Molecular Tumour Board (MTB): From Standard Therapy to Precision Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blueprint Service
2.2. CORM and MTB
- -
- Support and consolidation of the regional oncology network;
- -
- Encouragement and development of clinical and translational research, including phase I trials with innovative drugs that need an Italian Regulatory Agency of Drugs (AIFA) certification. The Department of Oncology of AOU of Marche is the only active Phase I Centre in the Marche region;
- -
- Support of research in oncological genetics for hereditary cancers. The AOU of the Marche region also includes the Highly Specialised Regional Reference Centre in cancer genetics and this represents a benefit for all the regional hospitals;
- -
- Promotion and consolidation of cancer diagnostic and therapeutic pathways;
- -
- Promotion of the regional model of PDTA.
2.3. Molecular Testing
- (1)
- Myriapod® NGS Cancer panel DNA Illumina® (CE IVD, Diatech Pharmacogenetics SRL, Jesi (AN), Italy), a DNA Gene Panel with 16 genes. The test allows for the identification of single nucleotide variants (SNV) and insertions and deletions (indels) in 16 genes of clinical-diagnostic relevance in major cancers (ALK, BRAF, EGFR, ERBB2, FGFR3, HRAS, IDH1, IDH2, KIT, KRAS, MET, NRAS, PDGFRA, PIK3CA, RET, ROS1), starting from DNA extracted from FFPE (formalin-fixed paraffin-embedded) tissue and ctDNA;
- (2)
- Myriapod® NGS Cancer Panel RNA Illumina® (CE IVD), a RNA Gene Panel with 10 genes. This is the panel dedicated to the study of gene fusions on 10 targets of interest (ALK, ROS1, RET, MET, PPARG, FGFR2, FGFR3, NTRK1, NTRK2, NTRK3) for the prediction of response to oncological drugs, starting from RNA extracted from FFPE tissue;
- (3)
- Real-Time RT-PCR NTRK analysis, using EasyPGX® ready NTRK Fusion (Diatech Pharmacogenetics SRL, Jesi (AN), Italy), and immune-histochemistry analysis, such as IHC DMMR and IHC PDL1;
- (4)
- FoundationOne® CDx, a DNA single tissue-based test with 324 genes. This is the first FDA-approved tissue-based broad companion diagnostic (CDx) that is clinically and analytically validated for all solid tumours. The test is designed to provide physicians with clinically actionable information to consider appropriate therapies for patients and to understand results with evidence of resistance based on the individual genomic profile of each patient’s cancer. Test results include microsatellite instability (MSI) and tumour mutational burden (TMB) to help inform immunotherapy decisions and loss of heterozygosity (LOH) for ovarian cancer patients;
- (5)
- FoundationOne®Liquid CDx analyses 324 genes from circulating cell-free DNA and is FDA-approved to report short variants in 311 genes.
