The Influence of History of Severe Periodontitis on Estimated Long-Term Marginal Bone Loss around Implants Restored with Fixed Segmented Full-Arch Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
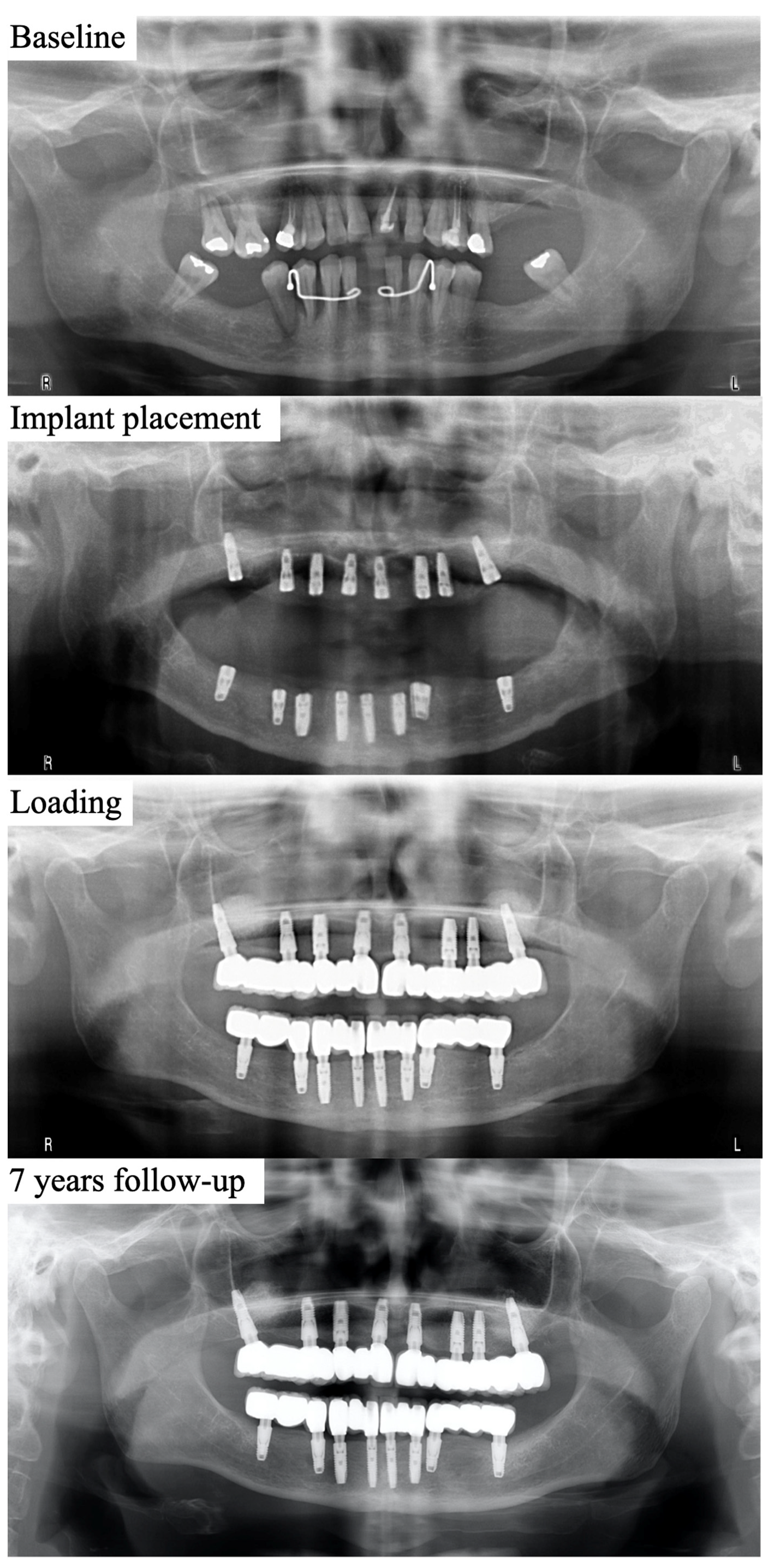
2.2. Surgical and Restorative Procedures
2.3. Radiographic Evaluation of MBL
2.4. Additional Data Recorded
2.5. Statistical Analysis
3. Results
4. Discussion
- 1.
