Left Atrial Appendage Occlusion versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation—One-Year Survival
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Endpoints
2.3. Statistical Analysis
2.4. Propensity Score Matching
3. Results
3.1. Study Population and Characteristics
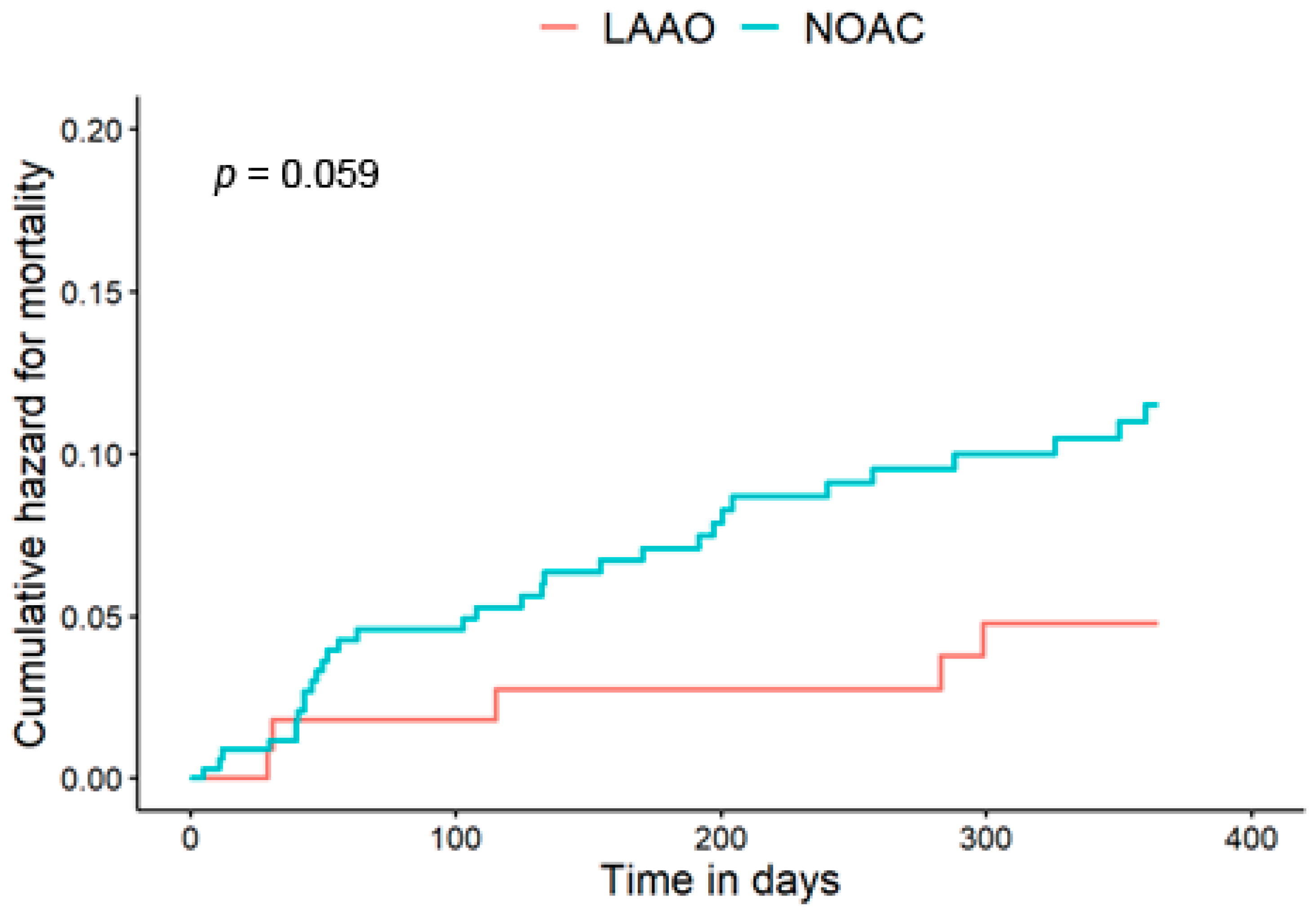
3.2. Primary Endpoint
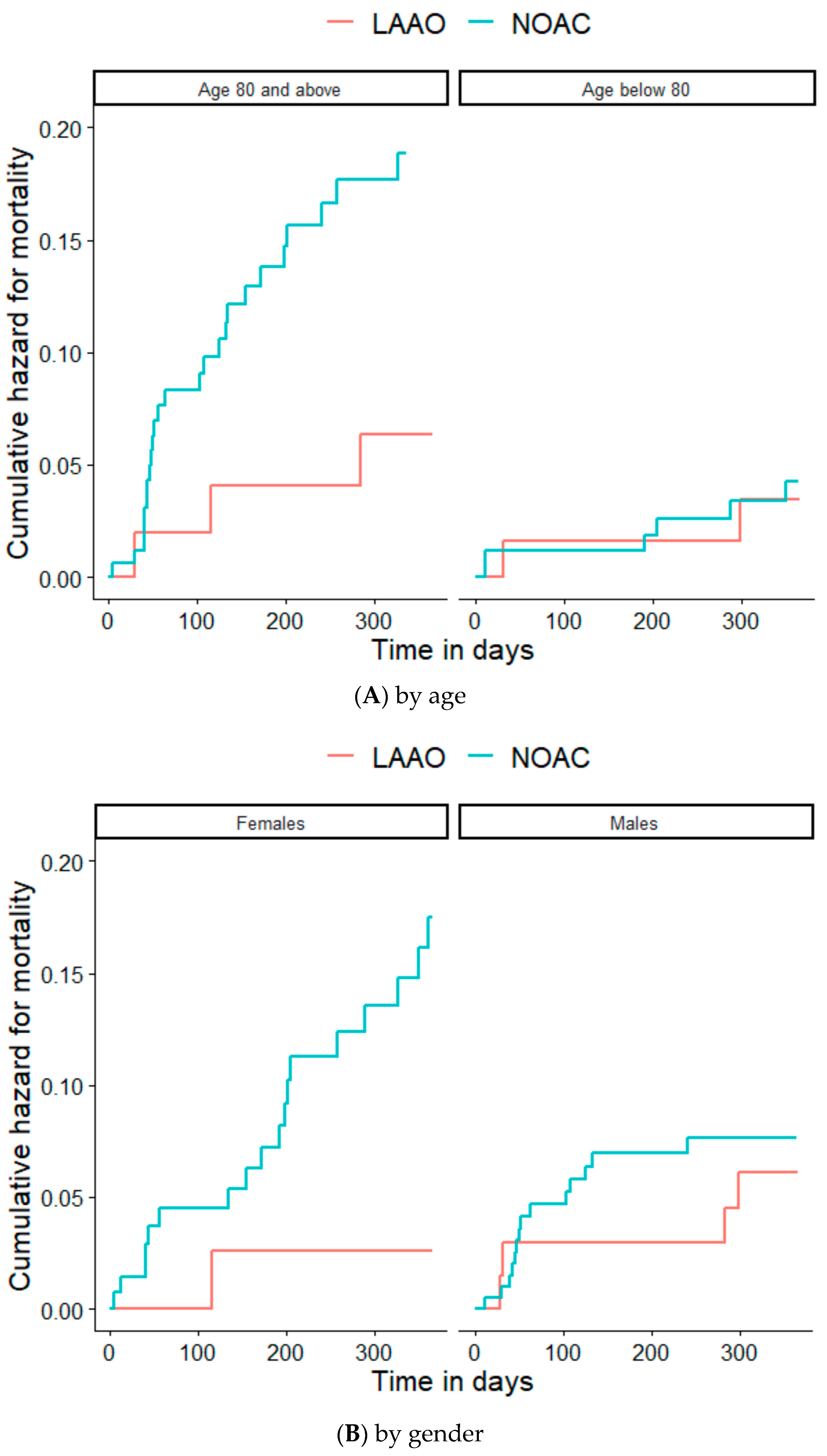
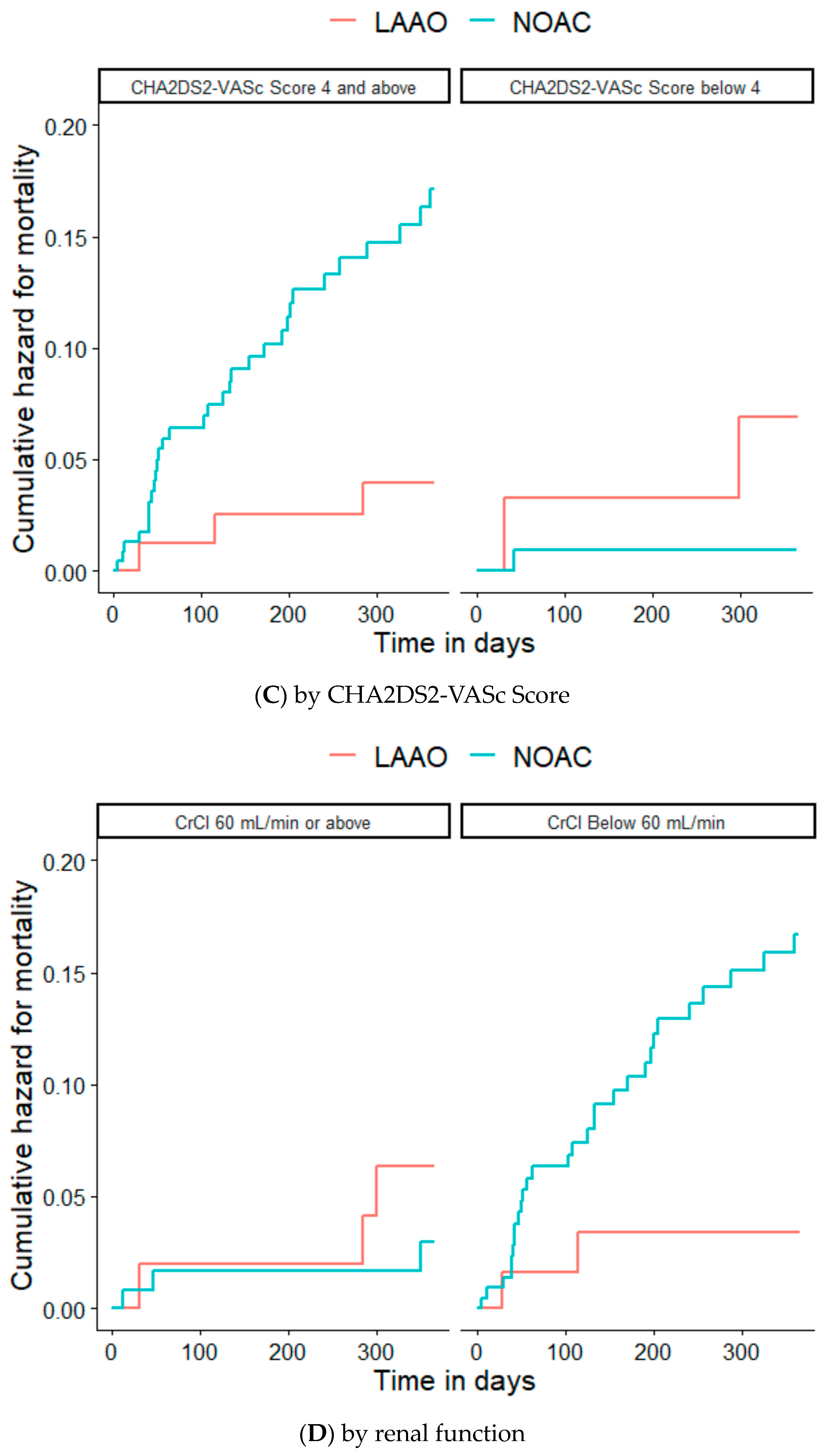
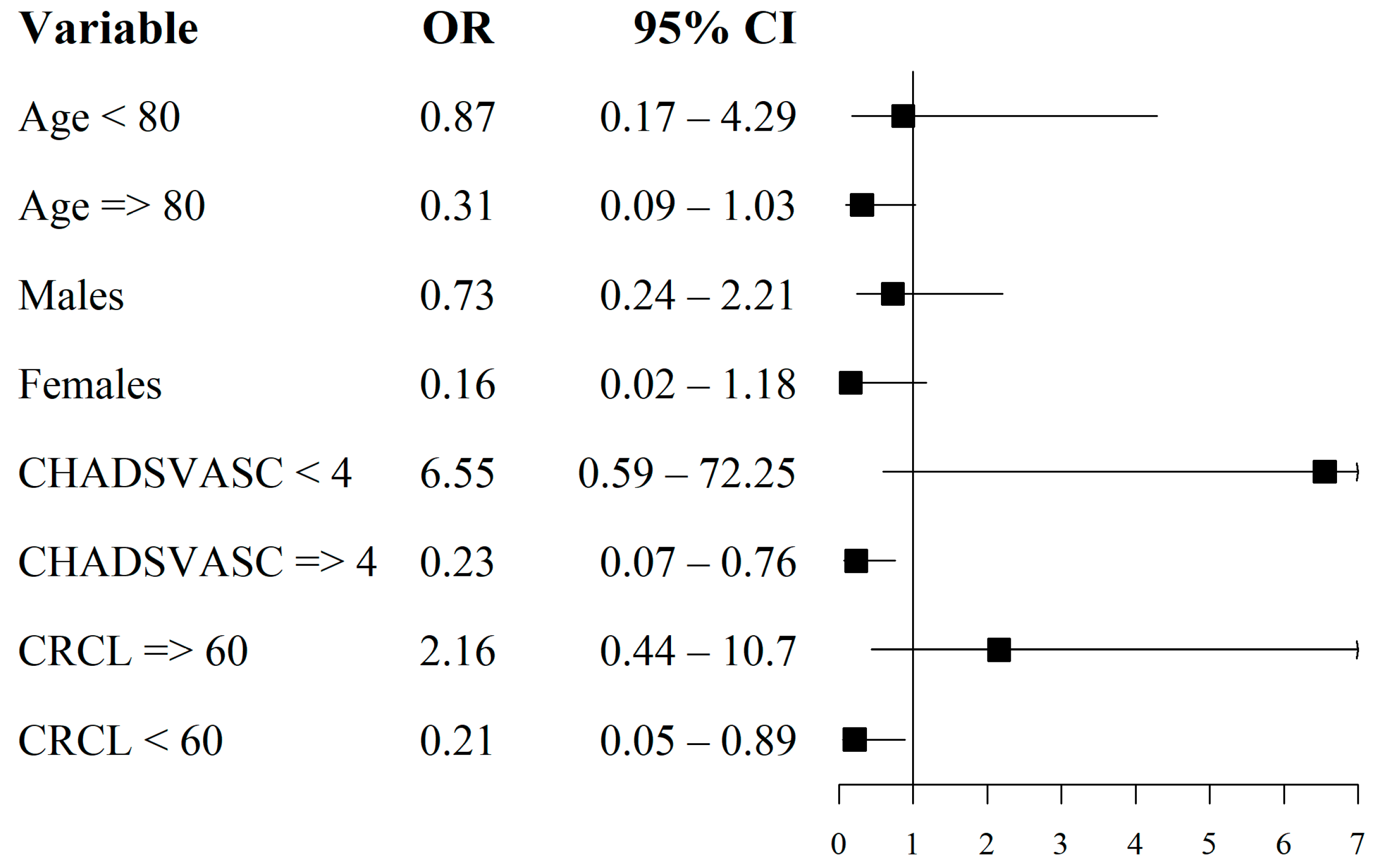
3.3. Secondary Endpoints
4. Discussion
4.1. Renal Failure
4.2. Prior Major Bleeding
4.3. Low Dose NOACs
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simon, G.; Wylie-Rosett, J.; et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Selvin, E.; Stevens, L.A.; Manzi, J.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Levey, A.S. Prevalence of Chronic Kidney Disease in the United States. JAMA 2007, 298, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.Z.; Prineas, R.J.; Go, A.S.; Xie, D.; Lash, J.P.; Rahman, M.; Ojo, A.; Teal, V.L.; Jensvold, N.G.; Robinson, N.L.; et al. Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am. Heart J. 2010, 159, 1102–1107. [Google Scholar] [CrossRef]
- Alonso, A.; Lopez, F.L.; Matsushita, K.; Loehr, L.R.; Agarwal, S.K.; Chen, L.Y.; Soliman, E.Z.; Astor, B.; Coresh, J. Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011, 123, 2946–2953. [Google Scholar] [CrossRef]
