Predictive Factors of Early Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis
Abstract
:1. Introduction
Learning Points
- Dupilumab is an effective and safe treatment for patients affected by moderate-to-severe AD.
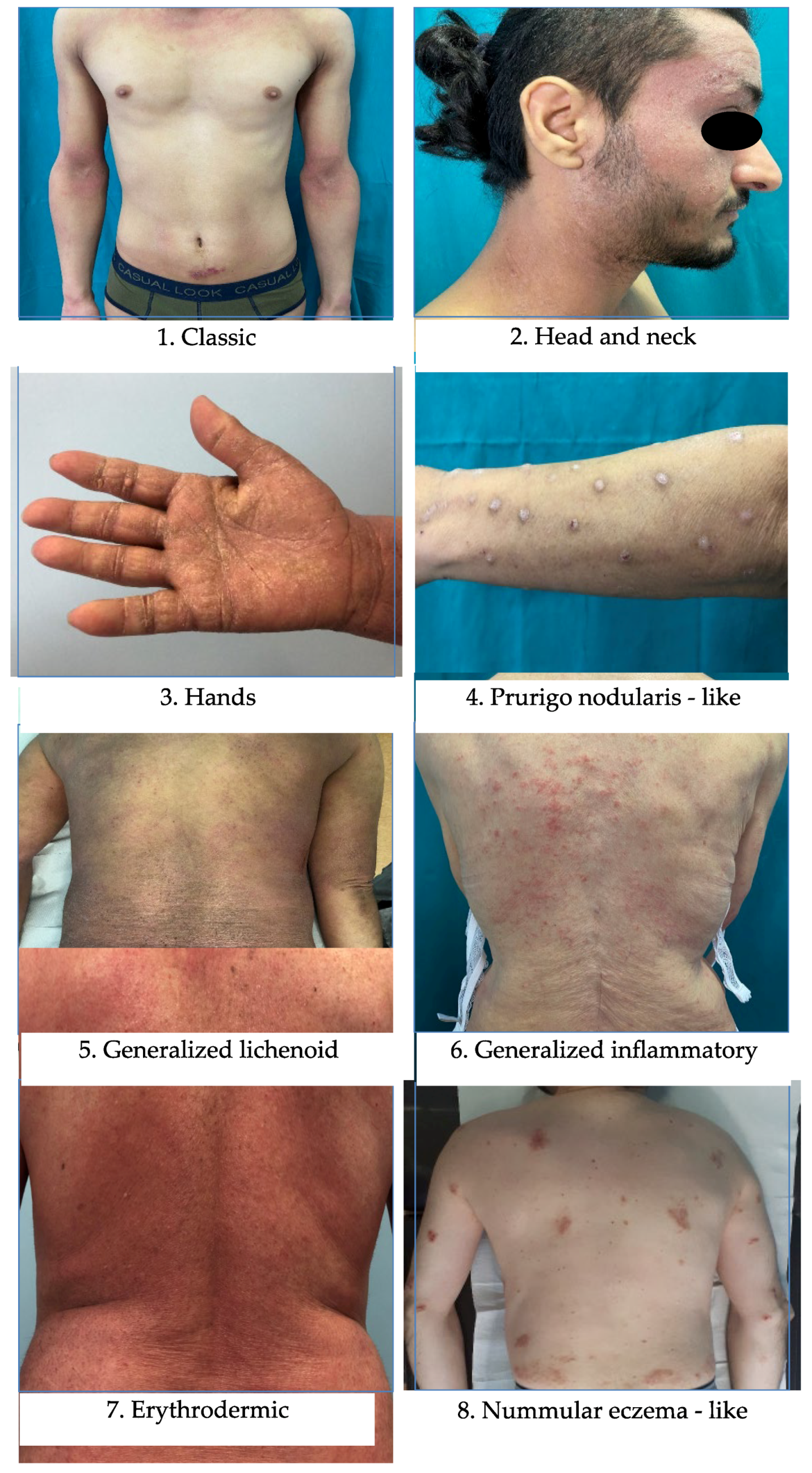
- Our findings demonstrate that patients affected by classic, generalized lichenoid and inflammatory phenotypes reach a mild level of disease earlier than patients with other non-classic phenotypes of AD.
- Patients with an EASI < 29 and particularly ≤24 respond earlier to dupilumab compared with those who have severe dermatitis with an EASI ≥ 29.
- Total serum IgE levels and eosinophil count values at baseline were not associated with an early clinical response.
- An association was found between presence of atopic blepharoconjunctivitis and facial involvement at baseline with the development, respectively, of blepharoconjunctivitis and facial redness dermatitis as adverse events.
- A timely administration of dupilumab, when EASI is <29, can effectively and rapidly bring the disease under control.
2. Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Demographics and Clinical Baseline Characteristics
3.2. Dupilumab Efficacy
3.3. Predictive Factors of Response at 4 Weeks
3.4. Safety and Discontinuation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef]
- Laughter, M.; Maymone, M.; Mashayekhi, S.; Arents, B.; Karimkhani, C.; Langan, S.; Dellavalle, R.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017*. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. New Insights into Atopic Dermatitis: Role of Skin Barrier and Immune Dysregulation. Allergol. Int. 2013, 62, 151–161. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Pappa, G.; Sgouros, D.; Theodoropoulos, K.; Kanelleas, A.; Bozi, E.; Gregoriou, S.; Krasagakis, K.; Katoulis, A.C. The IL-4/-13 Axis and Its Blocking in the Treatment of Atopic Dermatitis. J. Clin. Med. 2022, 11, 5633. [Google Scholar] [CrossRef]
- Makowska, K.; Nowaczyk, J.; Blicharz, L.; Waśkiel-Burnat, A.; Czuwara, J.; Olszewska, M.; Rudnicka, L. Immunopathogenesis of Atopic Dermatitis: Focus on Interleukins as Disease Drivers and Therapeutic Targets for Novel Treatments. Int. J. Mol. Sci. 2023, 24, 781. [Google Scholar] [CrossRef]
- Callen, J.; Chamlin, S.; Eichendfield, L.F.; Ellis, C.; Girardi, M.; Goldfarb, M.; Hanifin, J.; Lee, P.; Margolis, D.; Paller, A.S. A systematic review of the safety of topical therapies for atopic dermatitis. Br. J. Dermatol. 2007, 156, 203–221. [Google Scholar] [CrossRef]
- Le Floc’h, A.; Allinne, J.; Nagashima, K.; Scott, G.; Birchard, D.; Asrat, S.; Bai, Y.; Lim, W.K.; Martin, J.; Huang, T.; et al. Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy 2020, 75, 1188–1204. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- De Bruin-Weller, M.; Thaçi, D.; Smith, C.; Reich, K.; Cork, M.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar] [PubMed]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.-H.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Bansal, A.; Simpson, E.L.; Boguniewicz, M.; Blauvelt, A.; Siegfried, E.C.; Guttman-Yassky, E.; Hultsch, T.; Chen, Z.; Mina-Osorio, P.; et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: Post-hoc analyses from a randomized clinical trial. Am. J. Clin. Dermatol. 2020, 21, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef]
- Paller, A.S.; Simpson, E.L.; Siegfried, E.C.; Cork, M.J.; Wollenberg, A.; Arkwright, P.D.; Soong, W.E.; Gonzalez, M.; Schneider, L.C.; Sidbury, R.; et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022, 400, 908–919. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Neri, I.; Stingeni, L.; Boccaletti, V.; Piccolo, V.; Amoruso, G.F.; Malara, G.; Pasquale, R.D.; Di Brizzi, E.V.; et al. Dupilumab Treatment in Children Aged 6-11 Years with Atopic Dermatitis: A Multicentre, Real-Life Study. Pediatr. Drugs 2022, 24, 671–678. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Potestio, L.; Fontanella, G.; Picone, V.; Bennardo, L.; Scalvenzi, M.; Patruno, C. A 24-weeks real-world experience of dupilumab in adolescents with moderate-to-severe atopic dermatitis. Dermatol. Ther. 2022, 35, e15588. [Google Scholar] [CrossRef]
- David, E.; Ungar, B.; Renert-Yuval, Y.; Facheris, P.; del Duca, E.; Guttman-Yassky, E. The evolving landscape of biologic therapies for atopic dermatitis: Present and future perspective. Clin. Exp. Allergy 2023, 53, 156–172. [Google Scholar] [CrossRef]
- Nettis, E.; Ferrucci, S.M.; Pellacani, G.; Di Leo, E.; Argenziano, G.; Foti, C.; Rongioletti, F.; Patruno, C.; Ortoncelli, M.; Macchia, L.; et al. Dupilumab in atopic dermatitis: Predictors of treatment outcome and time to response. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e896–e898. [Google Scholar] [CrossRef]
- Dupilumab AIFA Summary of Product Characteristics. Available online: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_005884_045676_RCP.pdf&retry=0&sys=m0b1l3 (accessed on 14 September 2023).