- Firstly, there is a probabilistic issue. It is easier to lose more bone and implants when there are more implants placed. In fact, there are some studies affirming that to have more than four implants is a risk factor to develop peri-implantitis [28]. Then, since patients with periodontal disease usually lose more teeth, they would carry more implants and would be at higher risk of complications.
- 2.
- It is very well known that periodontitis and peri-implantitis are pathological processes that begin with soft tissue inflammation. New theories support the idea that a pro-inflammatory profile could be a requisite needed to go from a symbiotic to a dysbiotic status, which would subsequently allow microorganisms to play a pathological role [51,52]. In addition, some microorganisms are even able to manipulate the host to “play their game” [53]. Regardless, the altered pro-inflammatory response profile to specific bacteria in periodontally compromised patients will continue even if all teeth are removed and replaced with implants. This is because some periodontophatogens are kept in the oral mucosa and tongue over time [54,55,56]. However, the extraction of infected periodontal teeth reduces the pathological bacterial load, and, consequently, the bacteria-promoted inflammation.
- 3.
- Periodontal disease and peri-implantitis follow a different pattern regarding bone resorption. It is well known that periodontal bone resorption usually affects most patients’ teeth leading to a pattern of horizontal bone resorption. In contrast, peri-implantitis usually affects only one implant and leads to a vertical bone resorption that evolves to a circumferential defect. This even happens with a different bone loss pattern in each implant when several implants are affected by peri-implantitis in the same patient. This is the reason why epidemiologically in periodontitis “patient level” is used but in peri-implantitis there is a need to introduce the “implant level” factor.
- 4.
- This “implant level” factor becomes more obvious when the high heterogeneity of implant designs is considered, which leads to a high heterogeneity in the studies presented in the literature. It is very well known that some features of the implants condition the final outcome in terms of marginal bone loss, as extensively discussed in the literature. For instance, there is the type of implant connection. In our previous studies, a previous history of periodontitis was a significant factor in the progression of marginal bone loss [57]. However, external and internal connection implants were analyzed. In the current study we have analyzed only internal conical connection implants and obtained really good outcomes in terms of MBL. So, the type of implant–abutment connection plays a major role in marginal bone loss, that can even be superior to that of a previous history of periodontitis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eke, P.I.; Dye, B.A.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Theodoridis, C.; Grigoriadis, A.; Menexes, G.; Vouros, I. Outcomes of Implant Therapy in Patients with a History of Aggressive Periodontitis. A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2017, 21, 485–503. [Google Scholar] [CrossRef]
