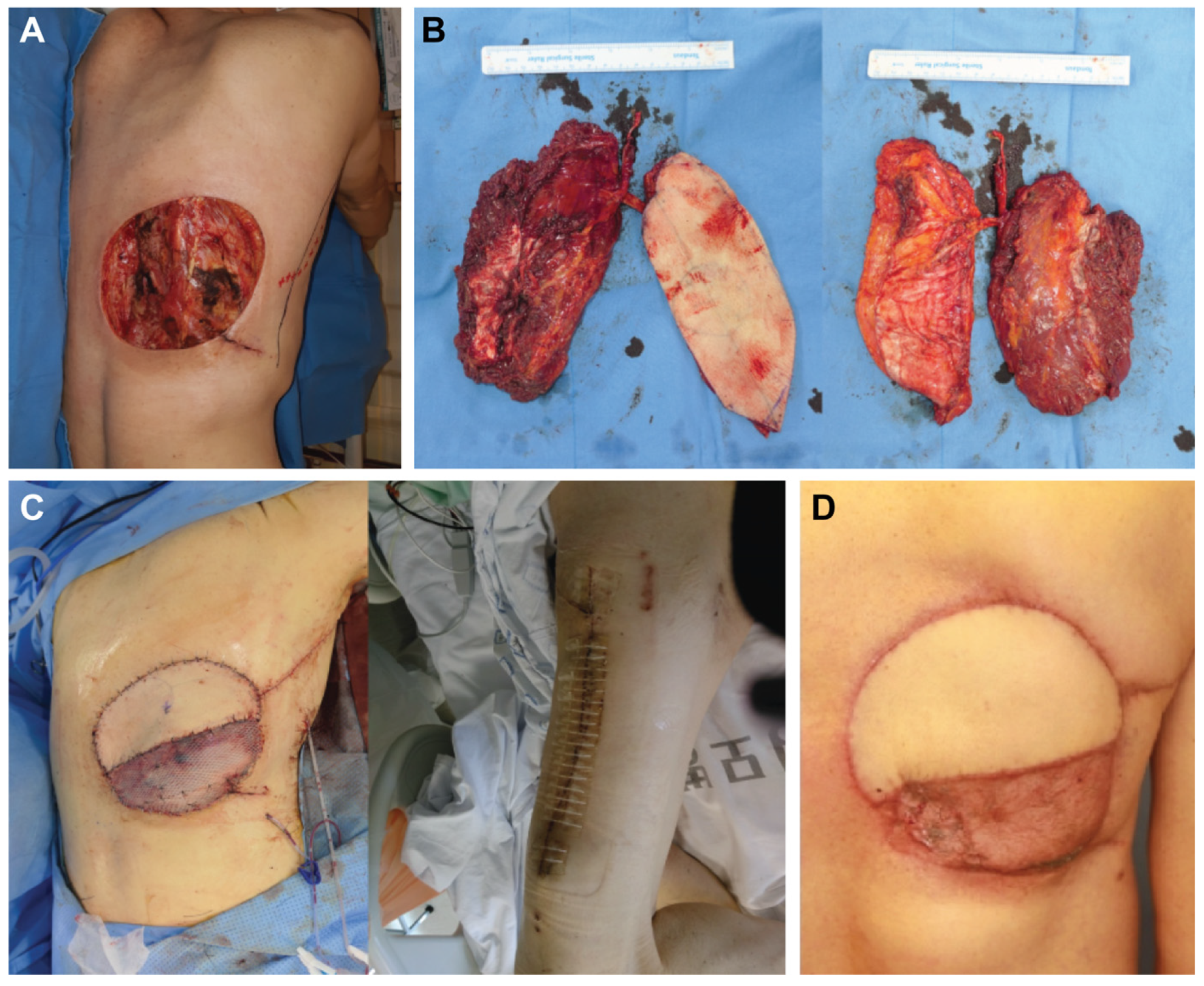
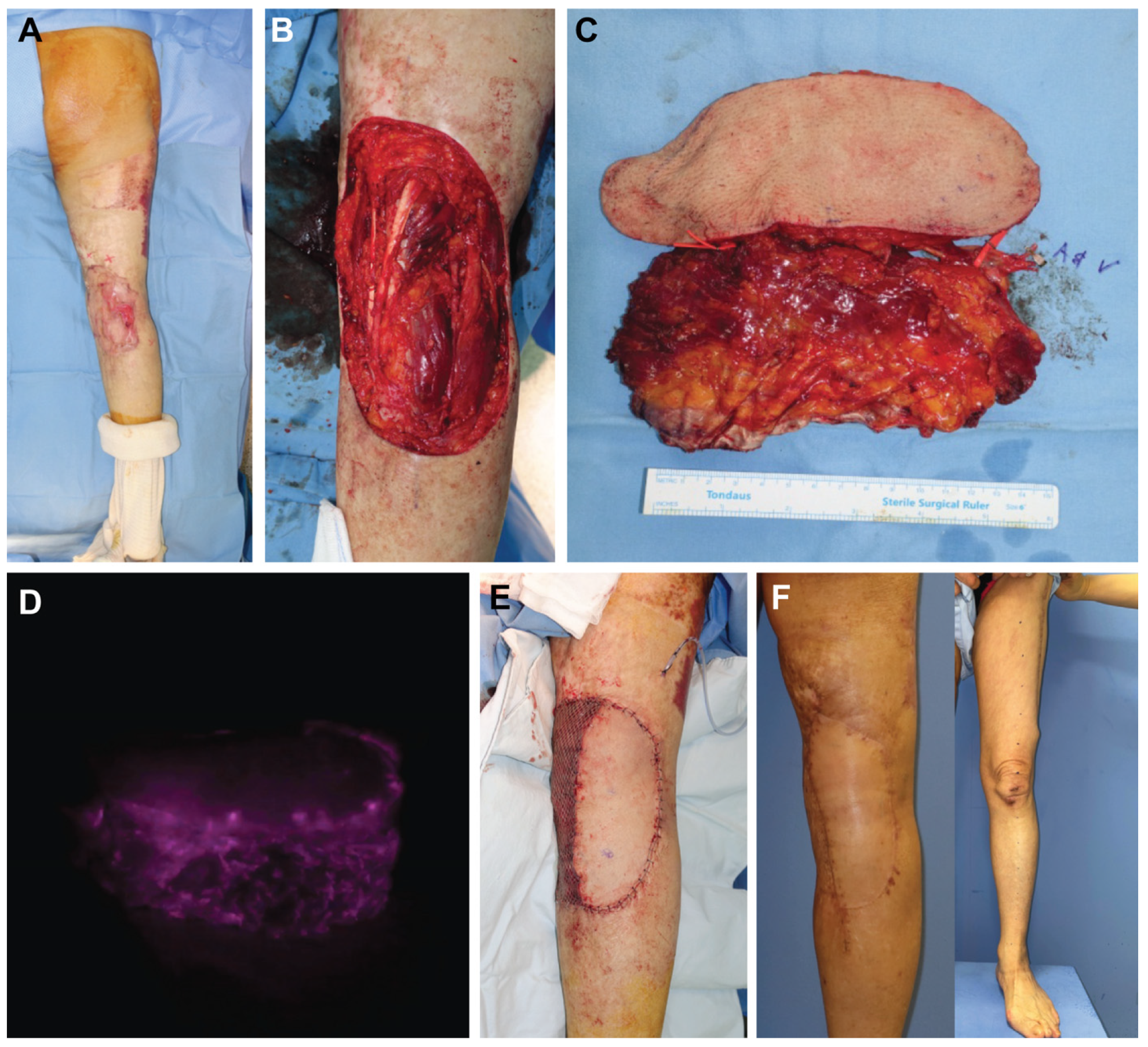
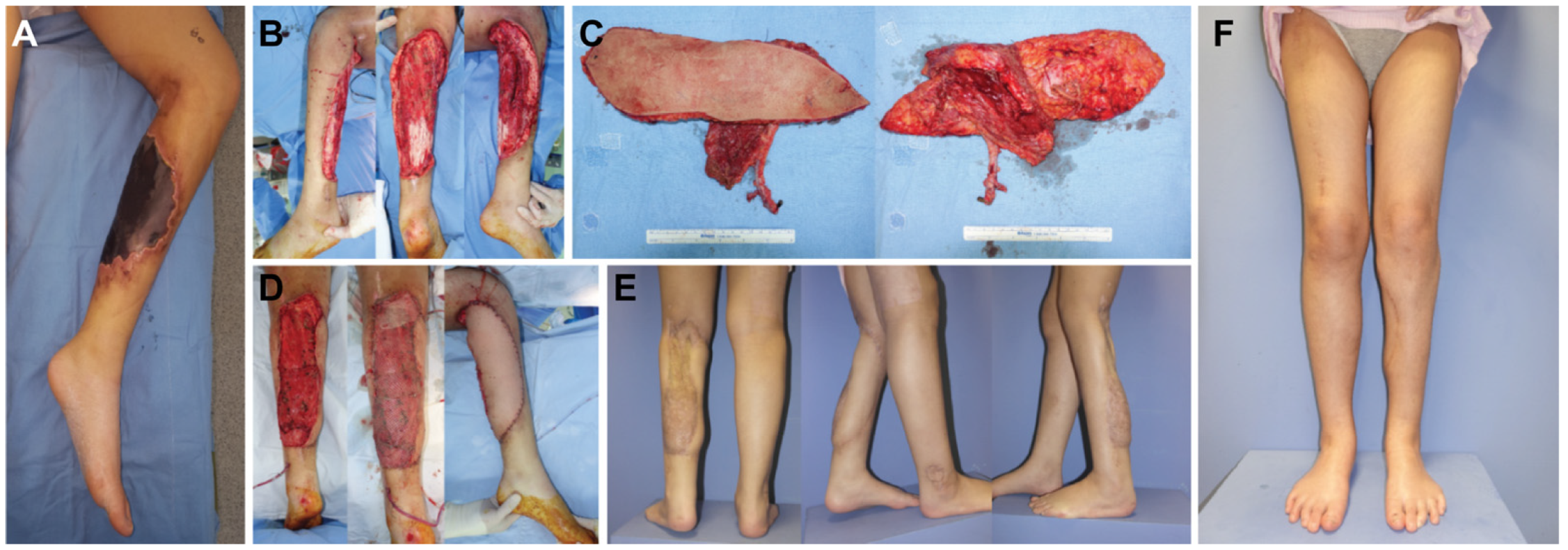
3.1. Case Reports
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kuo, Y.R.; Jeng, S.F.; Kuo, M.H.; Huang, M.N.; Liu, Y.T.; Chiang, Y.C.; Yeh, M.C.; Wei, F.C. Free anterolateral thigh flap for extremity reconstruction: Clinical experience and functional assessment of donor site. Plast. Reconstr. Surg. 2001, 107, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.C.; Jain, V.; Celik, N.; Chen, H.C.; Chuang, D.C.; Lin, C.H. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast. Reconstr. Surg. 2002, 109, 2219–2226, discussion 2227–2230. [Google Scholar] [CrossRef] [PubMed]
- Kimata, Y.; Uchiyama, K.; Ebihara, S.; Sakuraba, M.; Iida, H.; Nakatsuka, T.; Harii, K. Anterolateral thigh flap donor—Site complication and mortality. Plast. Reconstr. Surg. 2000, 106, 584–589. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, D.W.; Han, C.S. Outcomes of anterolateral thigh-free flaps and conversion from external to internal fixation with bone grafting in gustilo type IIIB open tibial fractures. Microsurgery 2012, 32, 431–437. [Google Scholar] [CrossRef]
- Kwee, M.M.; Rozen, W.M.; Ting, J.W.; Mirkazemi, M.; Leong, J.; Baillieu, C. Total scalp reconstruction with bilateral anterolateral thigh flaps. Microsurgery 2012, 32, 393–396. [Google Scholar] [CrossRef]
- Ng, S.W.; Fong, H.C.; Tan, B.K. Two sequential free flaps for coverage of a total knee implant. Arch. Plast. Surg. 2018, 45, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.Y.; Ki, S.H. Analysis of the chest wall reconstruction methods after malignant tumor resection. Arch. Plast. Surg. 2023, 50, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Sakuma, H.; Fujii, T.; Tanaka, I.; Matsui, J. Reconstruction of extensive diaphragmatic defects using the rectus abdominis muscle and fascial flap. Arch. Plast. Surg. 2023, 50, 166–170. [Google Scholar] [CrossRef]