- Baber, U.; Howard, V.J.; Halperin, J.L.; Soliman, E.Z.; Zhang, X.; McClellan, W.; Muntner, P. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ. Arrhythmia Electrophysiol. 2011, 4, 26–32. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.; Kamper, A.-L.; Hommel, K.; Køber, L.; Lane, D.A.; Lindhardsen, J.; Gislason, G.H.; Torp-Pedersen, C. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012, 367, 625–635. [Google Scholar] [CrossRef]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [CrossRef]
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly, S.J.; Pogue, J.; Hart, R.G.; A Pfeffer, M.; Hohnloser, S.H.; Chrolavicius, S.; Yusuf, S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): A randomised controlled trial. Lancet 2006, 367, 1903–1912. [Google Scholar]
- Shameem, R.; Ansell, J. Disadvantages of VKA and requirements for novel anticoagulants. Best Pract. Res. Clin. Haematol. 2013, 26, 103–114. [Google Scholar] [CrossRef]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.-C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011, 365, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland Jr, J.C.; Yancy, C.W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef]
- Freedman, B.; Potpara, T.S.; Lip, G.Y. Stroke prevention in atrial fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Chan, K.E.; Giugliano, R.P.; Patel, M.R.; Abramson, S.; Jardine, M.; Zhao, S.; Perkovic, V.; Maddux, F.W.; Piccini, J.P. Nonvitamin K Anticoagulant Agents in Patients With Advanced Chronic Kidney Disease or on Dialysis With AF. J. Am. Coll. Cardiol. 2016, 67, 2888–2899. [Google Scholar] [CrossRef]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2018, 14, e590–e597. [Google Scholar] [CrossRef]
