Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Outcomes
2.3. Follow-Up
2.4. Surgical Technique
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Overall Survival
3.3. Disease-Free Survival
3.4. Recurrence Rate
3.5. Surgery-Related Outcomes
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Lim, E.; Batchelor, T.J.P.; Dunning, J.; Shackcloth, M.; Anikin, V.; Naidu, B.; Belcher, E.; Loubani, M.; Zamvar, V.; Harris, R.A.; et al. Video-Assisted Thoracoscopic or Open Lobectomy in Early-Stage Lung Cancer. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Louie, B.E.; Wilson, J.L.; Kim, S.; Cerfolio, R.J.; Park, B.J.; Farivar, A.S.; Vallières, E.; Aye, R.W.; Burfeind, W.R., Jr.; Block, M.I. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2016, 102, 917–924. [Google Scholar] [CrossRef]
- Nelson, D.B.; Mehran, R.J.; Mitchell, K.G.; Rajaram, R.; Correa, A.M.; Bassett, R.L., Jr.; Antonoff, M.B.; Hofstetter, W.L.; Roth, J.A.; Sepesi, B.; et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann. Thorac. Surg. 2019, 108, 370–376. [Google Scholar] [CrossRef]
- Lee, B.E.; Korst, R.J.; Kletsman, E.; Rutledge, J.R. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: Are there outcomes advantages? J. Thorac. Cardiovasc. Surg. 2014, 147, 724–729. [Google Scholar] [CrossRef]
- Adams, R.D.; Bolton, W.D.; Stephenson, J.E.; Henry, G.; Robbins, E.T.; Sommers, E. Initial multicenter community robotic lobectomy experience: Comparisons to a national database. Ann. Thorac. Surg. 2014, 97, 1893–1898; discussion 1899–1900. [Google Scholar] [CrossRef]
- Kent, M.; Wang, T.; Whyte, R.; Curran, T.; Flores, R.; Gangadharan, S. Open, video-assisted thoracic surgery, and robotic lobectomy: Review of a national database. Ann. Thorac. Surg. 2014, 97, 236–242; discussion 242–244. [Google Scholar] [CrossRef]
- Novellis, P.; Maisonneuve, P.; Dieci, E.; Voulaz, E.; Bottoni, E.; Di Stefano, S.; Solinas, M.; Testori, A.; Cariboni, U.; Alloisio, M.; et al. Quality of Life, Postoperative Pain, and Lymph Node Dissection in a Robotic Approach Compared to VATS and OPEN for Early Stage Lung Cancer. J. Clin. Med. 2021, 10, 1687. [Google Scholar] [CrossRef]
