The FCGR2A Is Associated with the Presence of Atherosclerotic Plaques in the Carotid Arteries—A Case-Control Study
Abstract
1. Introduction
2. Material and Methods
3. Ethical Issues
Statistical Analysis
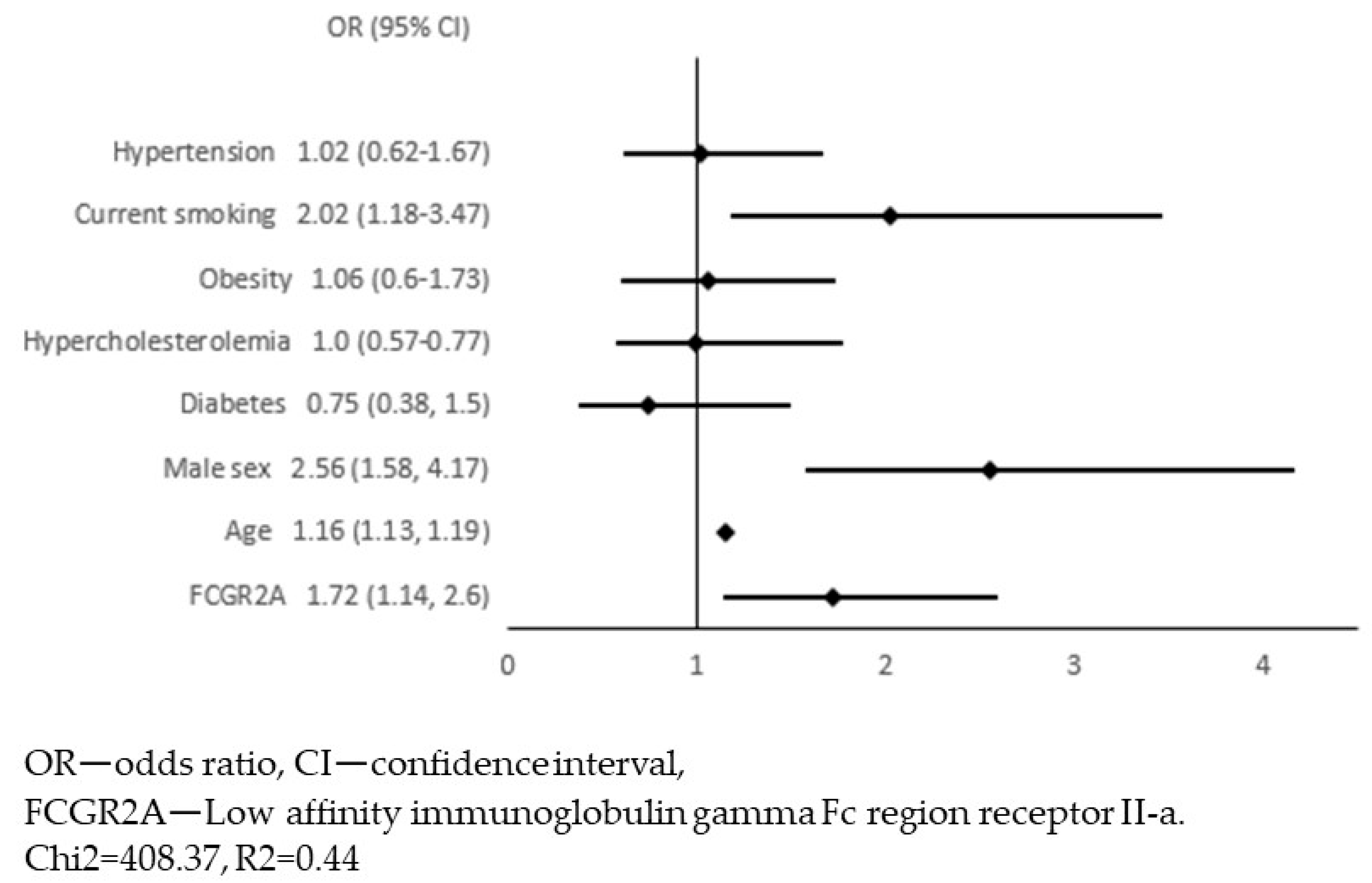
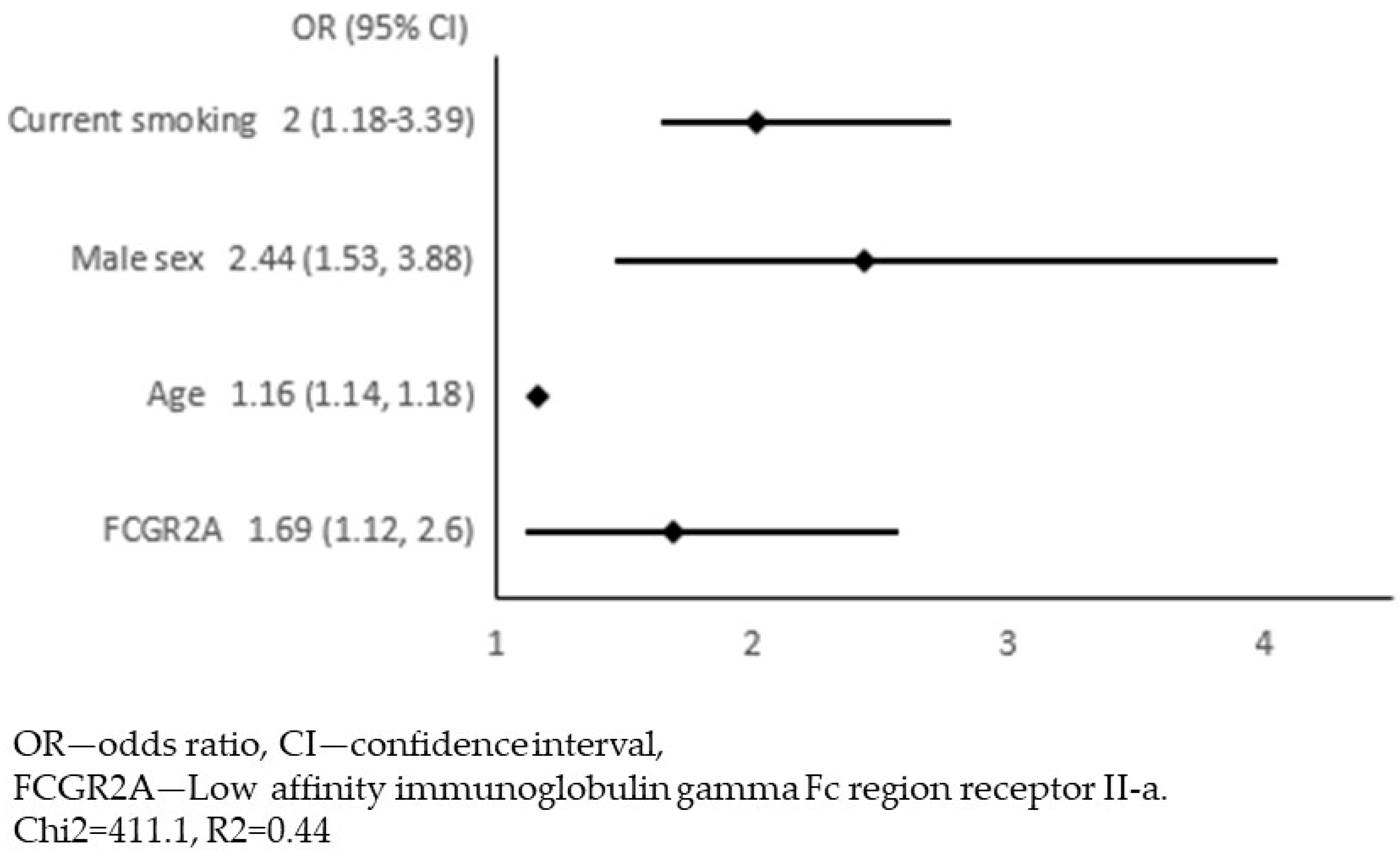
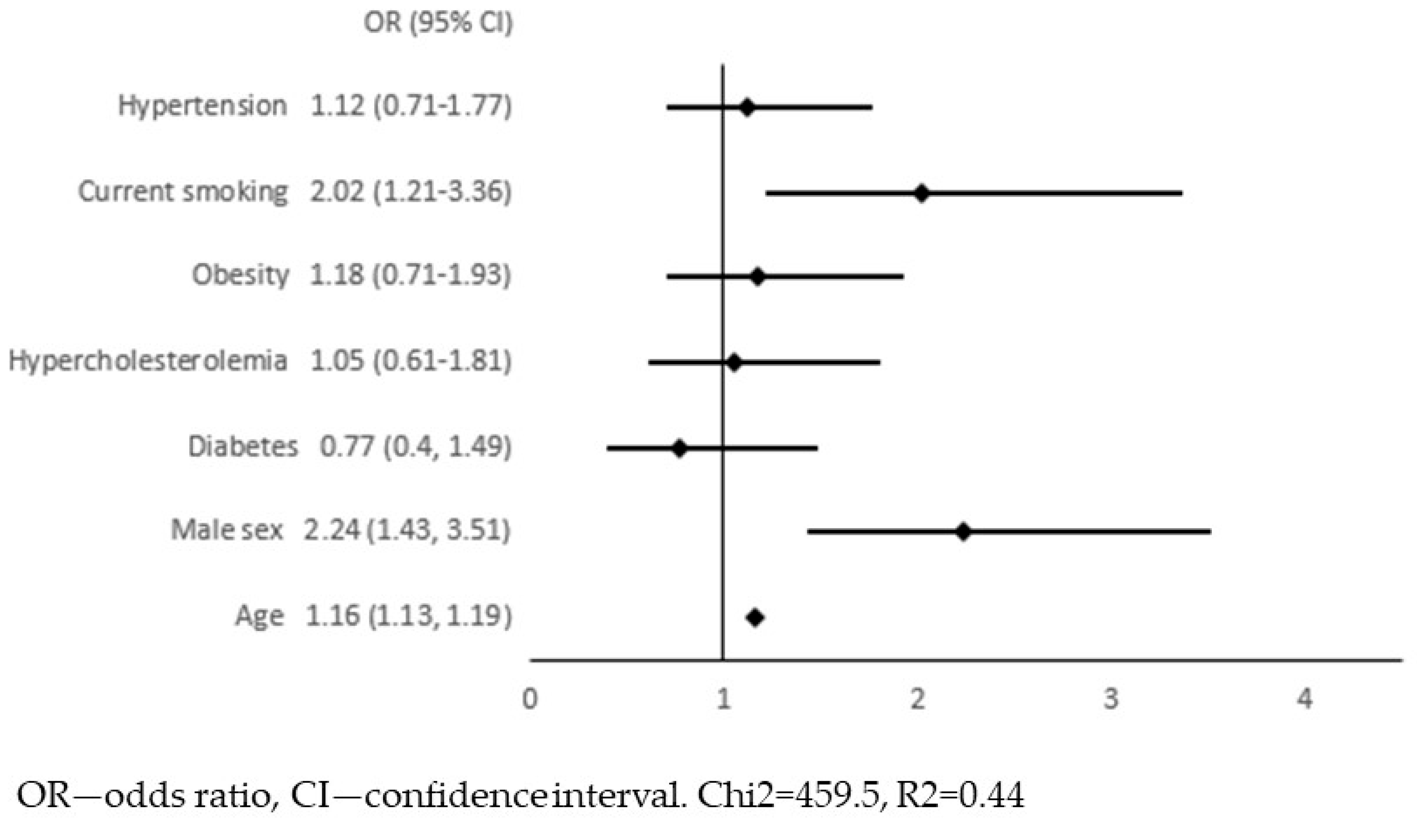
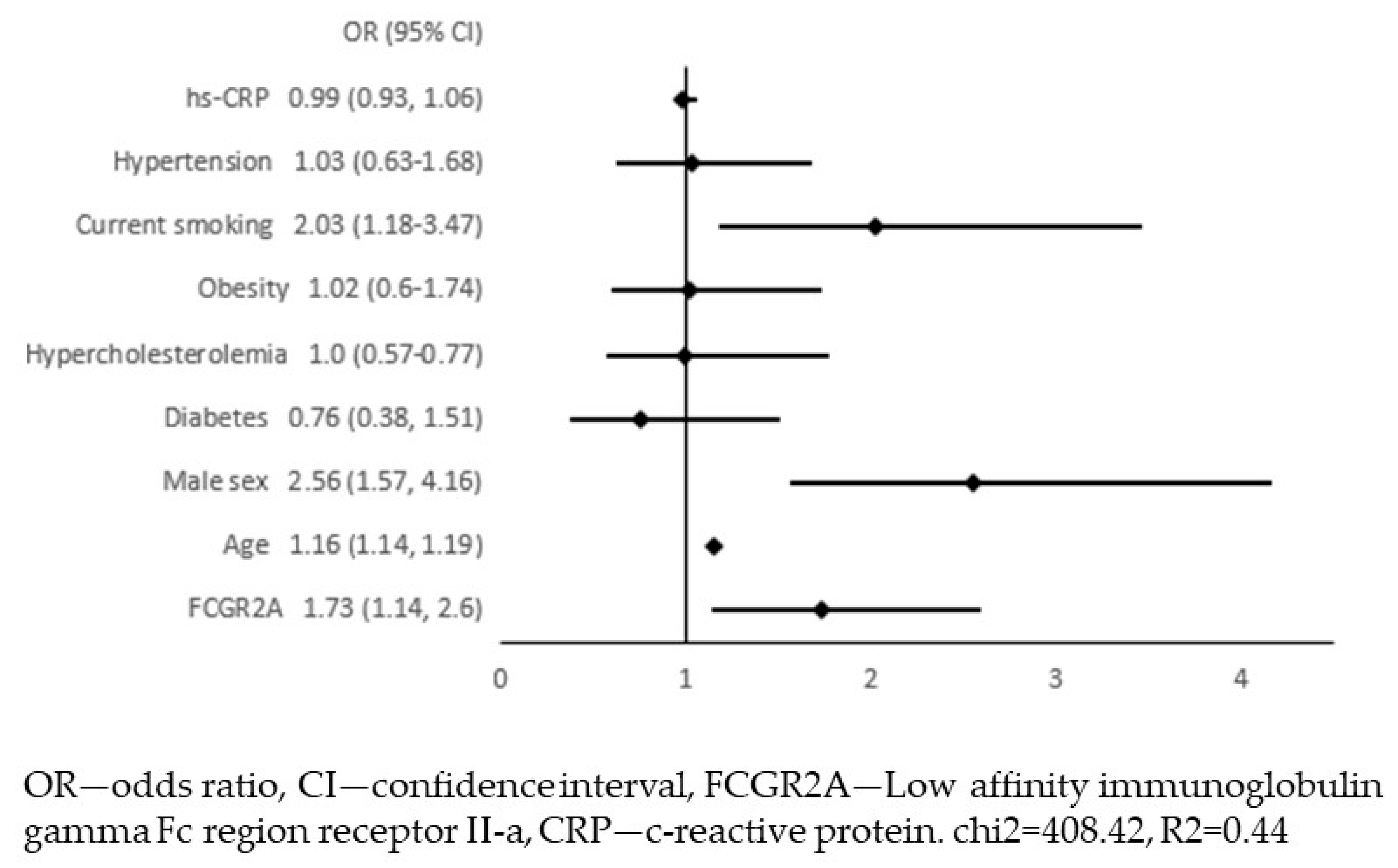
4. Results
5. Discussion
Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chimowitz, M.I.; Weiss, D.G.; Cohen, S.L.; Starling, M.R.; Hobson, R.W. Cardiac prognosis of patients with carotid stenosis and no history of coronary artery disease. Veterans Affairs Cooperative Study Group 167. Stroke 1994, 25, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.L. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: Results of a systematic review and analysis. Stroke 2009, 40, e573–e583. [Google Scholar] [CrossRef]
- Marquardt, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke 2010, 41, e11–e17. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.W.; Tucker, L.Y.; Rothenberg, K.A.; Lancaster, E.; Faruqi, R.M.; Kuang, H.C.; Flint, A.C.; Avins, A.L.; Nguyen-Huynh, M.N. Incidence of Ischemic Stroke in Patients With Asymptomatic Severe Carotid Stenosis Without Surgical Intervention. JAMA 2022, 327, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Goessens, B.M.; Visseren, F.L.; Kappelle, L.J.; Algra, A.; van der Graaf, Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: The SMART study. Stroke 2007, 38, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- De Weerd, M.; Greving, J.P.; Hedblad, B.; Lorenz, M.W.; Mathiesen, E.B.; O’Leary, D.H.; Rosvall, M.; Sitzer, M.; Buskens, E.; Bots, M.L. Prevalence of asymptomatic carotid artery stenosis in the general population: An individual participant data meta-analysis. Stroke 2010, 41, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, A.; Dubatówka, M.; Chlabicz, M.; Jamiołkowski, J.; Kondraciuk, M.; Szyszkowska, A.; Knapp, M.; Szpakowicz, A.; Łukasiewicz, A.; Kamiński, K. Disparities in the Prevalence and Risk Factors for Carotid and Lower Extremities Atherosclerosis in a General Population-Bialystok PLUS Study. J. Clin. Med. 2023, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef] [PubMed]
- Szwed, P.; Gąsecka, A.; Zawadka, M.; Eyileten, C.; Postuła, M.; Mazurek, T.; Szarpak, Ł.; Filipiak, K.J. Infections as Novel Risk Factors of Atherosclerotic Cardiovascular Diseases: Pathophysiological Links and Therapeutic Implications. J. Clin. Med. 2021, 10, 2539. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Song, F.; Wang, L.; Fu, Q.; Cao, S.; Gan, Y.; Zhang, W.; Yue, W.; Yan, F.; et al. Carotid Atherosclerosis Detected by Ultrasonography: A National Cross-Sectional Study. J. Am. Heart Assoc. 2018, 7, e008701. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://olink.com/products-services/explore/protein/?proteinID=OID20391 (accessed on 31 August 2023).
