Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy
Abstract
1. Introduction
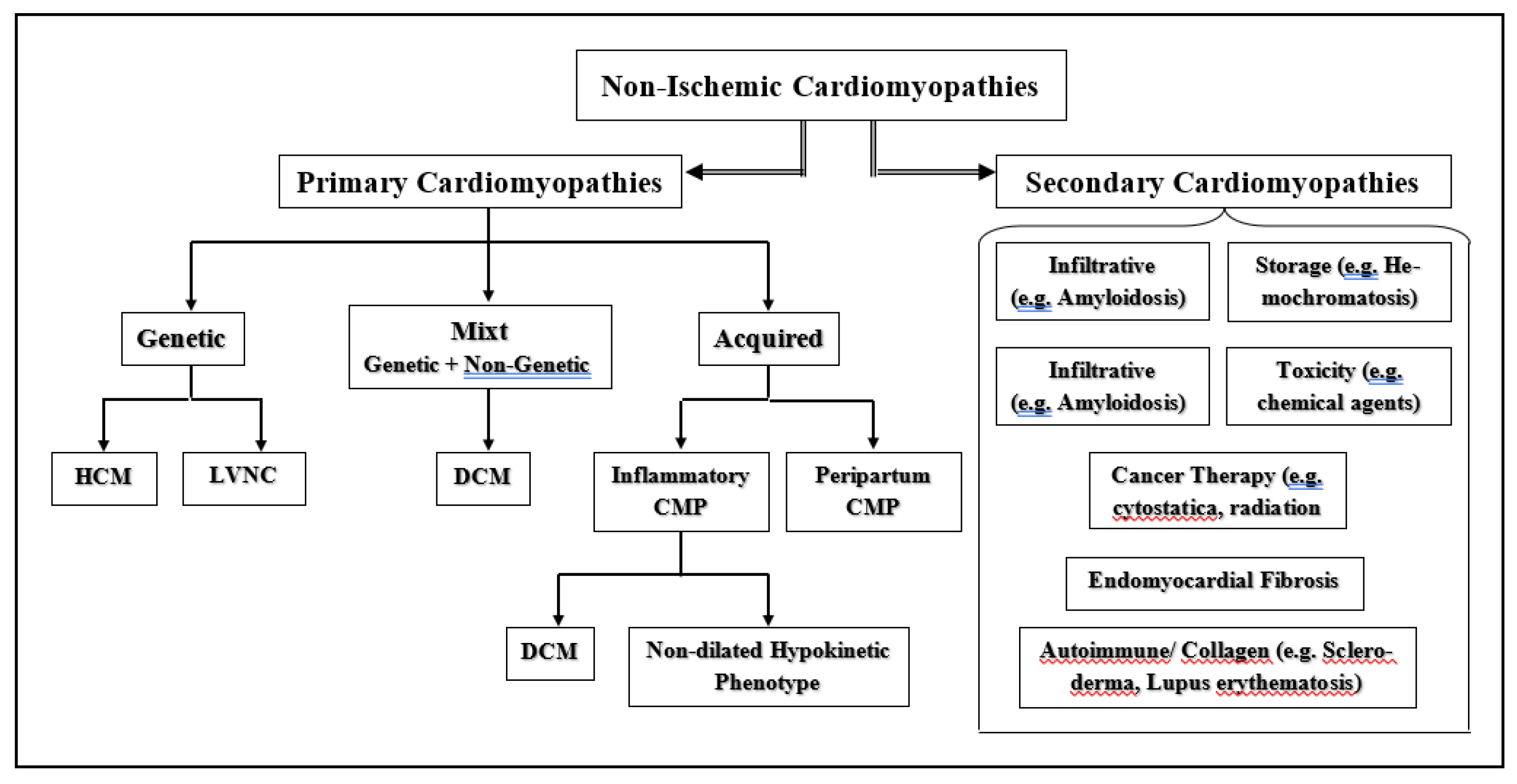
2. Non-Ischemic Cardiomyopathy-Induced Heart Failure
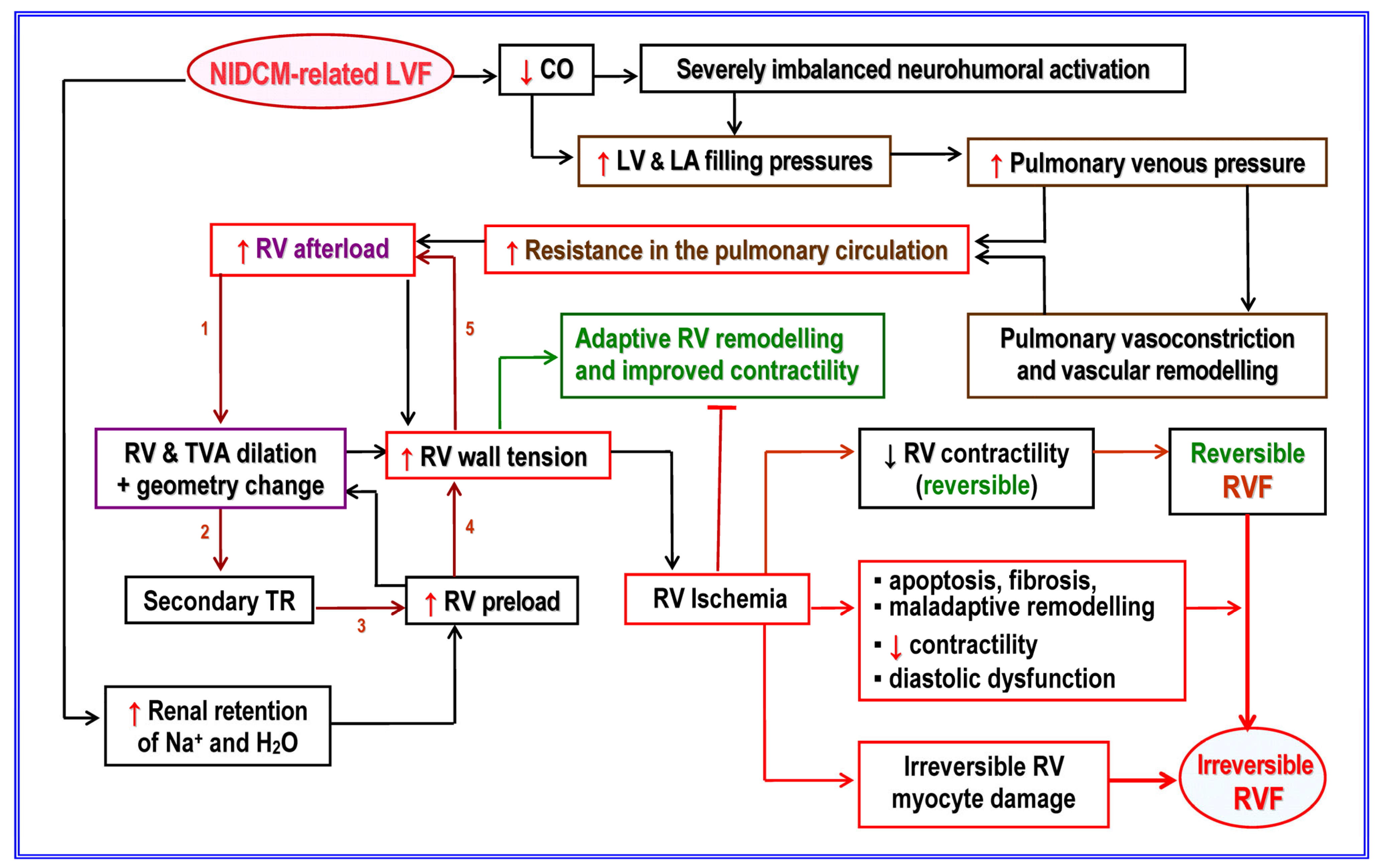
2.1. Etiopathogenic and Pathophysiological Particularities of NICM
2.2. Prediction of DCM-Related End-Stage Heart Failure