- Monje, A.; Alcoforado, G.; Padial-Molina, M.; Suarez, F.; Lin, G.-H.; Wang, H.-L. Generalized Aggressive Periodontitis as a Risk Factor for Dental Implant Failure: A Systematic Review and Meta-Analysis. J. Periodontol. 2014, 85, 1398–1407. [Google Scholar] [CrossRef]
- Kim, K.-K.; Sung, H.-M. Outcomes of Dental Implant Treatment in Patients with Generalized Aggressive Periodontitis: A Systematic Review. J. Adv. Prosthodont. 2012, 4, 210–217. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Baseviciene, N.; Wang, H.-L.; Tözüm, T.F. Effect of History of Periodontitis on Implant Success: Meta-Analysis and Systematic Review. Implant. Dent. 2014, 23, 687–696. [Google Scholar] [CrossRef]
- Graetz, C.; El-Sayed, K.F.; Geiken, A.; Plaumann, A.; Sälzer, S.; Behrens, E.; Wiltfang, J.; Dörfer, C.E. Effect of Periodontitis History on Implant Success: A Long-Term Evaluation during Supportive Periodontal Therapy in a University Setting. Clin. Oral Investig. 2018, 22, 235–244. [Google Scholar] [CrossRef]
- Rasperini, G.; Siciliano, V.I.; Cafiero, C.; Salvi, G.E.; Blasi, A.; Aglietta, M. Crestal Bone Changes at Teeth and Implants in Periodontally Healthy and Periodontally Compromised Patients. A 10-Year Comparative Case-Series Study. J. Periodontol. 2014, 85, e152–e159. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Huynh-Ba, G. History of Treated Periodontitis and Smoking as Risks for Implant Therapy. Int. J. Oral Maxillofac. Implant. 2009, 24, 39–68. [Google Scholar]
- Roccuzzo, M.; De Angelis, N.; Bonino, L.; Aglietta, M. Ten-Year Results of a Three-Arm Prospective Cohort Study on Implants in Periodontally Compromised Patients. Part 1: Implant Loss and Radiographic Bone Loss. Clin. Oral Implant. Res. 2010, 21, 490–496. [Google Scholar] [CrossRef]
- Carra, M.C.; Rangé, H.; Swerts, P.-J.; Tuand, K.; Vandamme, K.; Bouchard, P. Effectiveness of Implant-Supported Fixed Partial Denture in Patients with History of Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2022, 49 (Suppl. S24), 208–223. [Google Scholar] [CrossRef]
- Ravidà, A.; Rodriguez, M.V.; Saleh, M.H.A.; Galli, M.; Qazi, M.; Troiano, G.; Wang, H.-L.; Moreno, P.G. The Correlation between History of Periodontitis According to Staging and Grading and the Prevalence/Severity of Peri-Implantitis in Patients Enrolled in Maintenance Therapy. J. Periodontol. 2021, 92, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Leles, C.R.; Dias, D.R.; Nogueira, T.E.; McKenna, G.; Schimmel, M.; Jordão, L.M.R. Impact of Patient Characteristics on Edentulous Subjects’ Preferences for Prosthodontic Rehabilitation with Implants. Clin. Oral Implant. Res. 2019, 30, 285–292. [Google Scholar] [CrossRef]