- Yang, H.X.; Woo, K.M.; Sima, C.S.; Bains, M.S.; Adusumilli, P.S.; Huang, J.; Finley, D.J.; Rizk, N.P.; Rusch, V.W.; Jones, D.R.; et al. Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 2017, 265, 431–437. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; D’Souza, D.M.; Richardson, M.; Abdel-Rasoul, M.; Moffatt-Bruce, S.D.; Merritt, R.E. Long-Term Oncologic Outcomes After Robotic Lobectomy for Early-stage Non-Small-cell Lung Cancer Versus Video-assisted Thoracoscopic and Open Thoracotomy Approach. Clin. Lung Cancer 2020, 21, 214–224.e2. [Google Scholar] [CrossRef]
- Sesti, J.; Langan, R.C.; Bell, J.; Nguyen, A.; Turner, A.L.; Hilden, P.; Leshchuk, K.; Dabrowski, M.; Paul, S. A Comparative Analysis of Long-Term Survival of Robotic Versus Thoracoscopic Lobectomy. Ann. Thorac. Surg. 2020, 110, 1139–1146. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.J.; Petersen, R.H. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach—The Copenhagen experience. Ann. Cardiothorac. Surg. 2012, 1, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.; Chirieac, L.R.; D’Amico, T.A.; DeCamp, M.M.; Dilling, T.J.; Dobelbower, M.; et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 504–535. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; Waller, D.; Passlick, B.; Zielinski, M.; Lerut, T.; Weder, W. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardiothorac. Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Asamura, H.; Chansky, K.; Crowley, J.; Goldstraw, P.; Rusch, V.W.; Vansteenkiste, J.F.; Watanabe, H.; Wu, Y.L.; Zielinski, M.; Ball, D.; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 1675–1684. [Google Scholar] [CrossRef]
- Dong, S.; Du, J.; Li, W.; Zhang, S.; Zhong, X.; Zhang, L. Systematic mediastinal lymphadenectomy or mediastinal lymph node sampling in patients with pathological stage I NSCLC: A meta-analysis. World J. Surg. 2015, 39, 410–416. [Google Scholar] [CrossRef]
- Wu, Y.C.; Lin, C.F.; Hsu, W.H.; Huang, B.S.; Huang, M.H.; Wang, L.S. Long-term results of pathological stage I non-small cell lung cancer: Validation of using the number of totally removed lymph nodes as a staging control. Eur. J. Cardiothorac. Surg. 2003, 24, 994–1001. [Google Scholar] [CrossRef]
- Wu, Y.L.; Huang, Z.F.; Wang, S.Y.; Yang, X.N.; Ou, W. A randomized trial of systematic nodal dissection in resectable non-small cell lung cancer. Lung Cancer 2002, 36, 1–6. [Google Scholar] [CrossRef]
