Iron Chelation in Patients with Myelodysplastic Syndromes and Myeloproliferative Neoplasms—Real-World Data from the German Noninterventional Study EXCALIBUR
Abstract
:1. Introduction
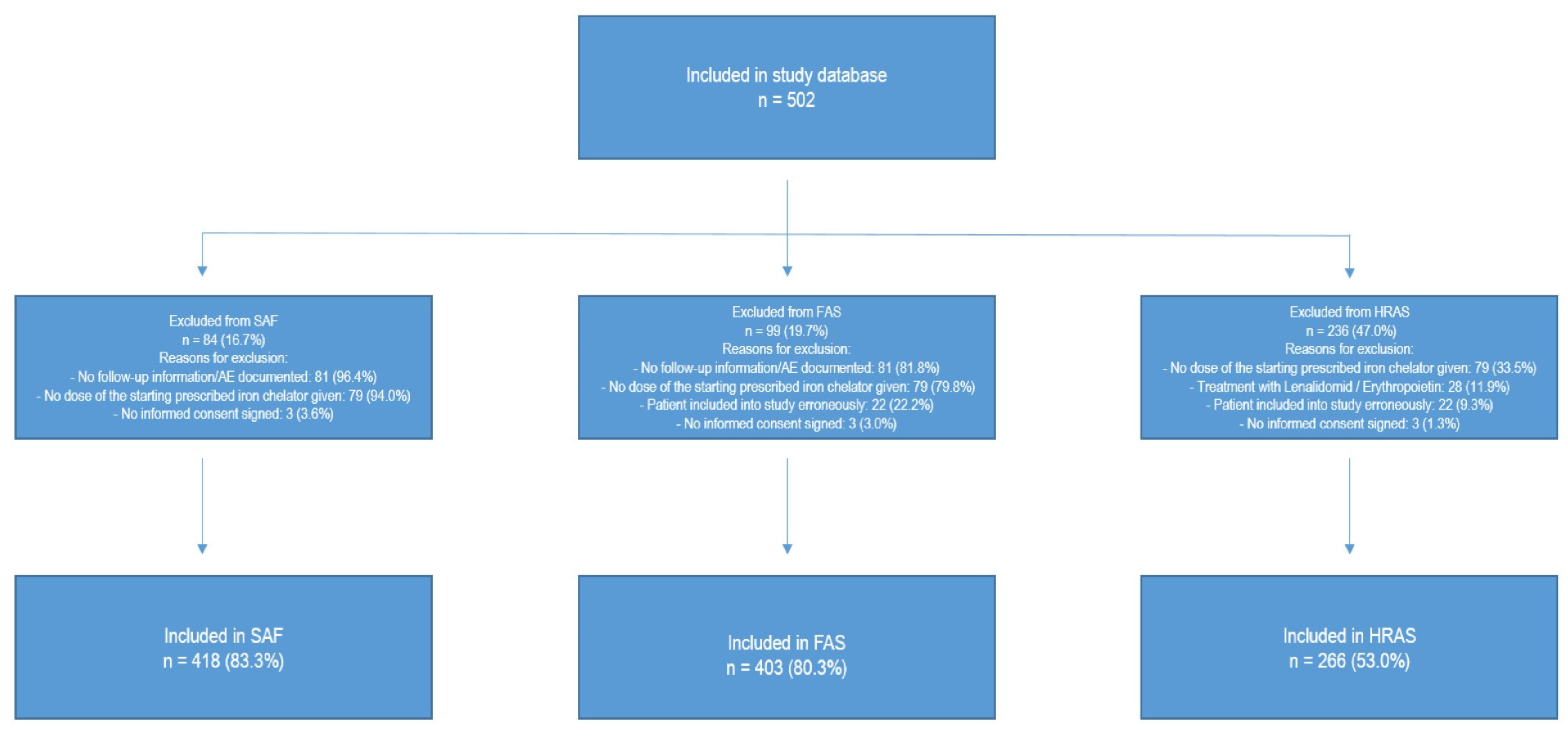
2. Materials and Methods
3. Results
3.1. Use and Switching of Iron Chelators
3.2. General Satisfaction with Iron Chelation Treatment
3.3. Safety
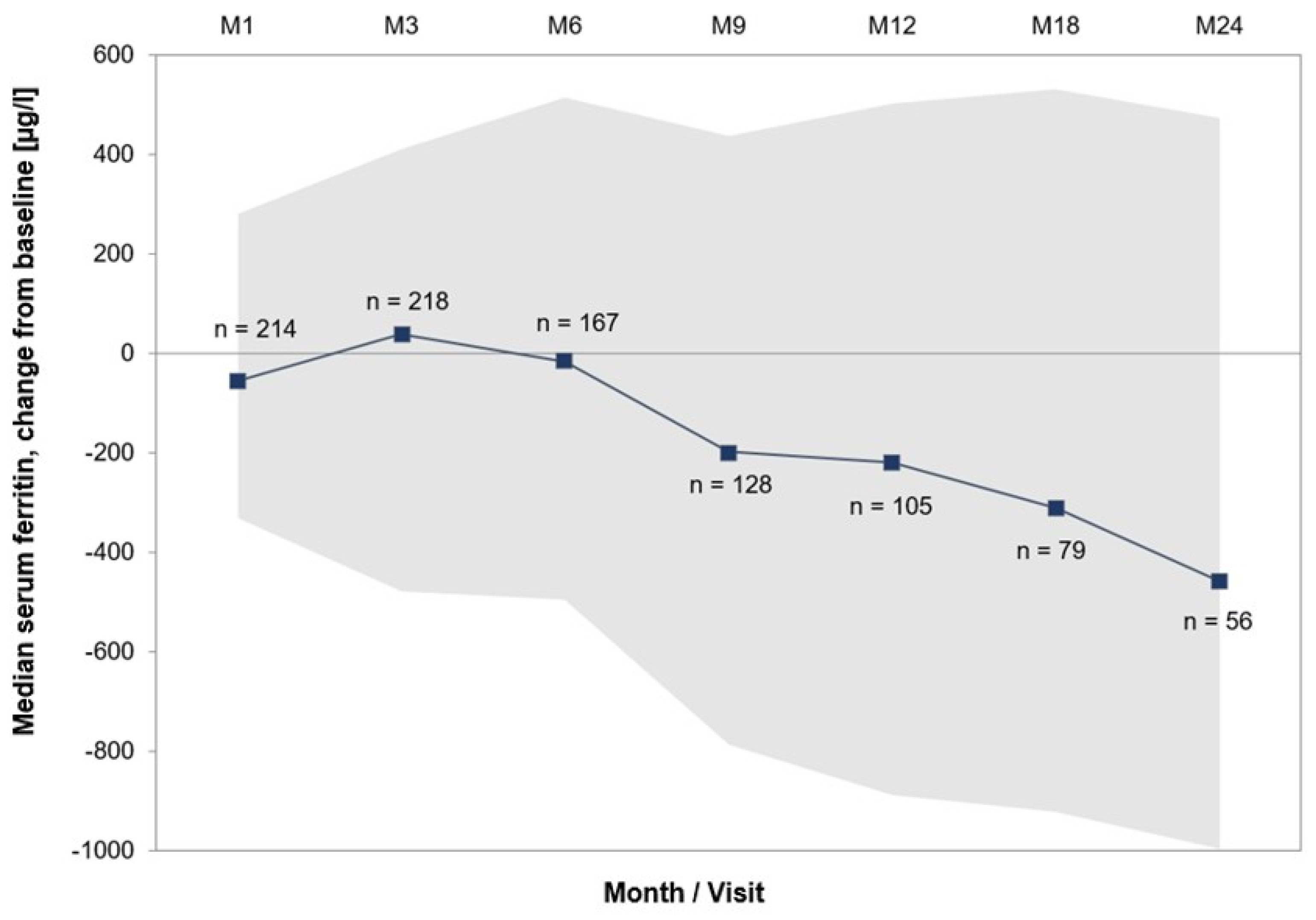
3.4. Effectiveness and Transfusion Dependence
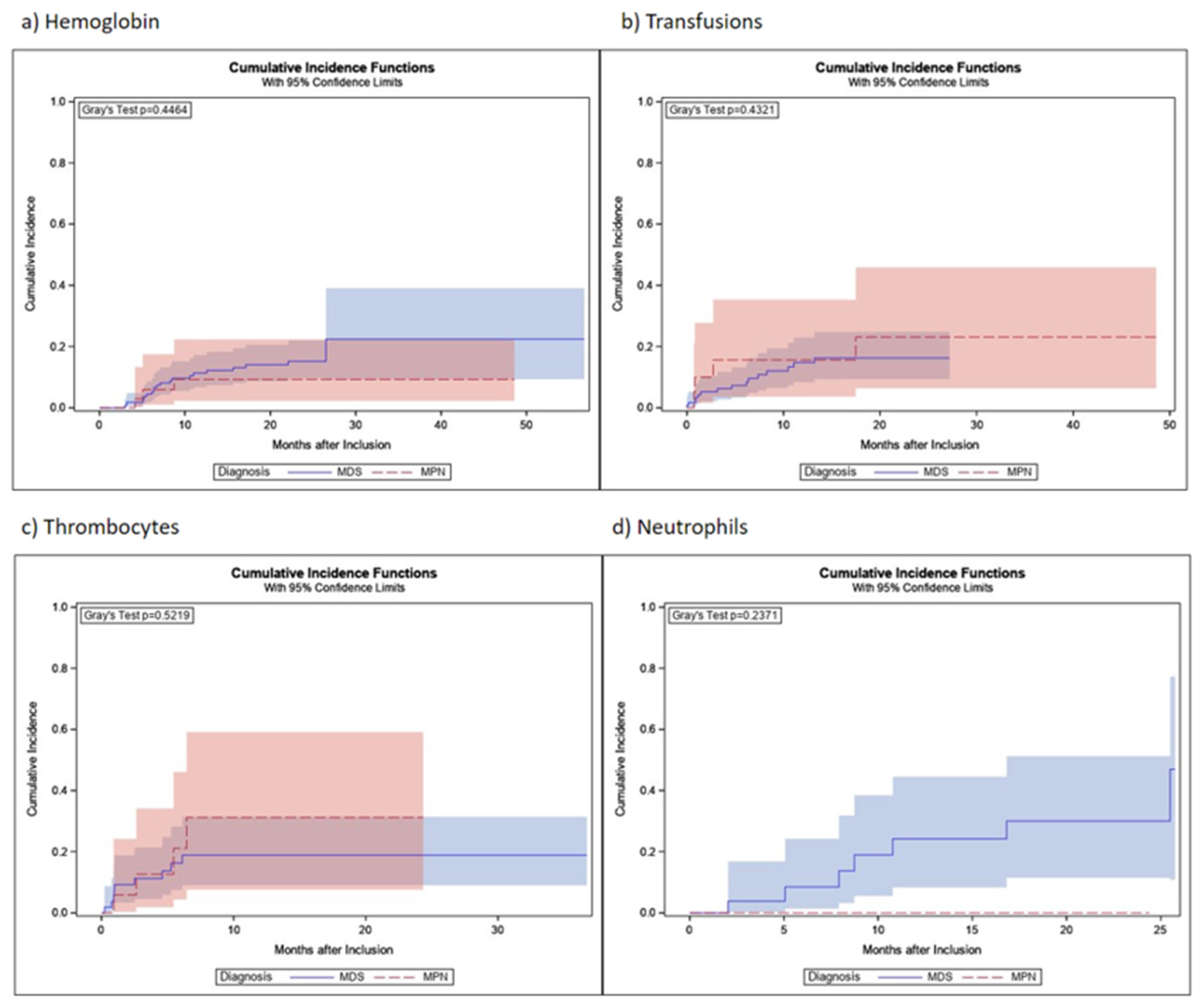
3.5. Hematological Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. The Evaluation of Iron Deficiency and Iron Overload. Dtsch. Ärzteblatt Int. 2021, 118, 847–856. [Google Scholar] [CrossRef]
- Steensma, D.P.; Gattermann, N. When is iron overload deleterious, and when and how should iron chelation therapy be administered in myelodysplastic syndromes? Best Pract. Res. Clin. Haematol. 2013, 26, 431–444, Erratum in: Best Pract. Res. Clin. Haematol. 2014, 27, e1. [Google Scholar] [CrossRef]