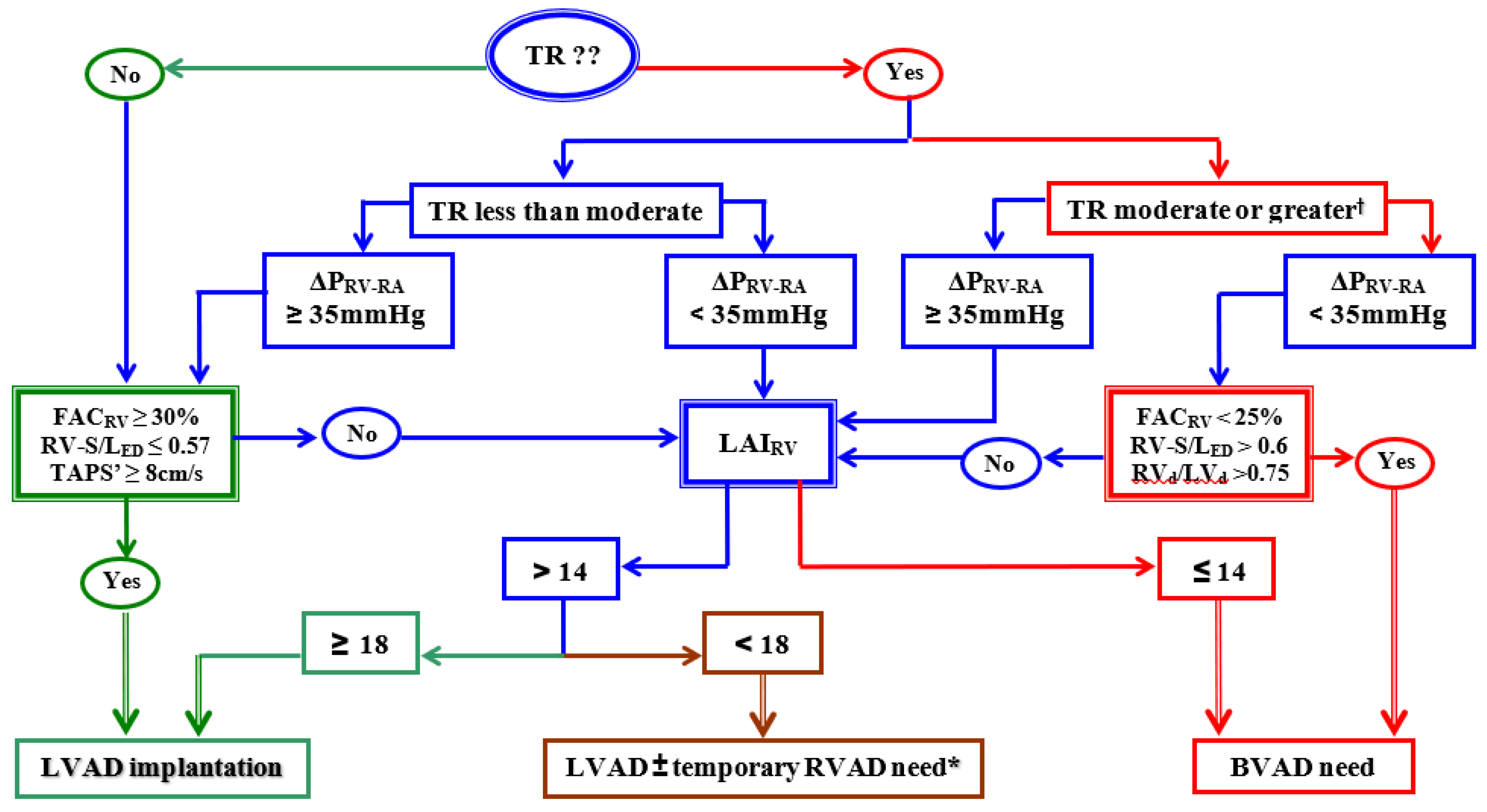
3. Patient Selection for Long-Term VAD Support
4. Biventricular Long-Term Mechanical Support
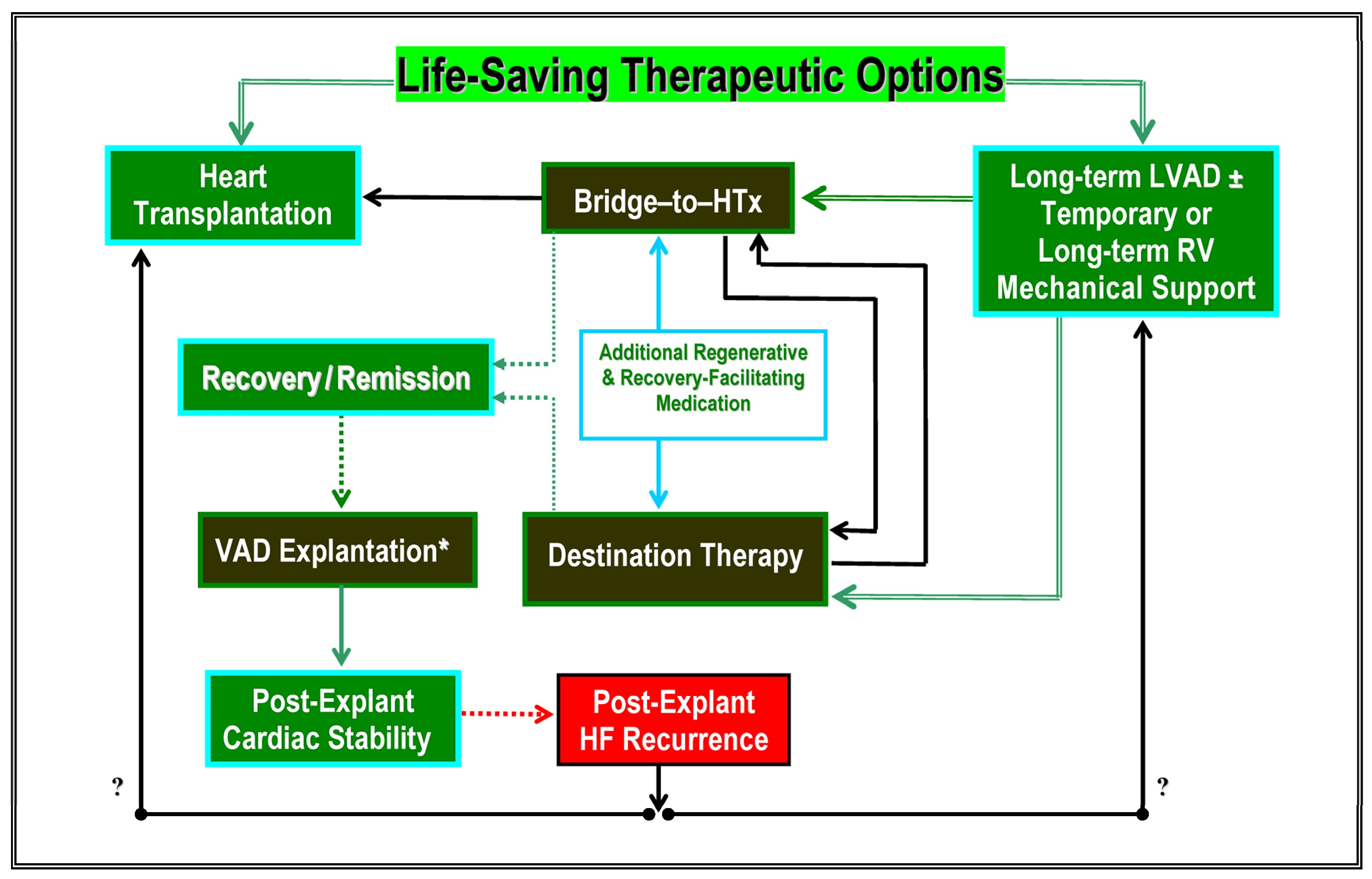
5. Cardiac Recovery during Long-Term Mechanical Support
5.1. Clinical Relevance of Chronic Heart Failure Reversal during VAD Support
5.2. Optimization of Ventricular Support and Heart Failure Therapy
5.3. Detection of Potential Weaning Candidates
5.4. Evaluation of Recovery
5.5. Prediction of Cardiac Stability without VAD Support
5.6. Decision-Making in Favor of Elective VAD Explantation
6. Conclusions and Future Tasks
- Can future research on molecular and cellular levels provide a platform for new adjunctive therapies (pharmacological and/or cell-based therapy, gene transfer, etc.) aimed to optimize recovery and increase the number of weaning candidates?
- Can future research on cellular, molecular, and genomic levels provide data which might allow the detection of patients with the potential for recovery before VAD implantation?