- D’Andrilli, A.; Venuta, F.; Rendina, E.A. The role of lymphadenectomy in lung cancer surgery. Thorac. Surg. Clin. 2012, 22, 227–237. [Google Scholar] [CrossRef]
- Hüyük, M.; Fiocco, M.; Postmus, P.E.; Cohen, D.; von der Thüsen, J.H. Systematic review and meta-analysis of the prognostic impact of lymph node micrometastasis and isolated tumour cells in patients with stage I–IIIA non-small cell lung cancer. Histopathology 2023, 82, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, N.Y.; Pyo, J.S. Prognostic roles of lymph node micrometastasis in non-small cell lung cancer. Pathol. Res. Pract. 2018, 214, 240–244. [Google Scholar] [CrossRef] [PubMed]



| Patient Characteristics | Total | VATS | RATS | p |
|---|---|---|---|---|
| Age (±SD 1) | 70 ± 10 | 69 ± 10 | 70 ± 10 | 0.059 |
| Women | 62.2% (n = 385) | 64.4% (n = 139) | 61% (n = 246) | 0.435 |
| Smoking habits | ||||
| Never | 14.4% (n = 89) | 11.6% (n = 25) | 15.9% (n = 64) | 0.152 |
| Former | 68.8% (n = 426) | 69.9% (n = 151) | 68.2% (n = 275) | 0.716 |
| Current | 16.2% (n = 100) | 18.5% (n = 40) | 14.9% (n = 60) | 0.253 |
| unknown | 0.6% (n = 4) | 0.0% (n = 0) | 1.0% (n = 4) | 0.304 |
| Performance status | 0.591 | |||
| <2 | 81.1% (n = 502) | 82.4% (n = 178) | 80.4% (n = 324) | |
| ≥2 | 18.9% (n = 117) | 17.6% (n = 38) | 19.6% (n = 79) | |
| Comorbidities | ||||
| COPD 2 | 20.7% (n = 128) | 25.0% (n = 54) | 18.4% (n = 74) | 0.061 |
| AF 3 | 6.0% (n = 37) | 5.6% (n = 12) | 6.2% (n = 25) | 0.860 |
| CAD/IHD 4 | 13.4% (n = 83) | 14.4% (n = 31) | 12.9% (n = 52) | 0.622 |
| CKD 5 | 4.2% (n = 26) | 3.7% (n = 8) | 4.5% (n = 18) | 0.834 |
| DM 6 | 11.8% (n = 73) | 14.4% (n = 31) | 10.4% (n = 42) | 0.153 |
| TIA/CVA 7 | 2.7% (n = 17) | 1.9% (n = 4) | 3.2% (n = 13) | 0.441 |
| Respiratory function | ||||
| FEV1 8 (median; IQR 9) | 91% (IQR 9: 30) | 92% (IQR 9: 30) | 90% (IQR 9: 30) | 0.195 |
| DLCO 10 (median; IQR 9) | 73% (IQR 9: 26) | 71% (IQR 9: 25) | 76% (IQR 9: 26) | 0.001 |
| Tumor location | ||||
| Right | ||||
| Upper | 33.6% (n = 208) | 35.2% (n = 76) | 32.8% (n = 132) | 0.592 |
| Middle | 11.3% (n = 70) | 12.1% (n = 26) | 10.9% (n = 44) | 0.691 |
| Lower | 21.0% (n = 130) | 19.4% (n = 42) | 21.8% (n = 88) | 0.535 |