- Reska, D.; Czajkowski, M.; Jurczuk, K.; Boldak, C.; Kwedlo, W.; Bauer, W.; Koszelew, J.; Kretowski, M. Integration of solutions and servicesfor multi-omics data analysis towards personalized medicine. Biocybern. Biomed. Eng. 2021, 41, 1646–1663. [Google Scholar] [CrossRef]
- Newling, M.; Sritharan, L.; van der Ham, A.J.; Hoepel, W.; Fiechter, R.H.; de Boer, L.; Zaat, S.A.J.; Bisoendial, R.J.; Baeten, D.L.P.; Everts, B.; et al. C-Reactive Protein Promotes Inflammation through FcγR-Induced Glycolytic Reprogramming of Human Macrophages. J. Immunol. 2019, 203, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cao, S.; Chen, M. Integrated analysis and exploration of potential shared gene signatures between carotid atherosclerosis and periodontitis. BMC Med. Genom. 2022, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Peczyńska, J.; Klonowska, B.; Żelazowska-Rutkowska, B.; Polkowska, A.; Noiszewska, K.; Bossowski, A.; Głowińska-Olszewska, B. The Relationship between Selected Inflammation and Oxidative Stress Biomarkers and Carotid Intima-Media Thickness (IMT) Value in Youth with Type 1 Diabetes Co-Existing with Early Microvascular Complications. J. Clin. Med. 2022, 11, 4732. [Google Scholar] [CrossRef] [PubMed]
- Kabłak-Ziembicka, A.; Przewłocki, T. Clinical Significance of Carotid Intima-Media Complex and Carotid Plaque Assessment by Ultrasound for the Prediction of Adverse Cardiovascular Events in Primary and Secondary Care Patients. J. Clin. Med. 2021, 10, 4628. [Google Scholar] [CrossRef]
- Available online: https://olink.com/resources-support/document-download-center/#whitepapers (accessed on 31 August 2023).
- Dai, Y.; Chen, W.; Huang, J.; Cui, T. Could Function as a Prognostic Marker and Correlate with Immune Infiltration in Head and Neck Squamous Cell Carcinoma. Biomed. Res. Int. 2021, 2021, 8874578. [Google Scholar] [CrossRef]
- Ning, Z.; Huang, Y.; Lu, H.; Zhou, Y.; Tu, T.; Ouyang, F.; Liu, Y.; Liu, Q. Novel Drug Targets for Atrial Fibrillation Identified Through Mendelian Randomization Analysis of the Blood Proteome. Cardiovasc. Drugs Ther. 2023. [CrossRef]
- Stein, M.P.; Edberg, J.C.; Kimberly, R.P.; Mangan, E.K.; Bharadwaj, D.; Mold, C.; Du Clos, T.W. C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J. Clin. Investig. 2000, 105, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Kroupis, C.; Theodorou, M.; Chaidaroglou, A.; Dalamaga, M.; Oliveira, S.C.; Cokkinos, D.V.; Degiannis, D.; Manginas, A. The association between a common FCGR2A polymorphism and C-reactive protein and coronary artery disease revisited. Genet. Test. Mol. Biomark. 2010, 14, 839–846. [Google Scholar] [CrossRef]