3. Results
3.1. Patients’ Characteristics
3.2. Genomic Profiling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garraway, L.A. Genomics-driven oncology: Framework for an emerging paradigm. J. Clin. Oncol. 2013, 31, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer 2020, 6, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat Commun. 2020, 11, 4965. [Google Scholar] [CrossRef] [PubMed]
- Naithani, N.; Sinha, S.; Misra, P.; Vasudevan, B.; Sahu, R. Precision medicine: Concept and tools. Med. J. Armed Forces India 2021, 77, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, L.; Hirshfield, K.M.; Rojas, V.; DiPaola, R.S.; Gibbon, D.; Hellmann, M.; Isani, S.; Leiser, A.; Riedlinger, G.M.; Wagreich, A.; et al. Use of comprehensive genomic profiling to direct point-of-care management of patients with gynecologic cancers. Gynecol Oncol. 2016, 141, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Hodson, R. Precision oncology. Nature 2020, 585, S1. [Google Scholar] [CrossRef]
- Charo, L.M.; Eskander, R.N.; Sicklick, J.; Kim, K.H.; Lim, H.J.; Okamura, R.; Lee, S.; Subramanian, R.; Schwab, R.; Shatsky, R.; et al. Real-World Data from a Molecular Tumor Board: Improved Outcomes in Breast and Gynecologic Cancers Patients With Precision Medicine. JCO Precis. Oncol. 2022, 6, e2000508. [Google Scholar] [CrossRef] [PubMed]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Fanta, P.T.; Boles, S.G.; Daniels, G.A.; Bazhenova, L.A.; Subramanian, R.; Coutinho, A.C.; Ojeda-Fournier, H.; et al. Molecular Tumor Board: The University of California San Diego Moores Cancer Center Experience. Oncologist 2014, 19, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, V.; Cognigni, V.; Lunerti, V.; Cicoli, C.; Ricci, G.; Papa, R.; Berardi, R. Diagnostic and therapeutic care pathways of cancer patients: A model of multidisciplinary management and patient-tailored healthcare at University Hospital in Italy. Hosp. Adm. Med. Pract. 2022, 1. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W.; Hutchcraft, M.L.; Weiss, H.L.; Wu, J.; Wang, C.; Liu, J.; Jayswal, R.; Buchanan, M.; Anderson, A.; Allison, D.B.; et al. Molecular Tumor Board-Assisted Care in an Advanced Cancer Population: Results of a Phase II Clinical Trial. JCO Precis. Oncol. 2022, 6, e2100524. [Google Scholar] [CrossRef] [PubMed]

