Preoperative Imaging Signs of Cerebral Malperfusion in Acute Type A Aortic Dissection: Influence on Outcomes and Prognostic Implications—A 20-Year Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Surgical Technique and Postoperative Management
2.3. Statistical Analysis
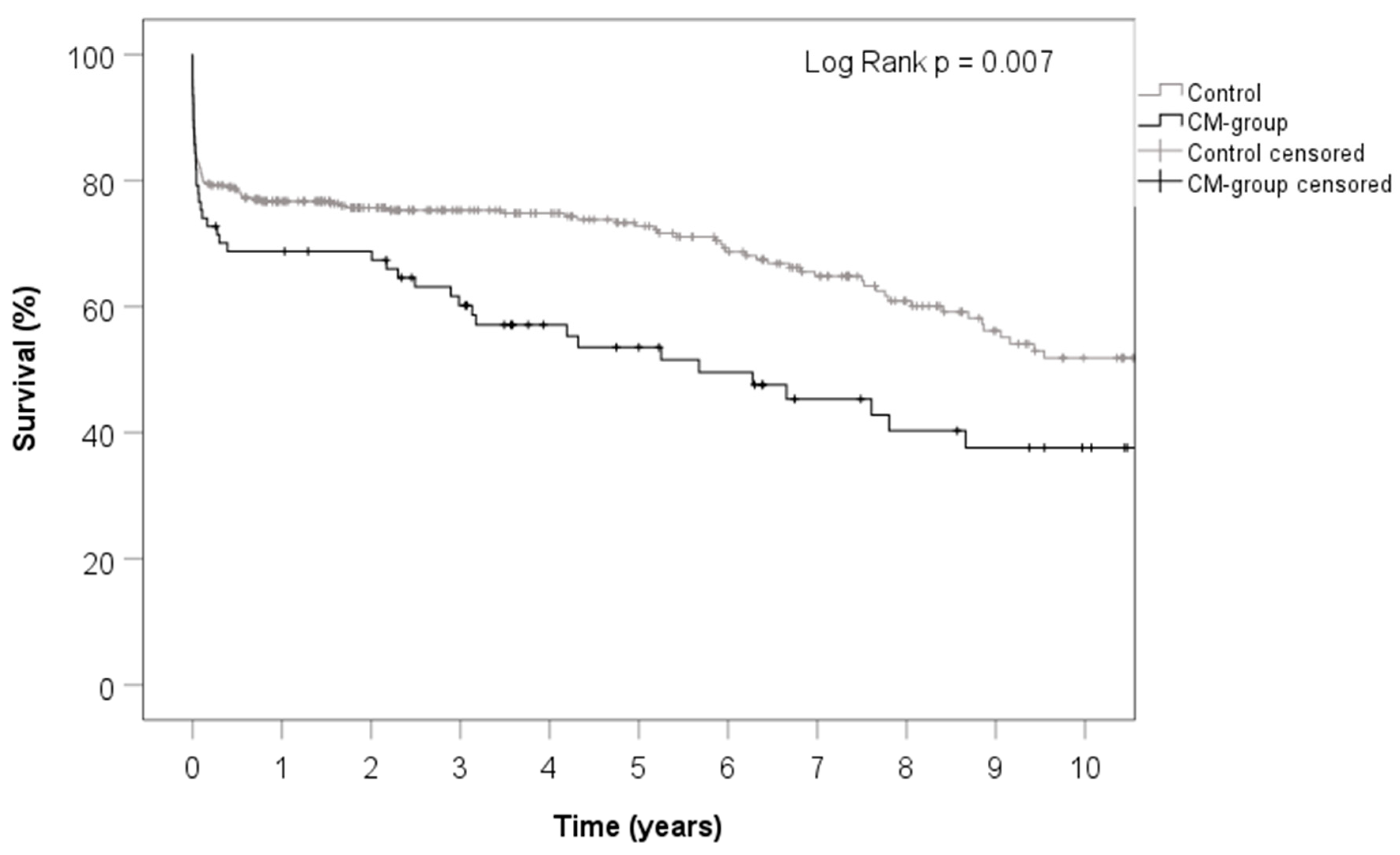
3. Results
3.1. Preoperative and Baseline Patients’ Characteristics
3.2. Intraoperative Details
3.3. Postoperative Outcomes
3.4. Risk Factors for Long-Term Mortality
4. Discussion
5. Strengths, Limitations, and Future Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biancari, F.; Dell’aquila, A.M.; Gatti, G.; Perrotti, A.; Hervé, A.; Touma, J.; Pettinari, M.; Peterss, S.; Buech, J.; Wisniewski, K.; et al. Interinstitutional analysis of the outcome after surgery for type A aortic dissection. Eur. J. Trauma Emerg. Surg. 2023, 49, 1791–1801. [Google Scholar] [CrossRef]
- Gemelli, M.; Di Tommaso, E.; Natali, R.; Dixon, L.K.; Mohamed Ahmed, E.; Rajakaruna, C.; Bruno, V.D. Validation of the German Registry for Acute Aortic Dissection Type A Score in predicting 30-day mortality after type A aortic dissection surgery. Eur. J. Cardio-Thorac. Surg. 2023, 63, ezad141. [Google Scholar] [CrossRef]
- Lee, T.C.; Kon, Z.; Cheema, F.H.; Grau-Sepulveda, M.V.; Englum, B.; Kim, S.; Chaudhuri, P.S.; Thourani, V.H.; Ailawadi, G.; Hughes, G.C.; et al. Contemporary management and outcomes of acute type A aortic dissection: An analysis of the STS adult cardiac surgery database. J. Card. Surg. 2018, 33, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, Z.; Zhang, Y.; Wu, W.; Liu, M.; Yang, Y.; Wang, J.; Lv, Q.; Zhang, L.; Li, Y.; et al. Clinical Analysis of Risk Factors for Mortality in Type A Acute Aortic Dissection: A Single Study From China. Front. Cardiovasc. Med. 2021, 8, 728568. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Di Bartolomeo, R.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, E334–E482. [Google Scholar] [CrossRef] [PubMed]
- Jaffar-Karballai, M.; Tran, T.T.; Oremakinde, O.; Zafar, S.; Harky, A. Malperfusion in Acute Type A Aortic Dissection: Management Strategies. Vasc. Endovasc. Surg. 2021, 55, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Kayali, F.; Jubouri, M.; Al-Tawil, M.; Tan, S.Z.C.P.; Williams, I.M.; Mohammed, I.; Velayudhan, B.; Bashir, M. Coronary artery involvement in type A aortic dissection: Fate of the coronaries. J. Card. Surg. 2022, 37, 5233–5242. [Google Scholar] [CrossRef]