- Gaster, R.S.; Bhatt, K.A.; Shelton, A.A.; Lee, G.K. Free transverse rectus abdominis myocutaneous flap reconstruction of a massive lumbosacral defect using superior gluteal artery perforator vessels. Microsurgery 2012, 32, 388–392. [Google Scholar] [CrossRef]
- Lee, Y.J.; Baek, S.E.; Lee, J.; Oh, D.Y.; Rhie, J.W.; Moon, S.H. Perforating vessel as an alternative option of a recipient selection for posterior trunk-free flap reconstruction. Microsurgery 2018, 38, 763–771. [Google Scholar] [CrossRef]
- Abraham, J.T.; Saint-Cyr, M. Keystone and pedicle perforator flaps in reconstructive surgery: New modifications and applications. Clin. Plast. Surg. 2017, 44, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.Y.; Yoon, J.M.; Ki, S.H. Reconstruction of a large chest wall defect using bilateral pectoralis major myocutaneous flaps and V-Y rotation advancement flaps: A case report. Arch. Plast. Surg. 2022, 49, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Klama-Baryla, A.; Labus, W.; Bergler-Czop, B.; Pietrauszka, K.; Niemiec, P. Platelet-Rich Plasma and Acellular Dermal Matrix in the Surgical Treatment of Hidradenitis Suppurativa: A Comparative Restrospective Study. J. Clin. Med. 2023, 14, 2112. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Labus, W.; Kitala, D.; Lorek, A.; Ochala-Gierek, G.; Zagorska, K.; Waniczek, D.; Szyluk, K.; Niemiec, P. Human Acellular Dermal Matrix in Reconstructive Surgery—A Review. Biomedicines 2022, 10, 2870. [Google Scholar] [CrossRef]
- Gierek, M.; Labus, W.; Slabon, A.; Ziokowska, K.; Ochala-Gierek, G.; Kitala, D.; Szyluk, K.; Niemiec, P. Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft-A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa. Bioengineering 2022, 9, 389. [Google Scholar] [CrossRef]
- Heo, C.Y.; Kang, B.; Jeong, J.H.; Kim, K.; Myung, Y. Acellular dermal matrix and bone cement sandwich technique for chest wall reconstruction. Arch. Plast. Surg. 2022, 49, 25–28. [Google Scholar] [CrossRef]