- Raaz, D.; Herrmann, M.; Ekici, A.B.; Klinghammer, L.; Lausen, B.; Voll, R.E.; Leusen, J.H.W.; van de Winkel, J.G.J.; Daniel, W.G.; Reis, A.; et al. FcgammaRIIa genotype is associated with acute coronary syndromes as first manifestation of coronary artery disease. Atherosclerosis 2009, 205, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, I.M.; Witteman, J.C.; Hofman, A.; Kluft, C.; de Maat, M.P. Genetic variation in Fcgamma receptor IIa protects against advanced peripheral atherosclerosis. The Rotterdam Study. Thromb. Haemost. 2004, 92, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Pessi, T.; Eklund, C.; Huhtala, H.; Raitakari, O.T.; Juonala, M.; Kähönen, M.; Viikari, J.S.A.; Lehtimäki, T.; Hurme, M. CRP and FCGR2A genes have an epistatic effect on carotid artery intima-media thickness: The Cardiovascular Risk in Young Finns Study. Int. J. Immunogenet. 2009, 36, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Clancy, R.; El Bannoudi, H.; Rasmussen, S.E.; Bornkamp, N.; Allen, N.; Dann, R.; Reynolds, H.; Buyon, J.P.; Berger, J.S. Human low-affinity IgG receptor FcγRIIA polymorphism H131R associates with subclinical atherosclerosis and increased platelet activity in systemic lupus erythematosus. J. Thromb. Haemost. 2019, 17, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; He, C.; Yang, S.; Zhou, M.; Zeng, C.; Luo, M.; Yu, J.; Hu, S.; Duan, Y. Bioinformatics and experimental analyses of glutamate receptor and its targets genes in myocardial and cerebral ischemia. BMC Genom. 2023, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, A.; Max, A.; Mongardon, N.; Grimaldi, D.; Pène, F.; Rousseau, C.; Chiche, J.D.; Bedos, J.P.; Vicaut, E. Protective effects of FCGR2A polymorphism in invasive pneumococcal diseases. Chest 2012, 142, 1474–1481. [Google Scholar] [CrossRef][Green Version]
- Beppler, J.; Koehler-Santos, P.; Pasqualim, G.; Matte, U.; Alho, C.S.; Dias, F.S.; Kowalski, T.W.; Velasco, I.T.; da Silva, F.P. Fc Gamma Receptor IIA (CD32A) R131 Polymorphism as a Marker of Genetic Susceptibility to Sepsis. Inflammation 2016, 39, 518–525. [Google Scholar] [CrossRef]
- Sinha, S.; Mishra, S.K.; Sharma, S.; Patibandla, P.K.; Mallick, P.K.; Sharma, S.K.; Mohanty, S.; Pati, S.S.; Mishra, S.K.; Ramteke, B.K.; et al. Polymorphisms of TNF-enhancer and gene for FcgammaRIIa correlate with the severity of falciparum malaria in the ethnically diverse Indian population. Malar. J. 2008, 7, 13. [Google Scholar] [CrossRef]