- Hanifin, J.M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S.J.; Graeber, M. The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp. Dermatol. 2001, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Reaney, M.; Mastey, V.; Eckert, L.; Abbé, A.; Nelson, L.; Clark, M.; Williams, N.; Chen, Z.; Ardeleanu, M.; et al. Peak Pruritus Numerical Rating Scale: Psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br. J. Dermatol. 2019, 181, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Badia, X.; Mascaró, J.M.; Lozano, R. Measuring health-related quality of life in patients with mild to moderate eczema and psoriasis: Clinical validity, reliability and sensitivity to change of the DLQI. The Cavide Research Group. Br. J. Dermatol. 1999, 141, 698–702. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Fuxench, Z.C.C.; Gelfand, J.M.; Margolis, D.J.; Boguniewicz, M.; Fonacier, L.; Grayson, M.H.; Simpson, E.L.; Ong, P.Y. Content and construct validity, predictors, and distribution of self-reported atopic dermatitis severity in US adults. Ann. Allergy Asthma Immunol. 2018, 121, 729–734.e4. [Google Scholar] [CrossRef]
- Hanifin, J.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Dermatovener 1980, 60, 44–47. [Google Scholar] [CrossRef]
- Salvador, J.S.; Romero-Pérez, D.; Encabo-Durán, B. Atopic dermatitis in adults: A diagnostic challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef]
- Wollenberg, A.; Beck, L.; Blauvelt, A.; Simpson, E.; Chen, Z.; Chen, Q.; Shumel, B.; Khokhar, F.; Hultsch, T.; Rizova, E.; et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: Results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br. J. Dermatol. 2020, 182, 1120–1135. [Google Scholar] [CrossRef]
- Ferrucci, S.; Angileri, L.; Tavecchio, S.; Fumagalli, S.; Iurlo, A.; Cattaneo, D.; Marzano, A.V.; Maronese, C.A. Elevation of peripheral blood eosinophils during dupilumab treatment for atopic dermatitis is associated with baseline comorbidities and development of facial redness dermatitis and ocular surface disease. J. Dematol. Treat. 2022, 33, 2587–2592. [Google Scholar] [CrossRef]
- Nettis, E.; Bonzano, L.; Patella, V.; Detoraki, A.; Trerotoli, P.; Lombardo, C. Dupilumab-Associated Conjunctivitis in Patients with Atopic Dermatitis: A Multicenter Real-Life Experience. J. Investig. Allergol. Clin. Immunol. 2020, 30, 201–204. [Google Scholar] [CrossRef]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef]
- Waldman, R.A.; DeWane, M.E.; Sloan, B.; Grant-Kels, J.M. Characterizing dupilumab facial redness: A multi-institution retrospective medical record review. J. Am. Acad. Dermatol. 2020, 82, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Constitution of the World Health Organization. 1946. Available online: https://apps.who.int/iris/handle/10665/268688 (accessed on 14 September 2023).
- Fargnoli, M.C.; Esposito, M.; Ferrucci, S.; Girolomoni, G.; Offidani, A.; Patrizi, A.; Peris, K.; Costanzo, A.; Malara, G.; Pellacani, G.; et al. Real-life experience on effectiveness and safety of dupilumab in adult patients with moderate-to-severe atopic dermatitis. J. Dermatol. Treat. 2019, 32, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Deleuran, M.; Bissonnette, R.; de Bruin-Weller, M.; Galus, R.; Nakahara, T.; Seo, S.J.; Khokhar, F.A.; Vakil, J.; Xiao, J.; et al. Dupilumab Provides Acceptable Safety and Sustained Efficacy for up to 4 Years in an Open-Label Study of Adults with Moderate-to-Severe Atopic Dermatitis. Am. J. Clin. Dermatol. 2022, 23, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Nettis, E.; Ferrucci, S.M.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Macchia, L.; et al. Use of Dupilumab in 543 Adult Patients with Moderate-to-Severe Atopic Dermatitis: A Multicenter, Retrospective Study. J. Investig. Allergol. Clin. Immunol. 2022, 32, 124–132. [Google Scholar] [CrossRef] [PubMed]
| Sex | |
| Men, n (%) | 276 (56.10%) |
| Women, n (%) | 216 (43.90%) |
| Age at diagnosis of AD, years, median (IQR) | 3 (0–14) |
| Age at dupilumab initiation, years, median (IQR) | 33 (22–50) |