- Santini, V.; Girelli, D.; Sanna, A.; Martinelli, N.; Duca, L.; Campostrini, N.; Cortelezzi, A.; Corbella, M.; Bosi, A.; Reda, G.; et al. Hepcidin Levels and Their Determinants in Different Types of Myelodysplastic Syndromes. PLoS ONE 2011, 6, e23109. [Google Scholar] [CrossRef]
- Zipperer, E.; Post, J.G.; Herkert, M.; Kündgen, A.; Fox, F.; Haas, R.; Gattermann, N.; Germing, U. Serum hepcidin measured with an improved ELISA correlates with parameters of iron metabolism in patients with myelodysplastic syndrome. Ann. Hematol. 2013, 92, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Gattermann, N. Iron overload in myelodysplastic syndromes (MDS). Int. J. Hematol. 2017, 107, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Origa, R.; Perrotta, S.; Kourakli, A.; Ruffo, G.B.; Kattamis, A.; Goh, A.; Cortoos, A.; Huang, V.; Weill, M.; et al. New film-coated tablet formulation of deferasirox is well tolerated in patients with thalassemia or lower-risk MDS: Results of the randomized, phase II ECLIPSE study. Am. J. Hematol. 2017, 92, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Della Porta, M.G.; Strupp, C.; Ambaglio, I.; Kuendgen, A.; Nachtkamp, K.; Travaglino, E.; Invernizzi, R.; Pascutto, C.; Lazzarino, M.; et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica 2011, 96, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Leitch, H.A. Improving clinical outcome in patients with myelodysplastic syndrome and iron overload using iron chelation therapy. Leuk. Res. 2007, 31, S7–S9. [Google Scholar] [CrossRef]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef]
- Nolte, F.; Nückel, H.; Schmidt, B.; Geer, T.; Rubanov, O.; Hebart, H.; Jarisch, A.; Albrecht, S.; Johr, C.; Schumann, C.; et al. Tolerability and efficacy of deferasirox in patients with transfusional iron overload: Results from a German 2-year non-interventional study. J. Cancer Res. Clin. Oncol. 2018, 144, 1531–1538. [Google Scholar] [CrossRef]
- Gattermann, N.; Finelli, C.; Della Porta, M.; Fenaux, P.; Ganser, A.; Guerci-Bresler, A.; Schmid, M.; Taylor, K.; Vassilieff, D.; Habr, D.; et al. Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: Results from the large 1-year EPIC study. Leuk. Res. 2010, 34, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Nolte, F.; Angelucci, E.; Beris, P.; Macwhannell, A.; Selleslag, D.; Schumann, C.; Xicoy, B.; Almeida, A.; Guerci-Bresler, A.; Sliwa, T.; et al. Clinical management of gastrointestinal disturbances in patients with myelodysplastic syndromes receiving iron chelation treatment with deferasirox. Leuk. Res. 2011, 35, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/exjade (accessed on 15 September 2023).
- Available online: https://klinischeforschung.novartis.de/dokumente/fachinformationen-desferal/ (accessed on 15 September 2023).