| Left | ||||
| Upper | 18.3% (n = 113) | 17.1% (n = 37) | 18.9% (n = 76) | 0.663 |
| Lower | 15.8% (n = 98) | 16.2% (n = 35) | 15.6% (n = 63) | 0.908 |
| Tumor size (mean; ±SD 1) | 28 ± 18 mm | 28 ± 16 mm | 29 ± 19 mm | 0.979 |
| Tumor histology | ||||
| Adenocarcinoma | 76.4% (n = 473) | 74.1% (n = 160) | 77.7% (n = 313) | 0.322 |
| Squamous cell carcinoma | 20.0% (n = 124) | 21.7% (n = 47) | 19.1% (n = 77) | 0.461 |
| Large cell carcinoma | 3.6% (n = 22) | 4.2% (n = 9) | 3.2% (n = 13) | 0.649 |
| Clinical stage | ||||
| I | 78.8% (n = 488) | 83.8% (n = 181) | 76.2% (n = 307) | 0.03 |
| II | 14.6% (n = 90) | 13.0% (n = 28) | 15.4% (n = 62) | 0.473 |
| III | 5.8% (n = 36) | 3.2% (n = 7) | 7.2% (n = 29) | 0.048 |
| Unknown | 0.8% (n = 5) | 0.0% (n = 0) | 1.2% (n = 5) | 0.169 |
| Pathological stage | ||||
| I | 65.6% (n = 406) | 63.9% (n = 138) | 66.5% (n = 268) | 0.535 |
| II | 20.5% (n = 127) | 22.2% (n = 48) | 19.6% (n = 79) | 0.466 |
| III | 13.9% (n = 86) | 13.9% (n = 30) | 13.9% (n = 56) | >0.999 |
| Final nodal status | ||||
| N1 | 9.5% (n = 59) | 8.3% (n = 18) | 10.2% (n = 41) | 0.556 |
| N2 | 9.4% (n = 58) | 10.6% (n = 23) | 8.7% (n = 35) | 0.470 |
| Neoadjuvant therapy | 1.6% (n = 10) | 1.9% (n = 4) | 1.5% (n = 6) | 0.746 |
| Chemotherapy | 1.5% (n = 9) | 1.4% (n = 3) | 1.5% (n = 6) | >0.999 |
| Chemo-radiotherapy | 0.2% (n = 1) | 0.5% (n = 1) | 0.0% (n = 0) | 0.349 |
| Survival | VATS | RATS | p |
|---|---|---|---|
| Overall Survival | 0.637 | ||
| 3 years | 82.3% | 75.9% | |
| 5 years | 68.5% | 70.5% | |
| OS 1 stage I | 0.436 | ||
| 3 years | 86.8% | 86.3% | |
| 5 years | 75.7% | 83.4% | |
| OS 1 stage II | 0.070 | ||
| 3 years | 77.0% | 58.2% | |
| 5 years | 68.7% | 51.7% | |
| OS 1 stage III | 0.412 | ||
| 3 years | 70.0% | 48.5% | |
| 5 years | 33.7% | 24.3% | |
| Disease-free Survival | <0.001 | ||
| 3 years | 81.2% | 92.4% | |
| 5 years | 77.6% | 90.3% | |
| DFS 2 stage I | 0.037 | ||
| 3 years | 88.9% | 94.4% | |
| 5 years | 85.2% | 91.8% | |
| DFS 2 stage II | 0.105 | ||
| 3 years | 77.7% | 92.6% | |
| 5 years | 73.4% | 92.6% | |
| DFS 2 stage III | 0.024 | ||
| 3 years | 51.1% | 82.4% | |
| 5 years | 37.7% | 82.4% |
| (A) | |||
|---|---|---|---|
| Variable | HR 1 | 95% CI 2 | p |
| Approach | |||