- Leles, C.R.; Ferreira, N.P.; Vieira, A.H.; Campos, A.C.V.; Silva, E.T. Factors Influencing Edentulous Patients’ Preferences for Prosthodontic Treatment. J. Oral Rehabil. 2011, 38, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Peršić, S.; Čelebić, A. Influence of Different Prosthodontic Rehabilitation Options on Oral Health-Related Quality of Life, Orofacial Esthetics and Chewing Function Based on Patient-Reported Outcomes. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2015, 24, 919–926. [Google Scholar] [CrossRef]
- Glantz, P.O.; Nilner, K. Biomechanical Aspects of Prosthetic Implant-Borne Reconstructions. Periodontology 2000 1998, 17, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Londhe, S.M.; Gowda, E.M.; Mandlik, V.B.; Shashidhar, M.P. Factors Associated with Abutment Screw Loosening in Single Implant Supported Crowns: A Cross-Sectional Study. Med. J. Armed Forces India 2020, 76, 37–40. [Google Scholar] [CrossRef]
- Wilson, T.G. The Positive Relationship between Excess Cement and Peri-Implant Disease: A Prospective Clinical Endoscopic Study. J. Periodontol. 2009, 80, 1388–1392. [Google Scholar] [CrossRef]
- Misch, C.E.; Silc, J.T. Key Implant Positions: Treatment Planning Using the Canine and First Molar Rules. Available online: https://www.dentistrytoday.com/sp-337104617/ (accessed on 16 October 2023).
- Sheridan, R.A.; Decker, A.M.; Plonka, A.B.; Wang, H.-L. The Role of Occlusion in Implant Therapy: A Comprehensive Updated Review. Implant. Dent. 2016, 25, 829–838. [Google Scholar] [CrossRef]
- Manfredini, D.; Poggio, C.E.; Lobbezoo, F. Is Bruxism a Risk Factor for Dental Implants? A Systematic Review of the Literature. Clin. Implant. Dent. Relat. Res. 2014, 16, 460–469. [Google Scholar] [CrossRef]
- Garaicoa-Pazmiño, C.; Suárez-López del Amo, F.; Monje, A.; Catena, A.; Ortega-Oller, I.; Galindo-Moreno, P.; Wang, H.-L. Influence of Crown/Implant Ratio on Marginal Bone Loss: A Systematic Review. J. Periodontol. 2014, 85, 1214–1221. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; Suárez, F.; ÓValle, F.; Spinato, S.; Catena, A. Prosthetic Abutment Height Is a Key Factor in Peri-Implant Marginal Bone Loss. J. Dent. Res. 2014, 93, 80S–85S. [Google Scholar] [CrossRef]
- Kwon, T.; Bain, P.A.; Levin, L. Systematic Review of Short- (5-10 Years) and Long-Term (10 Years or More) Survival and Success of Full-Arch Fixed Dental Hybrid Prostheses and Supporting Implants. J. Dent. 2014, 42, 1228–1241. [Google Scholar] [CrossRef]