- Angelucci, E.; Li, J.; Greenberg, P.; Wu, D.; Hou, M.; Figueroa, E.H.M.; Rodriguez, M.G.; Dong, X.; Ghosh, J.; Izquierdo, M.; et al. Iron Chelation in Transfusion-Dependent Patients With Low- to Intermediate-1–Risk Myelodysplastic Syndromes. Ann. Intern. Med. 2020, 172, 513–522. [Google Scholar] [CrossRef] [PubMed]
- List, A.F.; Baer, M.R.; Steensma, D.P.; Raza, A.; Esposito, J.; Martinez-Lopez, N.; Paley, C.; Feigert, J.; Besa, E. Deferasirox Reduces Serum Ferritin and Labile Plasma Iron in RBC Transfusion–Dependent Patients with Myelodysplastic Syndrome. J. Clin. Oncol. 2012, 30, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; MDS Foundation’s Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am. J. Hematol. 2008, 83, 858–861. [Google Scholar] [CrossRef]
- Angelucci, E.; Urru, S.A.M.; Pilo, F.; Piperno, A. Myelodysplastic syndromes and iron chelation therapy. Mediterr. J. Hematol. Infect. Dis. 2016, 9, e2017021. [Google Scholar] [CrossRef]
- Killick, S.B.; Ingram, W.; Culligan, D.; Enright, H.; Kell, J.; Payne, E.M.; Krishnamurthy, P.; Kulasekararaj, A.; Raghavan, M.; Stanworth, S.J.; et al. British Society for Haematology guidelines for the management of adult myelodysplastic syndromes. Br. J. Haematol. 2021, 194, 267–281. [Google Scholar] [CrossRef]
- Leitch, H.A.; Gattermann, N. Hematologic improvement with iron chelation therapy in myelodysplastic syndromes: Clinical data, potential mechanisms, and outstanding questions. Crit. Rev. Oncol. 2019, 141, 54–72. [Google Scholar] [CrossRef]
- Gattermann, N.; Finelli, C.; Della Porta, M.; Fenaux, P.; Stadler, M.; Guerci-Bresler, A.; Schmid, M.; Taylor, K.; Vassilieff, D.; Habr, D.; et al. Hematologic responses to deferasirox therapy in transfusion-dependent patients with myelodysplastic syndromes. Haematologica 2012, 97, 1364–1371. [Google Scholar] [CrossRef]
- Angelucci, E.; Santini, V.; Di Tucci, A.A.; Quaresmini, G.; Finelli, C.; Volpe, A.; Quarta, G.; Rivellini, F.; Sanpaolo, G.; Cilloni, D.; et al. Deferasirox for transfusion-dependent patients with myelodysplastic syndromes: Safety, efficacy, and beyond (GIMEMA MDS0306 Trial). Eur. J. Haematol. 2014, 92, 527–536. [Google Scholar] [CrossRef]
- Maurillo, L.; Breccia, M.; Buccisano, F.; Voso, M.T.; Niscola, P.; Trapè, G.; Tatarelli, C.; D’Addosio, A.; Latagliata, R.; Fenu, S.; et al. Deferasirox chelation therapy in patients with transfusion-dependent MDS: A ‘real-world’ report from two regional Italian registries: Gruppo Romano Mielodisplasie and Registro Basilicata. Eur. J. Haematol. 2015, 95, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Neukirchen, J.; Fox, F.; Kündgen, A.; Nachtkamp, K.; Strupp, C.; Haas, R.; Germing, U.; Gattermann, N. Improved survival in MDS patients receiving iron chelation therapy—A matched pair analysis of 188 patients from the Düsseldorf MDS registry. Leuk. Res. 2012, 36, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