| VATS | Reference | - | - |
| RATS | 1.23 | 0.83–1.81 | 0.293 |
| Sex | |||
| Female | Reference | - | - |
| Male | 1.62 | 1.09–2.37 | 0.015 |
| Age (continuous) | 1.05 | 1.03–1.08 | <0.001 |
| Pathology | |||
| Adenocarcinoma | Reference | - | - |
| Squamous cell carcinoma | 1.01 | 0.64–1.55 | 0.963 |
| Large cell carcinoma | 1.26 | 0.52–2.57 | 0.570 |
| Pathological stage | |||
| I | Reference | - | - |
| II | 1.8 | 1.13–2.82 | 0.011 |
| III | 4.54 | 2.93–6.97 | <0.001 |
| Induction therapy | |||
| no | Reference | - | - |
| yes | 2.29 | 0.56–6.21 | 0.165 |
| Comorbidities | |||
| Pulmonary 3 | 0.99 | 0.64–1.50 | 0.960 |
| Cardiovascular 4 | 1.12 | 0.76–1.66 | 0.572 |
| Diabetes | 1.11 | 0.69–1.72 | 0.663 |
| Renal failure | 0.36 | 0.11–0.89 | 0.051 |
| Respiratory function | |||
| FEV1 5 (continuous) | 0.99 | 0.99–1.00 | 0.168 |
| DLCO 6 (continuous) | 0.98 | 0.97–0.99 | 0.015 |
| (B) | |||
| Variable | HR 1 | 95% CI 2 | p |
| Approach | |||
| VATS | Reference | - | - |
| RATS | 0.46 | 0.27–0.78 | 0.004 |
| Sex | |||
| Female | Reference | - | - |
| Male | 2.02 | 1.20–3.37 | 0.008 |
| Age (continuous) | 0.99 | 0.96–1.01 | 0.205 |
| Pathology | |||
| Adenocarcinoma | Reference | - | - |
| Squamous cell carcinoma | 0.48 | 0.22–0.96 | 0.052 |
| Large cell carcinoma | 0.99 | 0.24–2.72 | 0.989 |
| Pathological stage | |||
| I | Reference | - | - |
| II | 2.27 | 1.12–4.45 | 0.02 |
| III | 6.44 | 2.82–14.12 | <0.001 |
| Induction therapy | |||
| no | Reference | - | - |
| yes | 1.44 | 0.23–4.92 | 0.625 |
| Nodal upstaging | |||
| no | Reference | - | - |
| yes | 1.23 | 0.60–2.56 | 0.582 |
| VATS | RATS | p | |
|---|---|---|---|
| Recurrence rate | |||
| Overall | 21.8% (n = 47) | 6.2% (n = 25) | <0.001 |
| Stage I | 12.3% (n = 17) | 4.1% (n = 11) | <0.001 |
| Stage II | 27.1% (n = 13) | 6.3% (n = 5) | <0.001 |
| Stage III | 56.7% (n = 17) | 15.8% (n = 9) | <0.001 |
| Recurrence site | |||
| local | 7.4% (n = 16) | 1.2% (n = 5) | <0.001 |
| distant | 11.6% (n = 25) | 4.5% (n = 18) | 0.001 |
| both | 2.8% (n = 6) | 0.5% (n = 2) | 0.024 |
| Stage I | |||
| local | 3.6% (n = 5) | 0.7% (n = 2) | 0.048 |
| distant | 6.5% (n = 9) | 3.0% (n = 8) | 0.117 |
| both | 2.2% (n = 3) | 0.4% (n = 1) | 0.117 |
| Stage II | |||