- Gomes, J.A.; Sartori, I.A.M.; Able, F.B.; de Oliveira Silva, T.S.; do Nascimento, C. Microbiological and Clinical Outcomes of Fixed Complete-Arch Mandibular Prostheses Supported by Immediate Implants in Individuals with History of Chronic Periodontitis. Clin. Oral Implant. Res. 2017, 28, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Catena, A.; Pérez-Sayáns, M.; Fernández-Barbero, J.E.; O’Valle, F.; Padial-Molina, M. Early Marginal Bone Loss around Dental Implants to Define Success in Implant Dentistry: A Retrospective Study. Clin. Implant. Dent. Relat. Res. 2022, 24, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Ravidà, A.; Catena, A.; O’Valle, F.; Padial-Molina, M.; Wang, H.-L. Limited Marginal Bone Loss in Implant-Supported Fixed Full-Arch Rehabilitations after 5 Years of Follow-Up. Clin. Oral Implant. Res. 2022, 33, 1224–1232. [Google Scholar] [CrossRef]
- Misch, C.E.; Perel, M.L.; Wang, H.L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant Success, Survival, and Failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-Implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Mengel, R.; Wendt, J.; Peleska, B. Prosthodontic Treatment Outcomes in Periodontally Compromised Patients: A 6- to 20-Year Long-Term Cohort Study. Int. J. Prosthodont. 2019, 32, 153–161. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the Periimplant Mucosa. Biological Width Revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the Microgap between Implant and Abutment: A Retrospective Histologic Evaluation in Monkeys. J. Periodontol. 2003, 74, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The Influence of Soft Tissue Thickness on Crestal Bone Changes around Implants: A 1-Year Prospective Controlled Clinical Trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Suárez-López Del Amo, F.; Lin, G.-H.; Monje, A.; Galindo-Moreno, P.; Wang, H.-L. Influence of Soft Tissue Thickness on Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Pico, A.; Martín-Lancharro, P.; Caneiro, L.; Nóvoa, L.; Batalla, P.; Blanco, J. Influence of Abutment Height and Implant Depth Position on Interproximal Peri-Implant Bone in Sites with Thin Mucosa: A 1-Year Randomized Clinical Trial. Clin. Oral Implant. Res. 2019, 30, 595–602. [Google Scholar] [CrossRef]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Zaffe, D. Biological Width Establishment around Dental Implants Is Influenced by Abutment Height Irrespective of Vertical Mucosal Thickness: A Cluster Randomized Controlled Trial. Clin. Oral Implant. Res. 2019, 30, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Dovigo, S.; Zaffe, D. Influence of Abutment Height and Vertical Mucosal Thickness on Early Marginal Bone Loss around Implants: A Randomised Clinical Trial with an 18-Month Post-Loading Clinical and Radiographic Evaluation. Int. J. Oral Implantol. 2020, 13, 279–290. [Google Scholar]