- Li, R.; Peng, H.; Chen, G.M.; Feng, C.C.; Zhang, Y.J.; Wen, P.F.; Qiu, L.J.; Leng, R.X.; Pan, H.F.; Ye, D.Q. Association of FCGR2A-R/H131 polymorphism with susceptibility to systemic lupus erythematosus among Asian population: A meta-analysis of 20 studies. Arch. Dermatol. Res. 2014, 306, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C.; Song, G.G. FCGR2A, FCGR3A, FCGR3B polymorphisms and susceptibility to rheumatoid arthritis: A meta-analysis. Clin. Exp. Rheumatol. 2015, 33, 647–654. [Google Scholar] [PubMed]
- McGovern, D.P.B.; The NIDDK IBD Genetics Consortium; Gardet, A.; Törkvist, L.; Goyette, P.; Essers, J.; Taylor, K.D.; Neale, B.M.; Ong, R.T.H.; Lagacé, C.; et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat. Genet. 2010, 42, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; I Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: A systematic review, meta-analysis, and modelling study. Lancet Glob. Health 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, M.; Jamiołkowski, J.; Chlabicz, M.; Łapińska, M.; Dubatówka, M.; Kondraciuk, M.; Hermanowicz, A.; Kamiński, K.A. How Unawareness of Weight Excess Can Increase Cardiovascular Risk? J. Clin. Med. 2022, 11, 4944. [Google Scholar] [CrossRef] [PubMed]
- Chlabicz, M.; Jamiołkowski, J.; Łaguna, W.; Sowa, P.; Paniczko, M.; Łapińska, M.; Szpakowicz, M.; Drobek, N.; Raczkowski, A.; Kamiński, K.A. A Similar Lifetime CV Risk and a Similar Cardiometabolic Profile in the Moderate and High Cardiovascular Risk Populations: A Population-Based Study. J. Clin. Med. 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Zieleniewska, N.A.; Szum-Jakubowska, A.; Chlabicz, M.; Jamiołkowski, J.; Kowalska, I.; Kamiński, K.A. The prevalence of diabetes and prediabetes: A population-based study. Pol. Arch. Intern. Med. 2023, 133, 16407. [Google Scholar] [CrossRef]
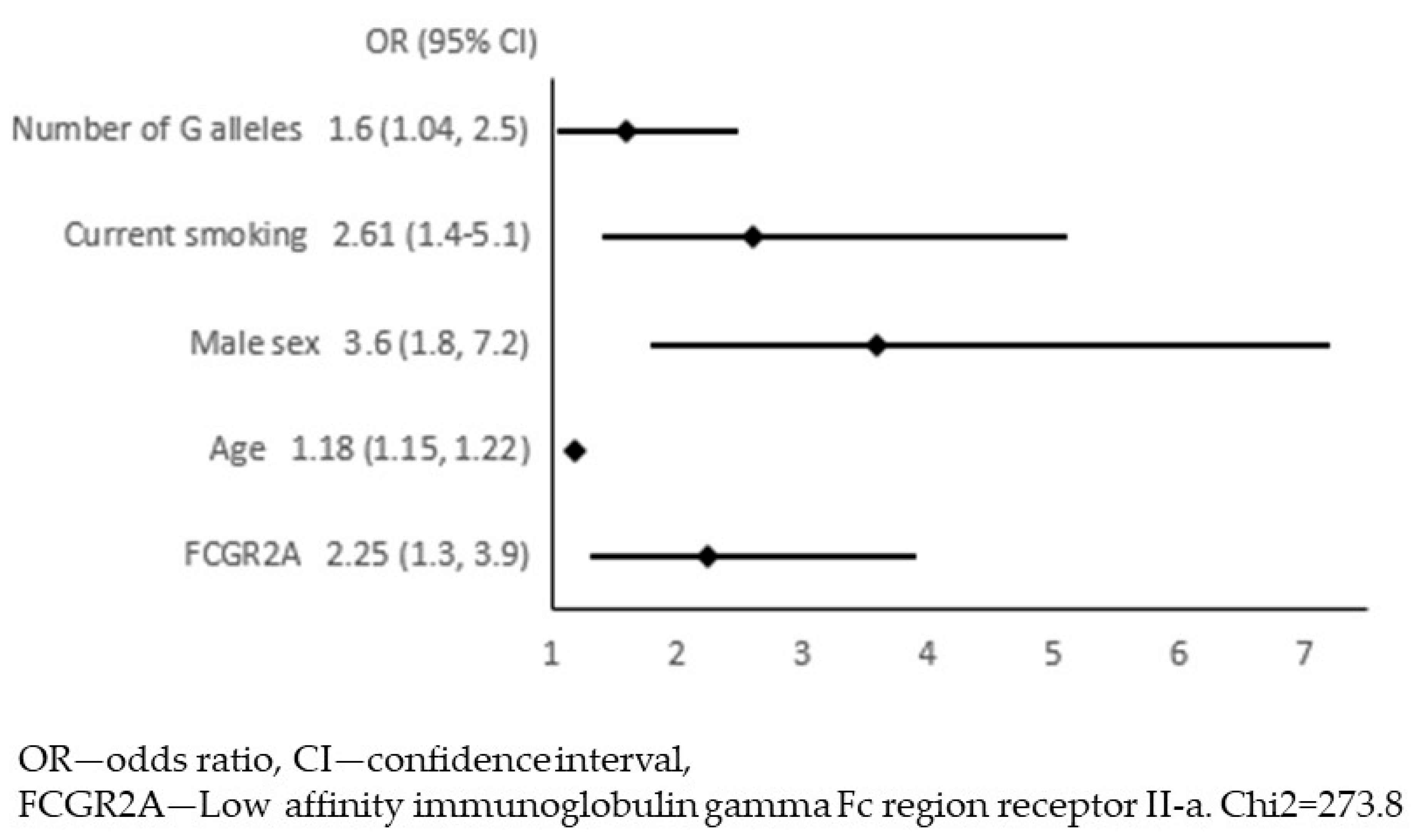
| Patients with APCA (n = 320) | Patients without APCA (n = 373) | p-Value | |
|---|---|---|---|
| Age (years) | 62 (53–69) | 38 (31–45) | <0.001 |
| Gender, men (n, %) | 145 (45.3%) | 156 (41.8%) | 0.356 |
| Current smoking (n, %) | 74 (23.1%) | 73 (19.6%) | 0.254 |
| Hypertension (n, %) | 206 (64.4%) | 107 (28.6%) | <0.001 |
| Diabetes (n, %) | 70 (21.9%) | 23 (6.2%) | <0.001 |
| Hypercholesterolaemia (n, %) | 273 (85.3%) | 220 (60%) | <0.001 |
| BP systolic (mmHg) | 128.5 (116.62–140.25) | 119.3 (108.5–130.5) | <0.001 |
| BP diastolic (mmHg) | 82 (76.5–88.75) | 80 (73.5–86.5) | 0.002 |
| BMI (kg/m2) | 27.8 (24.34–31.24) | 25.05 (22.59–28.36) | <0.001 |
| IMT (mm) | 0.71 (0.63–0.8) | 0.55 (0.51–0.62) | <0.001 |
| Biochemistry | |||
| Fasting glucose (mmol/L) | 5.72 (5.39–6.17) | 5.39 (5.06–5.72) | <0.001 |
| LDL-cholesterol (mmol/L) | 3.40 (2.59–4.03) | 3.05 (2.47–3.54) | <0.001 |
| HDL-cholesterol (mmol/L) | 1.52 (1.26–1.84) | 1.59 (1.32–1.89) | 0.184 |