- Koshima, I.; Hosoda, M.; Moriguchi, T.; Hamanaka, T.; Kawata, S.; Hata, T. A combined anterolateral thigh flap, anteromedial thigh flap, and vascularized iliac bone graft for a full-thickness defect of the mental region. Ann. Plast. Surg. 1993, 31, 175–180. [Google Scholar] [CrossRef]
- Koshima, I.; Yamamoto, H.; Hosoda, M.; Moriguchi, T.; Orita, Y.; Nagayama, H. Free combined composite flaps using the lateral circumflex femoral system for repair of massive defects of the head and neck regions: An introduction to the chimeric flap principle. Plast. Reconstr. Surg. 1993, 92, 411–420. [Google Scholar] [CrossRef]
- Kimata, Y.; Uchiyama, K.; Ebihara, S.; Yoshizumi, T.; Asai, M.; Saikawa, M.; Hayashi, R.; Jitsuiki, Y.; Majima, K.; Ohyama, W.; et al. Versatility of the free anterolateral thigh flap for reconstruction of head and neck defects. Arch. Otolaryngol. Head. Neck Surg. 1997, 123, 1325–1331. [Google Scholar] [CrossRef]
- Graboyes, E.M.; Hornig, J.D. Evolution of the anterolateral thigh free flap. Curr. Opin. Otolaryngol. Head. Neck Surg. 2017, 25, 416–421. [Google Scholar] [CrossRef]
- Yang, R.; Wu, X.; Kumar, P.A.; Xiong, Y.; Jiang, C.; Jian, X.; Guo, F. Application of chimerical ALT perforator flap with vastus lateralis muscle mass for the reconstruction of oral and submandibular defects after radical resection of tongue carcinoma: A retrospective cohort study. BMC Oral Health 2020, 20, 94. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, K.N.; Hong, J.P.; Park, S.W.; Park, C.R.; Yoon, C.S. Use of the chimeric anterolateral thigh free flap in lower extremity reconstruction. Microsurgery 2015, 35, 634–639. [Google Scholar] [CrossRef]
- Wong, C.H.; Wei, F.C. Anterolateral thigh flap. Head Neck 2010, 32, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, Y.J.; Oh, D.Y.; Jun, Y.J.; Rhie, J.W.; Moon, S.H. Reconstruction of wide soft tissue defects with extended anterolateral thigh perforator flap turbocharged technique with anteromedial thigh perforator. Microsurgery 2020, 40, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.T.; Wiraatmadja, E.S.; Mun, G.H. Free latissimus