- Ramanauskaite, A.; Schwarz, F.; Sader, R. Influence of Width of Keratinized Tissue on the Prevalence of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2022, 33 (Suppl. S23), 8–31. [Google Scholar] [CrossRef]
- Sanz, M.; Schwarz, F.; Herrera, D.; McClain, P.; Figuero, E.; Molina, A.; Monje, A.; Montero, E.; Pascual, A.; Ramanauskaite, A.; et al. Importance of Keratinized Mucosa around Dental Implants: Consensus Report of Group 1 of the DGI/SEPA/Osteology Workshop. Clin. Oral Implant. Res. 2022, 33 (Suppl. S23), 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ravidà, A.; Arena, C.; Tattan, M.; Caponio, V.C.A.; Saleh, M.H.A.; Wang, H.-L.; Troiano, G. The Role of Keratinized Mucosa Width as a Risk Factor for Peri-Implant Disease: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 287–300. [Google Scholar] [CrossRef]
- Vervaeke, S.; Dierens, M.; Besseler, J.; De Bruyn, H. The Influence of Initial Soft Tissue Thickness on Peri-Implant Bone Remodeling. Clin. Implant. Dent. Relat. Res. 2014, 16, 238–247. [Google Scholar] [CrossRef]
- Vervaeke, S.; Collaert, B.; Cosyn, J.; De Bruyn, H. A 9-Year Prospective Case Series Using Multivariate Analyses to Identify Predictors of Early and Late Peri-Implant Bone Loss. Clin. Implant. Dent. Relat. Res. 2016, 18, 30–39. [Google Scholar] [CrossRef]
- Spinato, S.; Galindo-Moreno, P.; Bernardello, F.; Zaffe, D. Minimum Abutment Height to Eliminate Bone Loss: Influence of Implant Neck Design and Platform Switching. Int. J. Oral Maxillofac. Implant. 2018, 33, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, L.; Lei, C.; Li, Y.; Li, D. Effects of Uncontrolled Periodontitis on Marginal Bone Alterations around Implants: A Case-Control Study. Clin. Implant. Dent. Relat. Res. 2017, 19, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Monje, A.; Ortega-Oller, I.; O’Valle, F.; Catena, A. Abutment Height Influences the Effect of Platform Switching on Peri-Implant Marginal Bone Loss. Clin. Oral Implant. Res. 2016, 27, 167–173. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Chen, C.-J.; Singh, M.; Weber, H.-P.; Gallucci, G.O. Success Criteria in Implant Dentistry. J. Dent. Res. 2012, 91, 242–248. [Google Scholar] [CrossRef]
- Cid, R.M.O.; Stanley, K.; Cordero, E.B.; Benfatti, C.A.M.; Bianchini, M.A. Influence of Cantilever Length and Type of Arch Antagonist on Bone Loss in Total Implant-Supported Prostheses. Acta Odontol. Latinoam. AOL 2014, 27, 131–136. [Google Scholar]
- Canullo, L.; Fedele, G.R.; Iannello, G.; Jepsen, S. Platform Switching and Marginal Bone-Level Alterations: The Results of a Randomized-Controlled Trial. Clin. Oral Implant. Res. 2010, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Iannello, G.; Peñarocha, M.; Garcia, B. Impact of Implant Diameter on Bone Level Changes around Platform Switched Implants: Preliminary Results of 18 Months Follow-up a Prospective Randomized Match-Paired Controlled Trial. Clin. Oral Implant. Res. 2012, 23, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Quirynen, M. Risk Indicators for Peri-Implantitis. A Narrative Review. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 15–44. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Periodontally Compromised vs. Periodontally Healthy Patients and Dental Implants: A Systematic Review and Meta-Analysis. J. Dent. 2014, 42, 1509–1527. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Hajishengallis, G. The Inflammophilic Character of the Periodontitis-Associated Microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Breaking Bad: Manipulation of the Host Response by Porphyromonas Gingivalis. Eur. J. Immunol. 2014, 44, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, N.; Van Essche, M.; Pauwels, M.; Teughels, W.; Quirynen, M. Do Periodontopathogens Disappear after Full-Mouth Tooth Extraction? J. Clin. Periodontol. 2009, 36, 1043–1047. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Aquino, D.R.; Cortelli, S.C.; Nobre Franco, G.C.; Fernandes, C.B.; Roman-Torres, C.V.G.; Costa, F.O. Detection of Periodontal Pathogens in Oral Mucous Membranes of Edentulous Individuals. J. Periodontol. 2008, 79, 1962–1965. [Google Scholar] [CrossRef]
- Sachdeo, A.; Haffajee, A.D.; Socransky, S.S. Biofilms in the Edentulous Oral Cavity. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2008, 17, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Fernández-Jiménez, A.; O’Valle, F.; Monje, A.; Silvestre, F.J.; Juodzbalys, G.; Sánchez-Fernández, E.; Catena, A. Influence of the Crown-Implant Connection on the Preservation of Peri-Implant Bone: A Retrospective Multifactorial Analysis. Int. J. Oral Maxillofac. Implant. 2015, 30, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Buser, D.; Chen, S.T.; Cochran, D.; Debruyn, H.; Jemt, T.; Koka, S.; Nevins, M.; Sennerby, L.; Simion, M.; et al. Statements from the Estepona Consensus Meeting on Peri-Implantitis, February 2–4, 2012. Clin. Implant. Dent. Relat. Res. 2012, 14, 781–782. [Google Scholar] [CrossRef]


| Variable | p Value | |||||
|---|---|---|---|---|---|---|
| Age (mean (min, max)) | 58.27 (44, 81) | - | ||||
| Gender (number of patients) | Women = 18 | Men = 17 | 0.87 | |||
| Implant location (number of implants) | Mandible = 122 | Maxilla = 220 | 0.09 | |||
| Maxillary sinus floor elevation (number of implants) | No = 273 | Yes = 69 | 0.001 | |||
| Implant diameter in mm (number of implants) | 3.5 = 94 | 4 = 96 | 4.5 = 139 | 5 = 13 | 0.001 | |
| Implant length in mm (number of implants) | 6 = 27 | 9 = 54 | 11 = 138 | 13 = 56 | 15 = 67 | 0.001 |
| Abutment height in mm (number of implants) | 1 = 40 | 2 = 177 | 4 = 93 | 6 = 32 | 0.001 | |
| Opposing Arch (number of implants) | ND = 51 | M = 122 | ISFB = 15 | ISOD = 154 | 0.001 | |