- Pacini, D.; Murana, G.; Di Marco, L.; Berardi, M.; Mariani, C.; Coppola, G.; Fiorentino, M.; Leone, A.; Di Bartolomeo, R. Cerebral perfusion issues in type A aortic dissection. J. Vis. Surg. 2018, 4, 77. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Li, T.; Xi, Z.; Wu, H.; Li, D. Surgical treatment of type A acute aortic dissection with cerebral malperfusion: A systematic review. J. Cardiothorac. Surg. 2022, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Friedrich, C.; Rusch, R.; Frank, D.; Hoffmann, G.; Lutter, G.; Berndt, R.; Cremer, J.; Haneya, A.; Puehler, T. Is total arch replacement associated with an increased risk after acute type A dissection? J. Thorac. Dis. 2020, 12, 5517–5531. [Google Scholar] [CrossRef] [PubMed]
- Pacini, D.; Leone, A.; Belotti, L.M.B.; Fortuna, D.; Gabbieri, D.; Zussa, C.; Contini, A.; Di Bartolomeo, R.; on behalf of RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Acute type A aortic dissection: Significance of multiorgan malperfusion. Eur. J. Cardio-Thoracic Surg. 2013, 43, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Bianco, V.; Patel, H.J.; Arnaoutakis, G.J.; Di Eusanio, M.; Chen, E.P.; Leshnower, B.; Sundt, T.M.; Sechtem, U.; Montgomery, D.G.; et al. Surgery for type A aortic dissection in patients with cerebral malperfusion: Results from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2021, 161, 1713–1720.e1. [Google Scholar] [CrossRef]
- Vendramin, I.; Isola, M.; Piani, D.; Onorati, F.; Salizzoni, S.; D’Onofrio, A.; Di Marco, L.; Gatti, G.; De Martino, M.; Faggian, G.; et al. Surgical management and outcomes in patients with acute type A aortic dissection and cerebral malperfusion. JTCVS Open 2022, 10, 22–33. [Google Scholar] [CrossRef]
- Fukuhara, S.; Norton, E.L.; Chaudhary, N.; Burris, N.; Shiomi, S.; Kim, K.M.; Patel, H.J.; Deeb, G.M.; Yang, B. Type A Aortic Dissection with Cerebral Malperfusion: New Insights. Ann. Thorac. Surg. 2021, 112, 501–509. [Google Scholar] [CrossRef]
- Gomibuchi, T.; Seto, T.; Naito, K.; Chino, S.; Mikoshiba, T.; Komatsu, M.; Tanaka, H.; Ichimura, H.; Yamamoto, T.; Nakahara, K.; et al. Strategies to improve outcomes for acute type A aortic dissection with cerebral malperfusion. Eur. J. Cardio-Thorac. Surg. 2021, 59, 666–673. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Patel, H.J.; Nienaber, C.A.; Montgomery, D.M.; Korach, A.; Sundt, T.M.; DeVincentiis, C.; Voehringer, M.; Peterson, M.D.; Myrmel, T.; et al. Patients with type A acute aortic dissection presenting with major brain injury: Should we operate on them? J. Thorac. Cardiovasc. Surg. 2013, 145 (Suppl. S3), S213–S221.e1. [Google Scholar] [CrossRef]
- Okita, Y.; Ikeno, Y.; Yokawa, K.; Koda, Y.; Henmi, S.; Gotake, Y.; Nakai, H.; Matsueda, T.; Inoue, T.; Tanaka, H. Direct perfusion of the carotid artery in patients with brain malperfusion secondary to acute aortic dissection. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 161–167. [Google Scholar] [CrossRef]