dorsi muscle-chimeric thoracodorsal artery perforator flaps for reconstruction of complicated defects: Does muscle still have a place in the domain of perforator flaps? Ann. Plast. Surg. 2015, 74, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Hallock, G.G. The role of free flaps for salvage of the exposed total ankle arthroplasty. Microsurgery 2017, 37, 34–37. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Ishiura, R. Free double-paddle superficial circumflex iliac perforator flap transfer for partial maxillectomy reconstruction: A case report. Microsurgery 2022, 42, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Low, O.-W.; Loh, T.; Lee, H.; Yap, Y.; Lim, J.; Lim, T.; Nallathamby, V. The superior lateral genicular artery flap for reconstruction of knee and proximal leg defect. Arch. Plast. Surg. 2022, 49, 108–114. [Google Scholar] [CrossRef]
- Cannady, S.B.; Mady, L.J.; Brody, R.M.; Shimunov, D.; Newman, J.G.; Chalian, A.C.; Rajasekaran, K.A.; Sheth, N.P.; Shanti, R.M. Anterolateral thigh osteomyocutaneous flap in head and neck: Lessons learned. Microsurgery 2022, 42, 117–124. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Meroni, M.; Knobe, M.; Fritsche, E. Versatility of perforator flaps for lower extremity defect coverage: Technical highlights and single center experience with 87 consecutive cases. Microsurgery 2022, 42, 548–556. [Google Scholar] [CrossRef]
- Hallock, G.G. Further clarification of the nomenclature for compound flaps. Plast. Reconstr. Surg. 2006, 117, 151e–160e. [Google Scholar] [CrossRef] [PubMed]
- Hallock, G.G. The complete nomenclature for combined perforator flaps. Plast. Reconstr. Surg. 2011, 127, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Engin, M.S.; Yildirim, K.; Kodalak, E.A.; Demir, A. Reconstruction of extensive orbital exenteration defects using an anterolateral thigh/vastus lateralis chimeric flap. J. Craniofac. Surg. 2017, 28, 638–642. [Google Scholar] [CrossRef] [PubMed]