| n = 403 | |
|---|---|
| Sex | |
| Male | 240 (59.6%) |
| Female | 163 (40.4%) |
| Median age at baseline in years (range) | 75 (24–92) |
| Primary diagnosis | |
| MDS | 246 (61.0%) |
| MPN | 65 (16.1%) |
| Acute leukemia | 20 (5.0%) |
| Acute myeloid leukemia (AML) | 19 (95.0%) |
| Acute lymphatic leukemia (ALL) | 1 (5.0%) |
| Lymphoma | 24 (6.0%) |
| Multiple myeloma | 10 (41.7%) |
| Non-Hodgkin lymphoma | 9 (37.5%) |
| Chronic lymphatic leukemia (CLL) | 5 (20.8%) |
| Hemoglobinopathies | 6 (1.5%) |
| Thalassemia | 5 (83.3%) |
| Sickle-cell anemia | 1 (16.7%) |
| Anemia, NOS (not MDS-related) | 16 (4.0%) |
| Solid tumor | 14 (3.5%) |
| Condition after stem cell transplantation/(radio-)chemotherapy | 6 (1.5%) |
| Other | 6 (1.5%) |
| Median time from first diagnosis to current iron chelation therapy in months (IQR) | 21.6 (8.6–50.0) |
| Patients with prior iron chelation therapy | 21 (5.2%) |
| Patients with concomitant diseases | 364 (90.3%) |
| Patients with concomitant medication | 379 (94.0%) |
| Transfusions | |
| Receipt of any transfusions before NIS entry | 400 (99.3%) |
| Median time from primary diagnosis to transfusions in months (IQR) | 2.8 (0.2–22.3) |
| Median time from start of transfusions to start of current iron chelation therapy in months (IQR) | 12.1 (5.7–24.3) |
| Number of erythrocyte concentrates since primary diagnosis until study entry | |
| 0 | 2 (0.5%) |
| <20 | 189 (46.9%) |
| 20–39 | 109 (27.0%) |
| 40–59 | 49 (12.2%) |
| 60–79 | 15 (3.7%) |
| ≥80 | 22 (5.5%) |
| Unknown | 17 (4.2%) |
| Specification of MDS | n = 246 |
| MDS-SLD | 17 (6.9%) |
| MDS-SLD-RS | 30 (12.2%) |
| MDS-MLD | 48 (19.5%) |
| MDS-MLD-RS | 20 (8.1%) |
| MDS del(5q) | 10 (4.1%) |
| MDS-EB I | 32 (13.0%) |
| MDS-EB II | 24 (9.8%) |
| MDS-U | 30 (12.2%) |
| CMML | 9 (3.7%) |
| MDS/MPN | 19 (7.7%) |
| MDS/MPN-RS | 7 (2.8%) |
| Specification of MPN | n = 65 |
| PMF | 41 (63.1%) |
| MPN-U | 14 (21.5%) |
| ET | 10 (15.4%) |
| n = 403 | |
|---|---|
| Number of patients with one treatment change | 48 (11.9%) |
| Deferasirox DT to deferasirox FCT | 40 (83.3%) |
| Deferasirox FCT to deferasirox DT | 3 (6.3%) |
| Deferasirox FCT to deferoxamine | 3 (6.3%) |
| Deferoxamine to deferasirox DT | 1 (2.1%) |
| Doxamine to deferasirox FCT | 1 (2.1%) |
| Number of patients with two treatment changes | 4 (1.0%) |
| n = 418 | |
|---|---|
| Patients with AEs | 387 (92.6%) |
| Patients with non-serious AEs | 256 (61.2%) |