- Baman, J.R.; Mansour, M.; Heist, E.K.; Huang, D.T.; Biton, Y. Percutaneous left atrial appendage occlusion in the prevention of stroke in atrial fibrillation: A systematic review. Heart Fail. Rev. 2018, 23, 191–208. [Google Scholar] [CrossRef]
- Ostermayer, S.H.; Reisman, M.; Kramer, P.H.; Matthews, R.V.; Gray, W.A.; Block, P.C.; Omran, H.; Bartorelli, A.L.; Della Bella, P.; Di Mario, C.; et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO System) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: Results from the international multi-center feasibility trials. J. Am. Coll. Cardiol. 2005, 46, 9–14. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Holmes, D. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: A randomized clinical trial. JAMA 2014, 312, 1988–1998. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K. 5-Year Outcomes after Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Reddy, V.Y. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Korsholm, K.; Damgaard, D.; Valentin, J.B.; Diener, H.C.; Camm, A.J.; Johnsen, S.P. Clinical Outcomes Associated with Left Atrial Appendage Occlusion versus Direct Oral Anticoagulation in Atrial Fibrillation. Cardiovasc. Interv. 2021, 14, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS Expert Consensus Statement on Transcatheter Left Atrial Appendage Closure. JACC: Cardiovasc. Interv. 2023, 16, 1384–1400. [Google Scholar]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.H.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. Europace 2020, 22, 184. [Google Scholar] [CrossRef]
- Korsholm, K.; Valentin, J.B.; Damgaard, D.; Diener, H.-C.; Camm, A.J.; Landmesser, U.; Hildick-Smith, D.; Johnsen, S.P.; Nielsen-Kudsk, J.E. Clinical outcomes of left atrial appendage occlusion versus direct oral anticoagulation in patients with atrial fibrillation and prior ischemic stroke: A propensity-score matched study. Int. J. Cardiol. 2022, 363, 56–63. [Google Scholar] [CrossRef]
- Zeitler, E.P.; Kearing, S.; Coylewright, M.; Nair, D.; Hsu, J.C.; Darden, D.; O’malley, A.J.; Russo, A.M.; Al-Khatib, S.M. Comparative Effectiveness of Left Atrial Appendage Occlusion Versus Oral Anticoagulation by Sex. Circulation 2023, 147, 586–596. [Google Scholar] [CrossRef]
- Lega, J.; Bertoletti, L.; Gremillet, C.; Boissier, C.; Mismetti, P.; Laporte, S. Consistency of safety profile of new oral anticoagulants in patients with renal failure. J. Thromb. Haemost. 2014, 12, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.E.; Lazarus, J.M.; Thadhani, R.; Hakim, R.M. Anticoagulant and Antiplatelet Usage Associates with Mortality among Hemodialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meersch, H.; De Bacquer, D.; De Vriese, A.S. Vitamin K antagonists for stroke