| Patient No. | Gender | Age | Diagnosis | Location | ALT Size (cm) | VL Size (cm) | Total Flap Size (cm) | Pedicle Length (cm) |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 63 | Sarcoma | Trunk | 16 × 6 | 15 × 10 | 27 × 20 | 5 |
| 2 | F | 63 | SCC | Knee joint | 14 × 8 | 18 × 10 | 26 × 25 | 5 |
| 3 | F | 16 | Infection | Lower leg | 14 × 8 | 10 × 8 | 22 × 18 | 7 |
| 4 | M | 58 | SCC | Lower leg | 15 × 10 | 11 × 8 | 25 × 18 | 9 |
| 5 | F | 38 | Trauma | Trunk | 13 × 9 | 10 × 9 | 23 × 18 | 6 |
| 6 | M | 28 | Scar contracture | Knee joint | 15 ×10 | 11 × 10 | 20 × 15 | 8 |
| 7 | F | 44 | Trauma | Lower leg | 15 ×10 | 10 × 9 | 25 × 10 | 6 |
| 8 | M | 32 | DFSP | trunk | 11 × 7 | 9 × 7 | 18 × 14 | 8 |
| 9 | F | 63 | Fibrosarcoma | Trunk | 13 × 10 | 10 × 9 | 23 × 17 | 10 |
| 10 | M | 72 | Osteosarcoma | Trunk | 12 × 8 | 13 × 10 | 25 × 16 | 7 |
| Average | 47.7 | 7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Kim, J.; Lee, C.R.; Kim, J.H.; Oh, D.Y.; Jun, Y.J.; Moon, S.-H. Anterolateral Thigh Chimeric Flap: An Alternative Reconstructive Option to Free Flaps for Large Soft Tissue Defects. J. Clin. Med. 2023, 12, 6723. https://doi.org/10.3390/jcm12216723
Lee YJ, Kim J, Lee CR, Kim JH, Oh DY, Jun YJ, Moon S-H. Anterolateral Thigh Chimeric Flap: An Alternative Reconstructive Option to Free Flaps for Large Soft Tissue Defects. Journal of Clinical Medicine. 2023; 12(21):6723. https://doi.org/10.3390/jcm12216723
Chicago/Turabian StyleLee, Yoon Jae, Junnyeon Kim, Chae Rim Lee, Jun Hyeok Kim, Deuk Young Oh, Young Joon Jun, and Suk-Ho Moon. 2023. "Anterolateral Thigh Chimeric Flap: An Alternative Reconstructive Option to Free Flaps for Large Soft Tissue Defects" Journal of Clinical Medicine 12, no. 21: 6723. https://doi.org/10.3390/jcm12216723
APA StyleLee, Y. J., Kim, J., Lee, C. R., Kim, J. H., Oh, D. Y., Jun, Y. J., & Moon, S.-H. (2023). Anterolateral Thigh Chimeric Flap: An Alternative Reconstructive Option to Free Flaps for Large Soft Tissue Defects. Journal of Clinical Medicine, 12(21), 6723. https://doi.org/10.3390/jcm12216723