| Patients without AEs | 31 (7.4%) |
| Patients with SAEs | 270 (64.6%) |
| Patients with non-serious ADRs | 220 (52.6%) |
| Patients with SADRs | 86 (20.6%) |
| Total | nsAE | SAE | nsADR | SADR | |
|---|---|---|---|---|---|
| n = 418 | n = 256 | n = 270 | n = 220 | n = 86 | |
| General disorders and administration site conditions | 202 (48.3%) | 109 (42.6%) | 82 (30.4%) | 53 (24.1%) | 11 (12.8%) |
| 51 (12.2%) | 17 (6.6%) | 25 (9.3%) | 7 (3.2%) | 3 (3.5%) |
| 41 (9.8%) | 31 (12.1%) | 2 (0.7%) | 8 (3.6%) | 1 (1.2%) |
| 36 (8.6%) | 16 (6.3%) | 18 (6.7%) | 4 (1.8%) | 0 (0.0%) |
| 23 (5.5%) | 17 (6.6%) | 2 (0.7%) | 4 (1.8%) | 0 (0.0%) |
| 19 (4.5%) | 0 (0.0%) | 16 (5.9%) | 0 (0.0%) | 3 (3.5%) |
| Gastrointestinal disorders | 187 (44.7%) | 80 (31.3%) | 44 (16.3%) | 113 (51.4%) | 16 (18.6%) |
| 82 (19.6%) | 22 (8.6%) | 4 (1.5%) | 57 (25.9%) | 6 (7.0%) |
| 50 (12.0%) | 26 (10.2%) | 3 (1.1%) | 22 (10.0%) | 3 (3.5%) |
| 28 (6.7%) | 12 (4.7%) | 1 (0.4%) | 14 (6.4%) | 1 (1.2%) |
| Infections and infestations | 151 (36.1%) | 71 (27.7%) | 105 (38.9%) | 5 (2.3%) | 7 (8.1%) |
| 34 (8.1%) | 0 (0.0%) | 34 (12.6%) | 0 (0.0%) | 0 (0.0%) |
| 26 (6.2%) | 12 (4.7%) | 17 (6.3%) | 0 (0.0%) | 0 (0.0%) |
| 23 (5.5%) | 21 (8.2%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Investigations | 141 (33.7%) | 43 (16.8%) | 44 (16.3%) | 72 (32.7%) | 25 (29.1%) |
| 41 (9.8%) | 7 (2.7%) | 0 (0.0%) | 29 (13.2%) | 7 (8.1%) |
| 36 (8.6%) | 7 (2.7%) | 21 (7.8%) | 7 (3.2%) | 6 (7.0%) |
| 17 (4.1%) | 2 (0.8%) | 0 (0.0%) | 14 (6.4%) | 1 (1.2%) |
| Respiratory, thoracic and mediastinal disorders | 93 (22.2%) | 67 (26.2%) | 31 (11.5%) | 6 (2.7%) | 3 (3.5%) |
| 32 (7.7%) | 25 (9.8%) | 7 (2.6%) | 1 (0.5%) | 0 (0.0%) |
| 24 (5.7%) | 18 (7.0%) | 5 (1.9%) | 1 (0.5%) | 0 (0.0%) |
| Musculoskeletal and connective tissue disorders | 74 (17.7%) | 52 (20.3%) | 15 (5.6%) | 11 (5.0%) | 0 (0.0%) |
| 15 (3.6%) | 13 (5.1%) | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Neoplasms benign, malignant and unspecified | 73 (17.5%) | 1 (0.4%) | 68 (25.2%) | 0 (0.0%) | 6 (7.0%) |
| 24 (5.7%) | 0 (0.0%) | 23 (8.5%) | 0 (0.0%) | 1 (1.2%) |
| 24 (5.7%) | 0 (0.0%) | 20 (7.4%) | 0 (0.0%) | 4 (4.7%) |
| 19 (4.5%) | 0 (0.0%) | 17 (6.3%) | 0 (0.0%) | 2 (2.3%) |
| Nervous system disorders | 70 (16.7%) | 39 (15.2%) | 24 (8.9%) | 16 (7.3%) | 3 (3.5%) |
| 39 (9.3%) | 26 (10.2%) | 4 (1.5%) | 10 (4.5%) | 2 (2.3%) |
| Blood and lymphatic system disorders | 66 (15.8%) | 15 (5.9%) | 43 (15.9%) | 6 (2.7%) | 12 (14.0%) |
| 25 (6.0%) | 0 (0.0%) | 19 (7.0%) | 0 (0.0%) | 6 (7.0%) |
| Skin and subcutaneous tissue disorders | 60 (14.4%) | 30 (11.7%) | 6 (2.2%) | 22 (10.0%) | 7 (8.1%) |