| Opposing Dentition (number of implants) | ND = 95 | RD = 19 | ISFB = 203 | TSFB = 25 | 0.001 | |
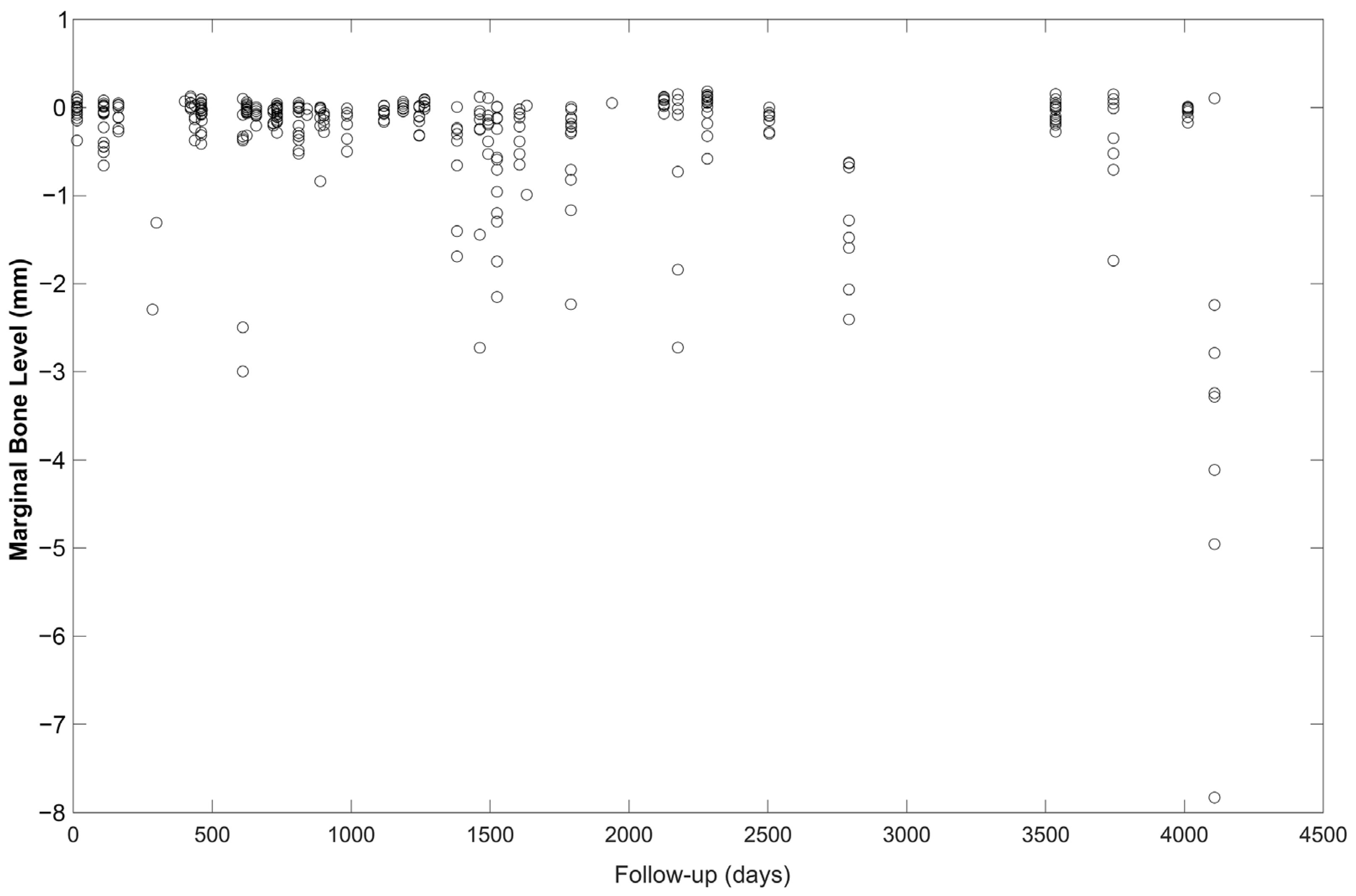
| Follow-up (mean (min, max)) in months | 48.10 (0.43, 136.93) |
| Variable | Mean | SE | Median | 95% CI |
|---|---|---|---|---|
| Crown Height (mm) | 13.04 | 0.18 | 12.54 | 12.69, 13.39 |
| Crown Implant Ratio | 1.464 | 0.035 | 1.270 | 1.396, 1.532 |
| Implants per Bridge (n) | 4.819 | 0.119 | 4.000 | 4.585, 5.052 |
| Crowns per Bridge (n) | 8.059 | 0.207 | 7.000 | 7.652, 8.465 |
| Bridge Ratio | 1.672 | 0.013 | 1.670 | 1.647, 1.679 |
| Variable | Mean | SE | 95% CI |
|---|---|---|---|
| MBL Mesial | −0.297 | 0.040 | −0.375, −0.218 |
| MBL Distal | −0.317 | 0.046 | −0.409, −0.226 |
| MBL Average | −0. 307 | 0.042 | −0.389, −0.224 |
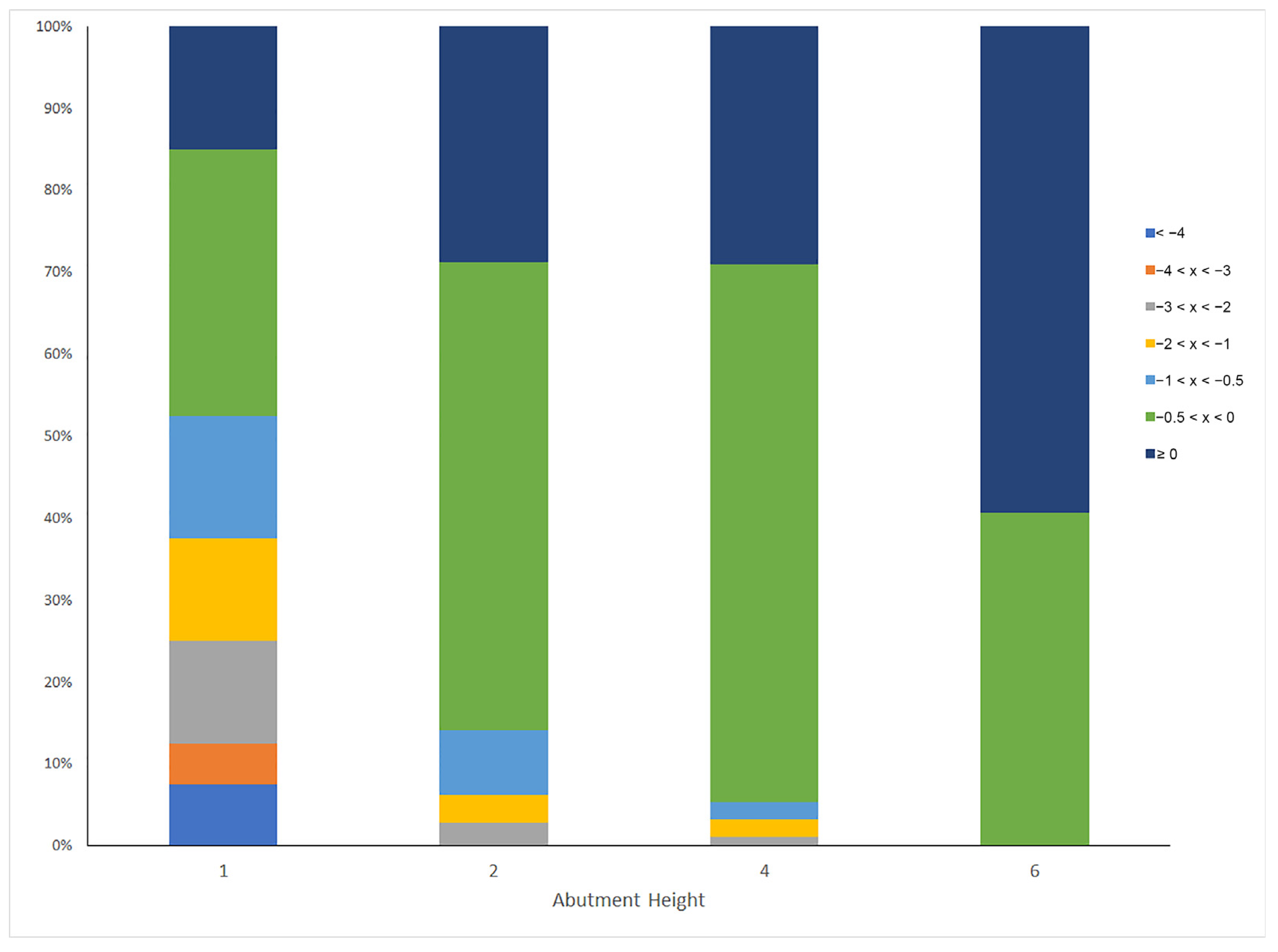
| Abutment Height (mm) | Ranges of MBL (mm) | N = 342 | ||||||
|---|---|---|---|---|---|---|---|---|
| <−4 | −4 < x < −3 | −3 < x < −2 | −2 < x < −1 | −1 < x < −0.5 | −0.5 < x < 0 | ≥0 | ||
| 1 | 7.5 | 5.0 | 12.5 | 12.5 | 15.0 | 32.5 | 15.0 | 40 |
| 2 | 0 | 0 | 2.8 | 3.4 | 7.9 | 57.1 | 28.8 | 177 |
| 4 | 0 | 0 | 1.1 | 2.2 | 2.2 | 65.6 | 29.0 | 93 |
| 6 | 0 | 0 | 0 | 0 | 0 | 40.6 | 59.4 | 32 |
| % of patients | 1 | 1 | 3 | 4 | 6 | 54 | 31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galindo-Moreno, P.; Catena, A.; Lopez-Chaichio, L.; Borges, T.; O’Valle, F.; Torrecillas-Martínez, L.; Padial-Molina, M. The Influence of History of Severe Periodontitis on Estimated Long-Term Marginal Bone Loss around Implants Restored with Fixed Segmented Full-Arch Rehabilitation. J. Clin. Med. 2023, 12, 6665. https://doi.org/10.3390/jcm12206665
Galindo-Moreno P, Catena A, Lopez-Chaichio L, Borges T, O’Valle F, Torrecillas-Martínez L, Padial-Molina M. The Influence of History of Severe Periodontitis on Estimated Long-Term Marginal Bone Loss around Implants Restored with Fixed Segmented Full-Arch Rehabilitation. Journal of Clinical Medicine. 2023; 12(20):6665. https://doi.org/10.3390/jcm12206665
Chicago/Turabian StyleGalindo-Moreno, Pablo, Andres Catena, Lucia Lopez-Chaichio, Tiago Borges, Francisco O’Valle, Laura Torrecillas-Martínez, and Miguel Padial-Molina. 2023. "The Influence of History of Severe Periodontitis on Estimated Long-Term Marginal Bone Loss around Implants Restored with Fixed Segmented Full-Arch Rehabilitation" Journal of Clinical Medicine 12, no. 20: 6665. https://doi.org/10.3390/jcm12206665
APA StyleGalindo-Moreno, P., Catena, A., Lopez-Chaichio, L., Borges, T., O’Valle, F., Torrecillas-Martínez, L., & Padial-Molina, M. (2023). The Influence of History of Severe Periodontitis on Estimated Long-Term Marginal Bone Loss around Implants Restored with Fixed Segmented Full-Arch Rehabilitation. Journal of Clinical Medicine, 12(20), 6665. https://doi.org/10.3390/jcm12206665