- Heran, M.K.; Balaji, N.; Cook, R.C. Novel Percutaneous Treatment of Cerebral Malperfusion Before Surgery for Acute Type A Dissection. Ann. Thorac. Surg. 2019, 108, e15–e17. [Google Scholar] [CrossRef]
- Norton, E.L.; Wu, X.; Kim, K.M.; Fukuhara, S.; Patel, H.J.; Deeb, G.M.; Yang, B. Is hemiarch replacement adequate in acute type A aortic dissection repair in patients with arch branch vessel dissection without cerebral malperfusion? J. Thorac. Cardiovasc. Surg. 2021, 161, 873–884.e2. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, O.; Engin, M. The role of neutrophil-lymphocyte platelet ratio in predicting in-hospital mortality after acute Type A aortic dissection operations. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Erdolu, B.; As, A.K. C-Reactive Protein and Neutrophil to Lymphocyte Ratio Values in Predicting Inhospital Death in Patients with Stanford Type A Acute Aortic Dissection. Heart Surg. Forum 2020, 23, E488–E492. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, S.; Eagle, K.A.; Nienaber, C.A.; Rampoldi, V.; Jonker, F.H.; De Vincentiis, C.; Frigiola, A.; Menicanti, L.; Tsai, T.; Froehlich, J.; et al. Role of age in acute type A aortic dissection outcome: Report from the International Registry of Acute Aortic Dissection (IRAD). J. Thorac. Cardiovasc. Surg. 2010, 140, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Salem, M.A.; Puehler, T.; Hoffmann, G.; Lutter, G.; Cremer, J.; Haneya, A. Sex-specific risk factors for early mortality and survival after surgery of acute aortic dissection type a: A retrospective observational study. J. Cardiothorac. Surg. 2020, 15, 145. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Das, A.S.; Stapleton, C.J.; Chandra, R.V.; Rabinov, J.D.; Patel, A.B.; Hirsch, J.A.; Leslie-Mazwi, T.M. Blood Pressure and Penumbral Sustenance in Stroke from Large Vessel Occlusion. Front. Neurol. 2017, 8, 317. [Google Scholar] [CrossRef]
| Variable | Total (n = 480) | No PSCM (n = 398/82.9%) | PSCM (n = 82/17.1%) | p-Value |
|---|---|---|---|---|
| Age (years) | 63 (53; 73) | 62 (53; 73) | 66 (56; 73) | 0.077 |
| Female gender | 170 (35.4%) | 139 (34.9%) | 31 (37.8%) | 0.619 |
| Body mass index [kg/m2] | 26.3 (24; 29.3) | 26.3 (24; 29.3) | 26.3 (23.4; 28.6) | 0.530 |
| Logistic EuroScore I | 27 (16; 42) | 24 (15; 39) | 47 (31; 64) | <0.001 |
| EuroScore II | 6.86 (4.07; 14.14) | 6.62 (3.92; 13.27) | 9.42 (4.96; 14.66) | 0.44 |
| LVEF [%] | 60 (55; 70) | 60 (55; 70) | 60 (55; 70) | 0.804 |
| Aneurysm Diameter [mm] | 52 (47; 60) | 52 (49; 60) | 48 (45; 53) | 0.048 |