| Injury, poisoning, and procedural complications | 50 (12.0%) | 21 (8.2%) | 22 (8.1%) | 10 (4.5%) | 2 (2.3%) |
| Renal and urinary disorders | 49 (11.7%) | 9 (3.5%) | 20 (7.4%) | 8 (3.6%) | 15 (17.4%) |
| 14 (3.3%) | 0 (0.0%) | 4 (1.5%) | 0 (0.0%) | 10 (11.6%) |
| Cardiac disorders | 45 (10.8%) | 3 (1.2%) | 41 (15.2%) | 3 (1.4%) | 3 (3.5%) |
| Metabolism and nutrition disorders | 43 (10.3%) | 26 (10.2%) | 12 (4.4%) | 8 (3.6%) | 2 (2.3%) |
| Vascular disorders | 36 (8.6%) | 20 (7.8%) | 17 (6.3%) | 2 (0.9%) | 0 (0.0%) |
| Psychiatric disorders | 23 (5.5%) | 19 (7.4%) | 4 (1.5%) | 1 (0.5%) | 0 (0.0%) |
| Hepatobiliary disorders | 17 (4.1%) | 3 (1.2%) | 9 (3.3%) | 0 (0.0%) | 6 (7.0%) |
| MDS | MPN | |
|---|---|---|
| Hemoglobin | ||
| Patients analyzed | 213 | 45 |
| Response | 21 | 3 |
| 6 | 0 |
| Death without response | 46 | 11 |
| Transfusions | ||
| Patients analyzed | 124 | 21 |
| Response | 15 | 4 |
| 7 | 1 |
| Death without response | 30 | 5 |
| Platelets | ||
| Patients analyzed | 58 | 18 |
| Response | 9 | 4 |
| 3 | 0 |
| Death without response | 18 | 5 |
| Neutrophils | ||
| Patients analyzed | 30 | 4 |
| Response | 7 | 0 |
| 0 | 0 |
| Death without response | 8 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, F.; Hauch, U.; Ketzler-Henkel, S.; von der Heyde, E.; Koenigsmann, M.; Lauseker, M.; Schulte, N.; Germing, U. Iron Chelation in Patients with Myelodysplastic Syndromes and Myeloproliferative Neoplasms—Real-World Data from the German Noninterventional Study EXCALIBUR. J. Clin. Med. 2023, 12, 6569. https://doi.org/10.3390/jcm12206569
Schulz F, Hauch U, Ketzler-Henkel S, von der Heyde E, Koenigsmann M, Lauseker M, Schulte N, Germing U. Iron Chelation in Patients with Myelodysplastic Syndromes and Myeloproliferative Neoplasms—Real-World Data from the German Noninterventional Study EXCALIBUR. Journal of Clinical Medicine. 2023; 12(20):6569. https://doi.org/10.3390/jcm12206569
Chicago/Turabian StyleSchulz, Felicitas, Ulrich Hauch, Sandra Ketzler-Henkel, Eyck von der Heyde, Michael Koenigsmann, Michael Lauseker, Nora Schulte, and Ulrich Germing. 2023. "Iron Chelation in Patients with Myelodysplastic Syndromes and Myeloproliferative Neoplasms—Real-World Data from the German Noninterventional Study EXCALIBUR" Journal of Clinical Medicine 12, no. 20: 6569. https://doi.org/10.3390/jcm12206569
APA StyleSchulz, F., Hauch, U., Ketzler-Henkel, S., von der Heyde, E., Koenigsmann, M., Lauseker, M., Schulte, N., & Germing, U. (2023). Iron Chelation in Patients with Myelodysplastic Syndromes and Myeloproliferative Neoplasms—Real-World Data from the German Noninterventional Study EXCALIBUR. Journal of Clinical Medicine, 12(20), 6569. https://doi.org/10.3390/jcm12206569