| local | 6.3% (n = 3) | 0.0% (n = 0) | 0.052 |
| distant | 16.7% (n = 8) | 6.3% (n = 5) | 0.075 |
| both | 4.2% (n = 2) | 0.0% (n = 0) | 0.141 |
| Stage III | |||
| local | 26.7% (n = 8) | 5.3% (n = 3) | 0.007 |
| distant | 26.7% (n = 8) | 8.8% (n = 5) | 0.054 |
| both | 3.3% (n = 1) | 1.8% (n = 1) | >0.999 |
| VATS | RATS | p | |
|---|---|---|---|
| LOS 1 (median; IQR 3) | 5 days (IQR 3: 5) | 5 days (IQR 3: 5) | 0.453 |
| LOD 2 (median; IQR 3) | 2 days (IQR 3: 3) | 2 days (IQR 3: 3) | 0.818 |
| Complication rate | 38.4% (n = 83) | 34.0% (n = 137) | 0.291 |
| In-hospital mortality | 0.0% (n = 0) | 0.25% (n = 1) * | >0.999 |
| 30-day mortality | 0.0% (n = 0) | 0.25% (n = 1) * | >0.999 |
| 90-day mortality | 0.0% (n = 0) | 0.25% (n = 1) * | >0.999 |
| Nodal stations harvested (median; IQR) | |||
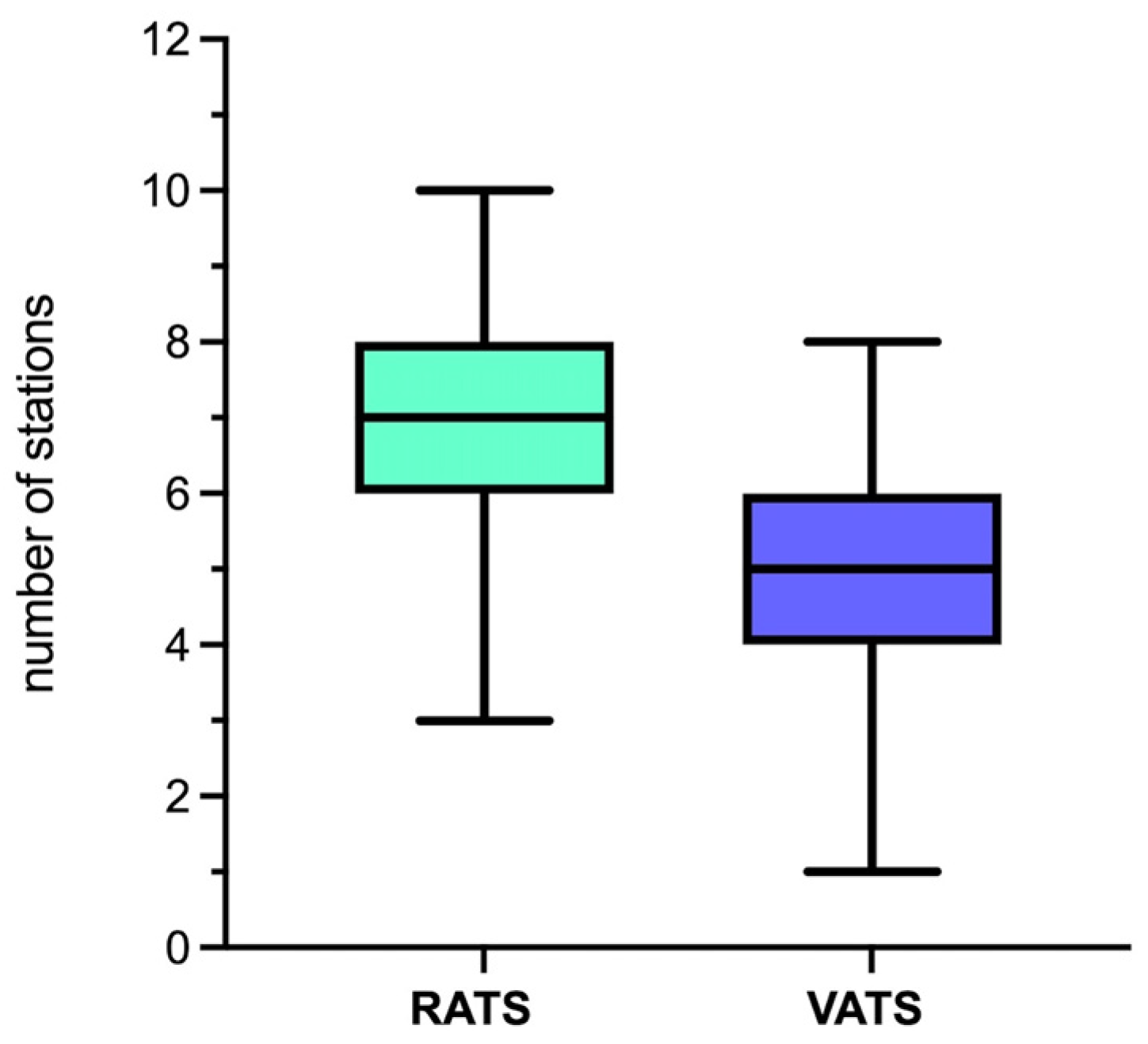
| Overall | 5 (IQR 3: 2) | 7 (IQR 3: 2) | <0.001 |
| Mediastinal (N2) | 3 (IQR 3: 1) | 4 (IQR 3: 1) | <0.001 |
| Hilar or intrapulmonary (N1) | 2 (IQR 3: 1) | 3 (IQR 3: 1) | <0.001 |
| Upstaging rate | 27.8% (n = 60) | 18.6% (n = 75) | 0.001 |
| Nodal upstaging | 16.7% (n = 36) | 13.2% (n = 53) | 0.233 |
| T–upstaging | 26.4% (n = 57) | 22.6% (n = 91) | 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, G.; Femia, F.; Lampridis, S.; Farinelli, E.; Maraschi, A.; Routledge, T.; Bille, A. Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer. J. Clin. Med. 2023, 12, 6609. https://doi.org/10.3390/jcm12206609
Fabbri G, Femia F, Lampridis S, Farinelli E, Maraschi A, Routledge T, Bille A. Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer. Journal of Clinical Medicine. 2023; 12(20):6609. https://doi.org/10.3390/jcm12206609
Chicago/Turabian StyleFabbri, Giulia, Federico Femia, Savvas Lampridis, Eleonora Farinelli, Alessandro Maraschi, Tom Routledge, and Andrea Bille. 2023. "Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer" Journal of Clinical Medicine 12, no. 20: 6609. https://doi.org/10.3390/jcm12206609
APA StyleFabbri, G., Femia, F., Lampridis, S., Farinelli, E., Maraschi, A., Routledge, T., & Bille, A. (2023). Long-Term Oncologic Outcomes in Robot-Assisted and Video-Assisted Lobectomies for Non-Small Cell Lung Cancer. Journal of Clinical Medicine, 12(20), 6609. https://doi.org/10.3390/jcm12206609