| DeBakey I | 380 (79.3%) | 314 (79.1%) | 66 (80.5%) | 0.776 |
| DeBakey II | 100 (20.7%) | 84 (20.9%) | 16 (19.5%) | 0.776 |
| Arterial hypertension | 312 (65%) | 251 (63.1%) | 61 (74.4%) | 0.050 |
| IDDM | 6 (1.3%) | 4 (1%) | 2 (2.4%) | 0.275 |
| Acute kidney failure | 9 (1.9%) | 8 (2%) | 1 (1.2%) | 1.000 |
| Chronic kidney failure | 51 (10.6%) | 43 (10.8%) | 8 (9.8%) | 0.774 |
| COPD | 33 (6.9%) | 30 (7.5%) | 3 (3.7%) | 0.206 |
| PAD | 15 (3.1%) | 12 (3%) | 3 (3.7%) | 0.729 |
| CAD | 75 (15.6%) | 62 (15.6%) | 13 (15.8%) | 0.537 |
| Bicuspid aortic valve | 23 (4.8%) | 22 (5.6%) | 1 (1.2%) | 0.297 |
| Marfan syndrome | 13 (2.7%) | 10 (2.5%) | 3 (3.7%) | 0.473 |
| Previous PCI | 33 (6.9%) | 30 (7.6%) | 3 (3.7%) | 0.213 |
| Previous thoracic intervention | 41 (8.5%) | 30 (7.5%) | 11 (13.4%) | 0.083 |
| Previous cardiac surgery | 14 (2.9%) | 8 (2%) | 6 (7.3%) | 0.020 |
| Pericardial tamponade | 78 (16.3%) | 65 (16.4%) | 13 (15.9%) | 0.901 |
| Acute MI (≤48 h) | 15 (3.1%) | 13 (3.3%) | 2 (2.4%) | 1.000 |
| Cardiogenic shock | 33 (6.9%) | 29 (7.3%) | 4 (4.9%) | 0.430 |
| CPR (≤48 h) | 40 (8.3%) | 37 (9.3%) | 3 (3.7%) | 0.093 |
| ICU transfer | 71 (14.8%) | 62 (15.6%) | 9 (11%) | 0.282 |
| Ventilated on admission | 52 (10.9%) | 44 (11.1%) | 8 (9.8%) | 0.725 |
| Atrial fibrillation | 56 (11.7%) | 43 (10.8%) | 13 (15.9%) | 0.195 |
| Aortic valve regurgitation | 157 (33.7%) | 131 (33.8%) | 26 (33.3%) | 0.302 |
| C-reactive protein (mg/dL) | 4.75 (1.28; 23.7) | 4.25 (1.2; 21.5) | 6.5 (2.1; 52.6) | 0.014 |
| Variable | Total (n = 480) | No PSCM (n = 398/82.9%) | PSCM (n = 82/17.1%) | p-Value |
|---|---|---|---|---|
| Surgery duration [min] | 281 (228; 347) | 280 (227; 349) | 289 (230; 335) | 0.993 |
| CPB [min] | 168 (135; 215) | 171 (135; 224) | 160 (139; 193) | 0.132 |
| Cross clamp duration [min] | 95 (72; 137) | 96 (72; 140) | 90 (71; 118) | 0.166 |
| Circulatory arrest [min] | 35 (26; 51) | 34 (26; 51) | 39 (28; 55) | 0.199 |
| RBC [unit] | 2 (0; 6) | 2 (0; 5) | 4 (2; 6) | <0.001 |
| FFP [unit] | 0 (0; 6) | 0 (0; 4) | 3 (0; 6) | 0.003 |
| Platelets [unit] | 2 (1; 2) | 2 (1; 2) | 2 (1; 2) | 0.035 |
| Supracoronary aortic replacement ONLY | 202 (42.1%) | 163 (41%) | 39 (47.6%) | 0.270 |
| Hemi-arch | 119 (24.8%) | 96 (24.1%) | 23 (28%) | 0.453 |
| Total-arch | 72 (15%) | 61 (15.3%) | 11 (13.4%) | 0.659 |
| Conduit/Bentall | 96 (20%) | 89 (22.4%) | 7 (8.5%) | 0.004 |
| David | 29 (6%) | 26 (6.5%) | 3 (3.7%) | 0.447 |
| Elephant-trunk | 13 (2.7%) | 11 (2.8%) | 2 (2.4%) | 1.000 |
| Aortic valve replacement | 91 (19%) | 86 (21.6%) | 5 (6.1%) | 0.001 |
| CABG | 37 (7.7%) | 34 (8.5%) | 3 (3.7%) | 0.131 |
| Arterial cannulation site | ||||