| Adults (n) | 451 |
| Adolescents (n) | 41 |
| Duration of AD, years, median (IQR) | 25.5 (17–39) |
| Familiarity for atopy, n (%) Missing data | 213 (55% of 384) 108 |
| Atopic comorbidities, n (%) | |
| Rhinitis and asthma | 375 (76.22%) |
| Conjunctivitis | 245 (49.80%) |
| Rhinitis and asthma and conjunctivitis | 237 (48.17%) |
| None | 109 (22.15%) |
| Atopic sensitization, n (%) | 399 (81%) |
| AD onset pattern: | |
| Early-onset persistent or relapsing, n (%) | 373 (76%) |
| Late-onset ‡, n (%) | 119 (24%) |
| AD type according to IgE: | |
| Intrinsic, n (%) | 42 (9.9%) |
| Extrinsic, n (%) | 384 (90.1%) |
| AD phenotype: | |
| Classic * | 279 (56.71%) |
| Non-classic ** | 213 (43.29%) |
| Generalized lichenoid and inflammatory | 138 |
| Prurigo nodularis | 41 |
| Nummular eczema | 19 |
| Erythrodermic | 15 |
| Previous treatments, n (%): | |
| Cyclosporine | 396 (80.49%) |
| Oral corticosteroids | 352 (71.54%) |
| Others # | 190 (38.62%) |
| Treatment at T0, n (%): | |
| Cyclosporine | 53 (11.23%) |
| Topical steroids | 237 (50.21%) |
| Cyclosporine and topical steroids | 34 (7.20%) |
| None | 148 (31.36%) |
| Baseline EASI, median (IQR) | 26 (24–30) |
| Baseline itch-NRS, median (IQR) | 9 (8–10) |
| Baseline sleep-NRS, median (IQR) | 8 (5–9) |
| Baseline DLQI, median (IQR) | 15 (11–20) |
| Baseline total serum IgE (kU/L), median (IQR) | 1287 (286–3906) |
| Missing data | 96 |
| Baseline blood eosinophil count (cells/mm3), median (IQR) | 400 (250–690) |
| Missing data | 65 |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Sex | M | 1 * | 0.0795 | - | |
| F | 1.40 (0.96–2.04) | ||||
| Age at AD diagnosis (years) | ≥65 * | 1 * | 0.1852 | - | |
| 46–65 | 1.30 (0.64–2.62) | ||||
| 19–45 | 1.31 (0.69–2.50) | ||||
| ≤18 | 2.44 (1.05–5.70) | ||||
| AD onset pattern | Late-onset * | 1 * | 0.6543 | - | |
| Early-onset relapsing | 1.27 (0.76–2.14) | ||||
| Early-onset persistent | 1.13 (0.72–1.78) | ||||
| AD phenotype | Nummular eczema * | 1 * | 0.0001 | 1 * | 0.0009 |
| Classic | 4.03 (1.56–10.41) | 6.92 (2.04–23.48) | |||
| Generalized lichenoid and inflammatory | 1.64 (0.62–4.32) | 4.22 (1.22–14.66) | |||
| Prurigo nodularis | 1.19 (0.40–3.56) | 2.28 (0.57–9.12) | |||
| Erythrodermic | 0.92 (0.23–3.63) | 1.97 (0.38–10.22) | |||
| Treatment at baseline | Cyclosporine + topical steroids * | 1 * | 0.0606 | - | |
| None | 1.58 (0.71–3.49) | ||||
| Cyclosporine only | 1.06 (0.43–2.62) | ||||
| Topical steroids only | 0.84 (0.40–1.79) | ||||
| Use of topical corticosteroids | Yes * | 1 * | 0.0140 | NS | |
| No | 1.64 (1.10–2.43) | ||||
| Baseline blood eosinophil count (cells/mm3) | ≥500 * | 1 * | 0.0102 | NS | |
| <500 | 1.68 (1.13–2.51) | ||||
| Baseline total serum IgE (kU/L) | ≥5001 * | 1 * | 0.5919 | - | |
| <100 | 1.27 (0.60–2.69) | ||||
| EASI at baseline | ≥29 * | 1 * | <0.0001 | 1 * | 0.0002 |
| ≤24 | 2.99 (1.90–4.70) | 3.13 (1.81–5.41) | |||
| 24–29 | 1.83 (1.15–2.91) | 1.79 (1.05–3.07) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrucci, S.; Casazza, G.; Zussino, M.; Tavecchio, S.; Marzano, A.V.; Tedeschi, M. Predictive Factors of Early Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis. J. Clin. Med. 2023, 12, 6575. https://doi.org/10.3390/jcm12206575
Ferrucci S, Casazza G, Zussino M, Tavecchio S, Marzano AV, Tedeschi M. Predictive Factors of Early Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis. Journal of Clinical Medicine. 2023; 12(20):6575. https://doi.org/10.3390/jcm12206575
Chicago/Turabian StyleFerrucci, Silvia, Giovanni Casazza, Martina Zussino, Simona Tavecchio, Angelo V. Marzano, and Micol Tedeschi. 2023. "Predictive Factors of Early Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis" Journal of Clinical Medicine 12, no. 20: 6575. https://doi.org/10.3390/jcm12206575
APA StyleFerrucci, S., Casazza, G., Zussino, M., Tavecchio, S., Marzano, A. V., & Tedeschi, M. (2023). Predictive Factors of Early Response to Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis. Journal of Clinical Medicine, 12(20), 6575. https://doi.org/10.3390/jcm12206575