| Triglycerides (mmol/L) | 1.16 (0.9–1.64) | 0.94 (0.68–1.38) | <0.001 |
| hsCRP (mg/L) | 0.83 (0.41–1.65) | 0.59 (0.27–1.44) | <0.001 |
| Interleukin-6 (pg/mL) | 3.07 (2.09–4.12) | 2.34 (1.83–3.51) | <0.001 |
| Pharmacotherapy | |||
| Beta-blockers | 104 (32.5%) | 25 (6.7%) | <0.001 |
| ACE-inhibitors or sartans | 100 (31.3%) | 34 (9.1%) | <0.001 |
| Diuretics | 32 (10%) | 10 (2.7%) | <0.001 |
| Lipid-lowering drugs | 80 (25%) | 16 (4.3%) | <0.001 |
| Antidiabetic drugs | 34 (10.6%) | 9 (2.4%) | <0.001 |
| Antiplatelet or anticoagulant drugs (%) | 65 (20.3%) | 8 (2.1%) | <0.001 |
| Variable | β | p |
|---|---|---|
| Univariate analysis | ||
| Age | 0.15 | <0.001 |
| Male sex | 0.07 | 0.055 |
| Diabetes | 0.09 | 0.02 |
| Hypercholesterolaemia | 0.04 | 0.26 |
| Obesity | 0.13 | <0.001 |
| Current smoking | −0.02 | 0.75 |
| Hypertension | 0.1 | 0.004 |
| Multivariate analysis, R2 = 0.028 | ||
| Age | 0.13 | 0.001 |
| Male sex | 0.1 | 0.01 |
| Genotype | Participants with APCA N = 310 | Participants without APCA N = 365 |
|---|---|---|
| AA | 89 (28.7%) | 123 (33.7%) |
| AG | 157 (50.6%) | 170 (46.6%) |
| GG | 64 (20.6%) | 72 (19.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szpakowicz, A.; Szum-Jakubowska, A.; Lisowska, A.; Dubatówka, M.; Raczkowski, A.; Czajkowski, M.; Szczerbiński, Ł.; Chlabicz, M.; Krętowski, A.; Kamiński, K.A. The FCGR2A Is Associated with the Presence of Atherosclerotic Plaques in the Carotid Arteries—A Case-Control Study. J. Clin. Med. 2023, 12, 6480. https://doi.org/10.3390/jcm12206480
Szpakowicz A, Szum-Jakubowska A, Lisowska A, Dubatówka M, Raczkowski A, Czajkowski M, Szczerbiński Ł, Chlabicz M, Krętowski A, Kamiński KA. The FCGR2A Is Associated with the Presence of Atherosclerotic Plaques in the Carotid Arteries—A Case-Control Study. Journal of Clinical Medicine. 2023; 12(20):6480. https://doi.org/10.3390/jcm12206480
Chicago/Turabian StyleSzpakowicz, Anna, Aleksandra Szum-Jakubowska, Anna Lisowska, Marlena Dubatówka, Andrzej Raczkowski, Marcin Czajkowski, Łukasz Szczerbiński, Małgorzata Chlabicz, Adam Krętowski, and Karol Adam Kamiński. 2023. "The FCGR2A Is Associated with the Presence of Atherosclerotic Plaques in the Carotid Arteries—A Case-Control Study" Journal of Clinical Medicine 12, no. 20: 6480. https://doi.org/10.3390/jcm12206480
APA StyleSzpakowicz, A., Szum-Jakubowska, A., Lisowska, A., Dubatówka, M., Raczkowski, A., Czajkowski, M., Szczerbiński, Ł., Chlabicz, M., Krętowski, A., & Kamiński, K. A. (2023). The FCGR2A Is Associated with the Presence of Atherosclerotic Plaques in the Carotid Arteries—A Case-Control Study. Journal of Clinical Medicine, 12(20), 6480. https://doi.org/10.3390/jcm12206480