prevention in hemodialysis patients with atrial fibrillation: A systematic review and meta-analysis. Am. Heart J. 2017, 184, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Zhang, X.; Eckard, A.; Bhave, N.; Schaubel, D.E.; He, K.; Nallamothu, B.K. Outcomes Associated with Apixaban use in Patients with End-Stage Kidney Disease and Atrial Fibrillation in the United States. Circulation 2018, 138, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Monz, B.U.; Clemens, A.; Brueckmann, M.; Lip, G.Y.H. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: A cross-sectional analysis using the General Practice Research Database. BMJ Open 2012, 2, e001768. [Google Scholar] [CrossRef]
- Bertaglia, E.; Anselmino, M.; Zorzi, A.; Russo, V.; Toso, E.; Peruzza, F.; Rapacciuolo, A.; Migliore, F.; Gaita, F.; Cucchini, U.; et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int. J. Cardiol. 2017, 249, 179–183. [Google Scholar] [CrossRef]
- Wang, K.-L.; Lopes, R.D.; Patel, M.R.; Büller, H.R.; Tan, D.S.-Y.; Chiang, C.-E.; Giugliano, R.P. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: A meta-analysis of randomized controlled trials. Eur. Heart J. 2018, 40, 1492–1500. [Google Scholar] [CrossRef]
- Sharma, S.P.; Park, P.; Lakkireddy, D. Left Atrial Appendages Occlusion: Current Status and Prospective. Korean Circ. J. 2018, 48, 692–704. [Google Scholar] [CrossRef]
| LAAO N = 114 | NOACs N = 342 | P | |
|---|---|---|---|
| Age | 77.9 ± 7.44 | 77.1 ± 11.2 | 0.409 |