| Femoral artery | 81 (17.3%) | 62 (16%) | 19 (23.5%) | 0.283 |
| Ascending aorta | 93 (19.9%) | 74 (19.1%) | 19 (23.5%) | 0.283 |
| Aortic arch | 13 (2.8%) | 10 (2.6%) | 3 (3.7%) | 0.283 |
| Subclavian artery | 2 (0.4%) | 2 (0.5%) | 0 (0%) | 0.283 |
| Apex | 5 (1.1%) | 4 (1%) | 1 (1.2%) | 0.283 |
| Pulmonary vein | 274 (58.5%) | 235 (60.7%) | 39 (48.1%) | 0.283 |
| Venous cannulation site | ||||
| Right Atrium | 455 (97.2%) | 378 (97.7%) | 77 (95.1%) | 0.086 |
| bicaval | 4 (0.9%) | 4 (1%) | 0 (0%) | 0.086 |
| Femoral vein | 9 (1.9%) | 5 (1.3%) | 4 (4.9%) | 0.086 |
| Variable | Total (n = 480) | No PSCM (n = 398/82.9%) | PSCM (n = 82/17.1%) | p-Value |
|---|---|---|---|---|
| Postoperative inotropic therapy | 64 (14%) | 54 (14.3%) | 10 (12.7%) | 0.213 |
| 48 h chest tube output [mL] | 910 (500; 1650) | 950 (500; 1700) | 900 (500; 1600) | 0.987 |
| 24 h RBC [unit] | 0 (0; 2) | 0 (0; 2) | 1 (0; 2) | 0.525 |
| 24 h FFP [unit] | 0 (0; 4) | 0 (0; 4) | 0 (0; 4) | 0.894 |
| 24 h Platelets [unit] | 0 (0; 0) | 0 (0; 0) | 0 (0; 1) | 0.424 |
| Total RBC given [unit] | 3 (0; 8) | 2 (0; 8) | 4 (0; 8) | 0.314 |
| Total FFP [unit] | 1.5 (0; 6) | 2 (0; 6) | 0 (0; 4) | 0.189 |
| Total platelets [unit] | 0 (0; 2) | 0 (0; 2) | 0 (0; 1) | 0.042 |
| Ventilation [h] | 17 (60; 189) | 43 (16; 158) | 108.5 (44; 277) | <0.001 |
| ICU stay [d] | 5 (2; 11) | 5 (2; 11) | 7 (4; 13) | 0.013 |
| ICU re-admission | 39 (8.2%) | 33 (8.4%) | 6 (7.3%) | 0.737 |
| Reintubation | 77 (16.3%) | 63 (16.1%) | 14 (17.1%) | 0.830 |
| Tracheotomy | 110 (23.2%) | 81 (20.7%) | 29 (35.4%) | 0.004 |
| Delirium | 93 (19.8%) | 81 (20.9%) | 12 (14.6%) | 0.197 |
| MI | 6 (1.3%) | 6 (1.5%) | 0 (0%) | 0.596 |
| New neurological deficits | 105 (22.2%) | 76 (19.4%) | 29 (35.4%) | 0.002 |
| CPR | 29 (6.1%) | 25 (6.4%) | 4 (4.9%) | 0.603 |
| Pneumonia | 67 (14.2%) | 49 (12.5%) | 18 (22%) | 0.026 |
| Sepsis | 21 (4.4%) | 16 (4.1%) | 5 (6.1%) | 0.385 |
| TEVAR(EVAR) | 31 (6.5%) | 27 (6.9%) | 4 (4.9%) | 0.522 |
| Re-thoracotomy | 89 (18.7%) | 78 (19.7%) | 11 (13.4%) | 0.180 |
| Wound healing deficits | 7 (1.5%) | 6 (1.5%) | 1 (1.2%) | 1.000 |
| AKI KDIGO | 102 (21.7%) | 85 (21.9%) | 17 (20.7%) | 0.815 |
| Postoperative AF | 46 (9.8%) | 37 (9.5%) | 9 (11.1%) | 0.654 |
| New pacer | 23 (4.9%) | 20 (5.1%) | 3 (3.7%) | 0.780 |
| Postoperative C-reactive protein (mg/dL) | 110.5 (47; 173.1) | 102 (42.55; 161.38) | 141.6 (91.8; 193.65) | 0.005 |
| Postoperative platelets count | 127.5 (101.25; 157) | 130 (104; 161) | 111 (90; 141) | 0.004 |
| Variable | Total (n = 480) | No Cerebral Malperfusion (n = 398/82.9%) | Cerebral Malperfusion (n = 82/17.1%) | p-Value |
|---|---|---|---|---|
| In-hospital mortality | 77 (16.1%) | 61 (15.3%) | 16 (19.8%) | 0.323 |