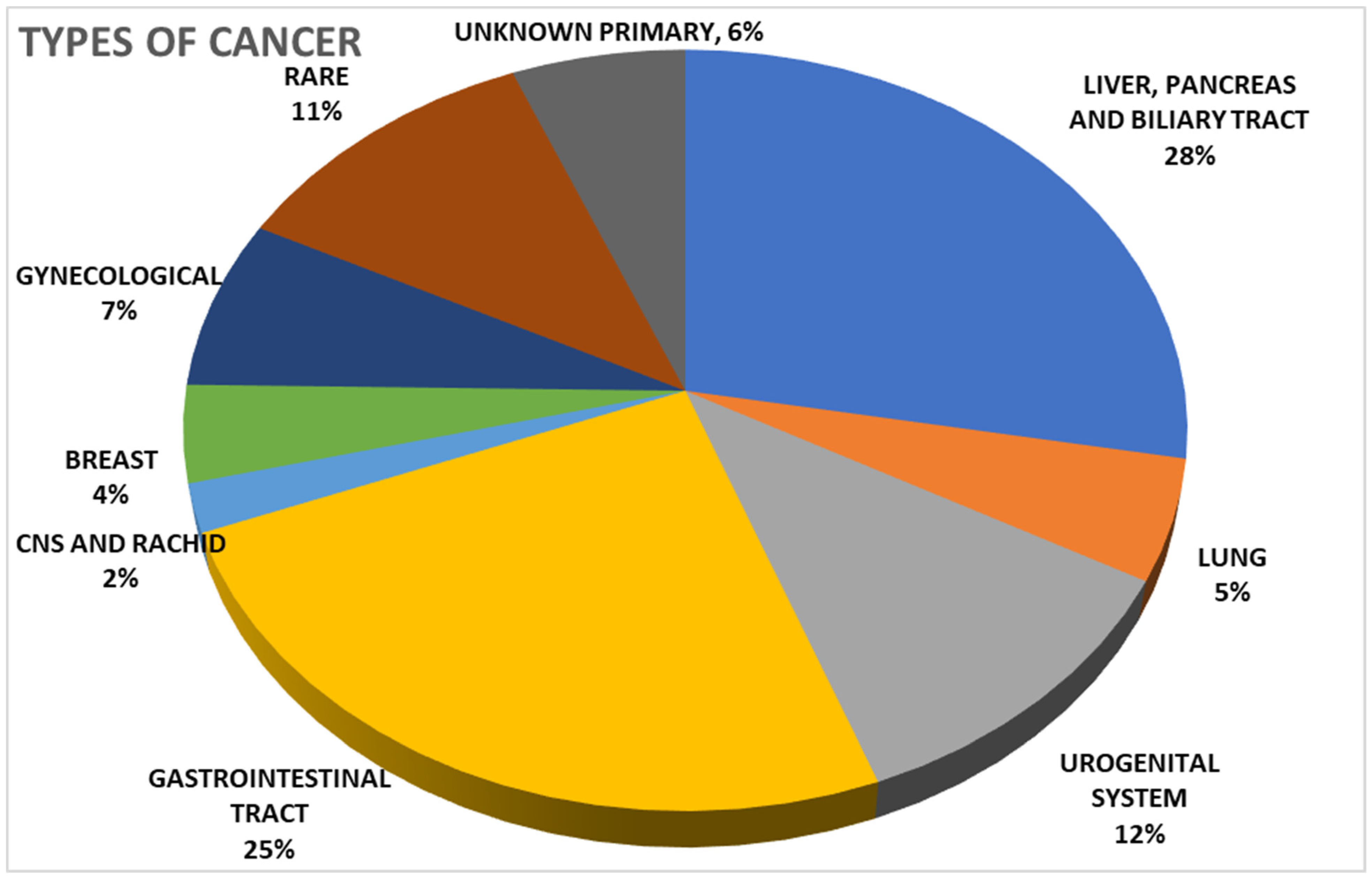


| Period | June 2021–May 2023 |
|---|---|
| Number of meetings | ~50 |
| Age | 60.6 Years Range, 22–83 years |
| Gender, N (%) | Male, 49 (50.5%) Female, 48 (49.5%) |
| Number of Physicians who presented ≥ 1 case | 35 |
| Diagnosis, N | |
| Breast cancers | 4 |
| Pancreas and biliary tract cancers | 27 |
| Gastrointestinal cancers | 24 |
| Lung cancers | 5 |
| Gynaecological cancers | 7 |
| Rare cancers (sarcoma, NET) | 11 |
| Urogenital System cancers | 11 |
| CNS and Rachid cancers | 2 |
| Cancer of Unknown Primary (CUP) | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballatore, Z.; Bozzi, F.; Cardea, S.; Savino, F.D.; Migliore, A.; Tarantino, V.; Chiodi, N.; Ambrosini, E.; Bianchi, F.; Goteri, G.; et al. Molecular Tumour Board (MTB): From Standard Therapy to Precision Medicine. J. Clin. Med. 2023, 12, 6666. https://doi.org/10.3390/jcm12206666
Ballatore Z, Bozzi F, Cardea S, Savino FD, Migliore A, Tarantino V, Chiodi N, Ambrosini E, Bianchi F, Goteri G, et al. Molecular Tumour Board (MTB): From Standard Therapy to Precision Medicine. Journal of Clinical Medicine. 2023; 12(20):6666. https://doi.org/10.3390/jcm12206666
Chicago/Turabian StyleBallatore, Zelmira, Francesco Bozzi, Sara Cardea, Francesco Domenico Savino, Antonella Migliore, Valentina Tarantino, Natalia Chiodi, Elisa Ambrosini, Francesca Bianchi, Gaia Goteri, and et al. 2023. "Molecular Tumour Board (MTB): From Standard Therapy to Precision Medicine" Journal of Clinical Medicine 12, no. 20: 6666. https://doi.org/10.3390/jcm12206666
APA StyleBallatore, Z., Bozzi, F., Cardea, S., Savino, F. D., Migliore, A., Tarantino, V., Chiodi, N., Ambrosini, E., Bianchi, F., Goteri, G., Filosa, A., Barbisan, F., Bartoli, E., Papa, R., & Berardi, R. (2023). Molecular Tumour Board (MTB): From Standard Therapy to Precision Medicine. Journal of Clinical Medicine, 12(20), 6666. https://doi.org/10.3390/jcm12206666