| Sex: Male | 70 (61.4%) | 202 (59.1%) | 0.741 |
| Hypertension | 98 (86.0%) | 279 (81.6%) | 0.353 |
| Diabetes Mellitus | 50 (43.9%) | 137 (40.1%) | 0.545 |
| Ischemic Heart Disease | 62 (54.4%) | 173 (50.6%) | 0.552 |
| Congestive Heart Failure | 44 (38.6%) | 124 (36.3%) | 0.737 |
| Ejection fraction (%) | 52.5 ± 7.27 | 52.9 ± 11.1 | 0.674 |
| Prior bleeding | 85 (74.6%) | 256 (74.9%) | 1.000 |
| Prior stroke or TIA | 49 (43.0%) | 127 (37.1%) | 0.317 |
| CrCl | 58.3 ± 31.6 | 54.4 ± 29.5 | 0.243 |
| CrCl ≤ 60 mL/min | 62 (54.4%) | 217 (63.5%) | 0.108 |
| CHA2DS2-VASc | 4.17 ± 1.29 | 3.96 ± 1.57 | 0.173 |
| CHA2DS2-VASc ≥ 4 | 83 (72.8%) | 230 (67.3%) | 0.322 |
| HAS-BLED | 4.14 ± 1.04 | 3.95 ± 1.35 | 0.113 |
| HAS-BLED ≥ 4 | 86 (75.4%) | 236 (69.0%) | 0.235 |
| Prior Aspirin treatment | 49 (43.0%) | 126 (36.8%) | 0.291 |
| HR (95% CI) | P | |
|---|---|---|
| Age above 80 | 3.23 (1.25, 8.39) | 0.016 |
| Sex: Female | 1.48 (0.51, 4.33) | 0.474 |
| Group: LAAO | 0.38 (0.14, 0.99) | 0.048 |
| Congestive Heart Failure | 3.87 (1.30, 11.54) | 0.015 |
| Hypertension | 0.39 (0.10, 1.43) | 0.154 |
| Diabetes Mellitus | 1.63 (0.52, 5.15) | 0.404 |
| Ischemic Heart Disease | 1.36 (0.67, 2.77) | 0.401 |
| Ejection fraction * | 0.99 (0.96, 1.02) | 0.655 |
| Prior bleeding | 4.39 (0.79, 24.32) | 0.09 |
| Stroke or TIA | 1.30 (0.42, 4.00) | 0.645 |
| CrCl ** | 0.98 (0.96, 1.00) | 0.123 |
| CHA2DS2-VASc | 1.08 (0.48, 2.42) | 0.856 |
| HAS-BLED | 0.89 (0.46, 1.73) | 0.741 |