| Cause of death | ||||
| Cardiac | 44 (50%) | 34 (49.3%) | 10 (52.6%) | 0.064 |
| Cerebrovascular | 8 (9.1%) | 4 (5.8%) | 4 (21.1%) | 0.064 |
| Sepsis | 4 (4.5%) | 2 (2.9%) | 2 (10.5%) | 0.064 |
| Multi-organ failure | 28 (31.8%) | 25 (36.2%) | 3 (15.8%) | 0.064 |
| Unknown | 4 (4.5%) | 4 (5.8%) | 0 (0%) | 0.064 |
| Surgery till death [d] | 3 (1; 12) | 3 (1; 11) | 6 (1; 16) | 0.223 |
| 7-day mortality | 56 (11.8%) | 46 (11.7%) | 10 (12.2%) | 0.900 |
| 30-day mortality | 86 (18%) | 68 (17.2%) | 18 (22%) | 0.310 |
| Risk Factor | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Age (years) | 1.041 | [1.026–1.057] | <0.001 |
| Female gender | 0.949 | [0.682–1.321] | 0.758 |
| PSCM | 1.747 | [1.204–2.534] | 0.003 |
| Arterial hypertension | 0.595 | [0.433–0.817] | 0.001 |
| PAD | 1.890 | [0.917–3.896] | 0.085 |
| Ventilated on admission | 1.677 | [1.050–2.679] | 0.031 |
| CPR (<48 h) | 2.610 | [1.622–4.202] | <0.001 |
| CPB (min) | 1.007 | [1.004–1.009] | <0.001 |
| RBC (unit) | 1.043 | [1.000–1.088] | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tawil, M.; Salem, M.; Friedrich, C.; Diraz, S.; Broll, A.; Rezahie, N.; Schoettler, J.; de Silva, N.; Puehler, T.; Cremer, J.; et al. Preoperative Imaging Signs of Cerebral Malperfusion in Acute Type A Aortic Dissection: Influence on Outcomes and Prognostic Implications—A 20-Year Experience. J. Clin. Med. 2023, 12, 6659. https://doi.org/10.3390/jcm12206659
Al-Tawil M, Salem M, Friedrich C, Diraz S, Broll A, Rezahie N, Schoettler J, de Silva N, Puehler T, Cremer J, et al. Preoperative Imaging Signs of Cerebral Malperfusion in Acute Type A Aortic Dissection: Influence on Outcomes and Prognostic Implications—A 20-Year Experience. Journal of Clinical Medicine. 2023; 12(20):6659. https://doi.org/10.3390/jcm12206659
Chicago/Turabian StyleAl-Tawil, Mohammed, Mohamed Salem, Christine Friedrich, Shirin Diraz, Alexandra Broll, Najma Rezahie, Jan Schoettler, Nora de Silva, Thomas Puehler, Jochen Cremer, and et al. 2023. "Preoperative Imaging Signs of Cerebral Malperfusion in Acute Type A Aortic Dissection: Influence on Outcomes and Prognostic Implications—A 20-Year Experience" Journal of Clinical Medicine 12, no. 20: 6659. https://doi.org/10.3390/jcm12206659
APA StyleAl-Tawil, M., Salem, M., Friedrich, C., Diraz, S., Broll, A., Rezahie, N., Schoettler, J., de Silva, N., Puehler, T., Cremer, J., & Haneya, A. (2023). Preoperative Imaging Signs of Cerebral Malperfusion in Acute Type A Aortic Dissection: Influence on Outcomes and Prognostic Implications—A 20-Year Experience. Journal of Clinical Medicine, 12(20), 6659. https://doi.org/10.3390/jcm12206659