| Aspirin treatment | 1.11 (0.48, 2.58) | 0.807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiosano, S.; Banai, A.; Mulla, W.; Goldenberg, I.; Bayshtok, G.; Amit, U.; Shlomo, N.; Nof, E.; Rosso, R.; Glikson, M.; et al. Left Atrial Appendage Occlusion versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation—One-Year Survival. J. Clin. Med. 2023, 12, 6693. https://doi.org/10.3390/jcm12206693
Tiosano S, Banai A, Mulla W, Goldenberg I, Bayshtok G, Amit U, Shlomo N, Nof E, Rosso R, Glikson M, et al. Left Atrial Appendage Occlusion versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation—One-Year Survival. Journal of Clinical Medicine. 2023; 12(20):6693. https://doi.org/10.3390/jcm12206693
Chicago/Turabian StyleTiosano, Shmuel, Ariel Banai, Wesam Mulla, Ido Goldenberg, Gabriella Bayshtok, Uri Amit, Nir Shlomo, Eyal Nof, Raphael Rosso, Michael Glikson, and et al. 2023. "Left Atrial Appendage Occlusion versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation—One-Year Survival" Journal of Clinical Medicine 12, no. 20: 6693. https://doi.org/10.3390/jcm12206693
APA StyleTiosano, S., Banai, A., Mulla, W., Goldenberg, I., Bayshtok, G., Amit, U., Shlomo, N., Nof, E., Rosso, R., Glikson, M., Guetta, V., Barbash, I., & Beinart, R. (2023). Left Atrial Appendage Occlusion versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation—One-Year Survival. Journal of Clinical Medicine, 12(20), 6693. https://doi.org/10.3390/jcm12206693