Funding
Data Availability Statement
Conflicts of Interest
References
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M.; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.S.; Sundareswaran, K.S.; Pham, D.T.; Kapur, N.K.; Pereira, N.L.; Strueber, M.; Farrar, D.J.; DeNofrio, D.; Rogers, J.G. Preoperative Determinants of Quality of Life and Functional Capacity Response to Left Ventricular Assist Device Therapy. J. Card. Fail. 2016, 22, 797–805. [Google Scholar] [CrossRef]
- Bellavia, D.; Iacovoni, A.; Scardulla, C.; Moja, L.; Pilato, M.; Kushwaha, S.S.; Senni, M.; Clemenza, F.; Agnese, V.; Falletta, C.; et al. Prediction of right ventricular failure after ventricular assist device implant: Systematic review and meta-analysis of observational studies. Eur. J. Heart Fail. 2017, 19, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Tang, Y.; Nassif, M.E.; Novak, E.; Jones, P.G.; LaRue, S.; Spertus, J.A.; Mann, D.L. The HeartMate risk score identifies patients with similar mortality risk across all INTERMACS profiles in a large multicenter analysis. J. Am. Coll. Cardiol. Heart Fail. 2016, 4, 950–958. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; Priori, S.G.; et al. Dilated cardiomyopathy. Nat. Rev. 2019, 5, 32. [Google Scholar] [CrossRef]
- Seferović, P.M.; Polovina, M.M.; Coats, A.J.S. Heart failure in dilated non-ischaemic cardiomyopathy. Eur. Heart J. Suppl. 2019, 21, M40–M43. [Google Scholar] [CrossRef]
- Pinto, J.M.; Elliott, P.M.; Arbustini, E.; Adler, Y.; Anastasakis, A.; Duboc, D.; Gimeno, J.; de Groote, P.; Imazio, M.; Heymans, S.; et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016, 37, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Amirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Klingel, K.; Pedrotti, P.; Rimoldi, O.E.; Camici, P.G.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Heart Fail. 2020, 13, 663–687. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Tavazzi, L.; Serio, A.; Grasso, M.; Favalli, V.; Bellazzi, R.; Tajik, J.A.; Bonow, R.O.; Fuster, V.; et al. The MOGE(S) Classification of Cardiomyopathy for Clinicians. J. Am. Coll. Cardiol. 2014, 64, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, H.; Song, J. Accurate Classification of Non-ischemic Cardiomyopathy. Curr. Cardiol. Rep. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Merlo, M.; Dominguez, F.; Wang, P.; Henkens, M.; Adriaens, M.; Hazebroek, M.; Mase, M.; Escobar, L.E.; Sinagra, G.; et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2021, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, T.; Neglia, D.; Bionda, A.; Pino, B.D.; Bigazzi, F.; Puntoni, M.; Startari, U.; Morales, A.; Minichilli, F.; Bianchi, F.; et al. Inflammatory Markers and Serum Lipids in Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2005, 96, 1718–1720. [Google Scholar] [CrossRef]
- Dandel, M.; Wallukat, G.; Englert, A.; Hetzer, R. Immunoadsorption therapy for dilated cardiomyopathy and pulmonary arterial hypertension. Atheroscler. Suppl. 2013, 14, 203–211. [Google Scholar] [CrossRef]
- Lappé, J.M.; Pelfrey, C.M.; Tang, W.W. Recent Insights Into the Role of Autoimmunity in Idiopathic Dilated Cardiomyopathy. J. Card. Fail. 2008, 14, 521–530. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Baba, A.; Nagatomo, Y. Autoimmune Mechanisms Underlying Dilated Cardiomyopathy. Circ. J. 2009, 73, 602–607. [Google Scholar] [CrossRef]
- Caforio, A.L.; Mahon, N.J.; Tona, F.; McKenna, W.J. Circulating cardiac autoantibodies in dilated cardiomyopathy and miocarditis: Pathogenic and clinical significance. Eur. J. Heart Fail. 2002, 4, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Jahns, R.; Boivin, V.; Hein, L. Direct evidence for beta-1 adrenergic directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Investig. 2004, 113, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Prasad, S.V.N. Autoantibodies and Cardiomyopathy: Focus on Beta-1 Adrenergic Receptor Autoantibodies. J. Cardiovasc. Pharmacol. 2022, 80, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Nagatamo, Y.; Tang, W. Autoantibodies and Cardiovascular Dysfunction: Cause or Consequence? Curr. Heart Fail. Rep. 2014, 11, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.A.; Hernandez, A.F. Targeting anti-beta-1-adrenergic receptor antibodies for dilated cardiomyopathy. Eur. J. Heart Fail. 2013, 15, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Düngen, H.-D.; Dordevic, A.; Felix, S.B. β1-Adrenoreceptor Autoantibodies in Heart Failure: Physiology and Therapeutic Implications. Circ. Heart Fail. 2020, 13, e006155. [Google Scholar] [CrossRef] [PubMed]
- Jahns, R.; Boivin, V.; Siegmund, C.; Inselmann, G.; Lohse, M.J.; Boege, F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation 1999, 99, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. Pathogenic roles of cardiac autoantibodies in dilated cardiomyopathy. Trends Mol. Med. 2005, 11, 322–326. [Google Scholar] [CrossRef]
- Iwata, M.; Yoshikawa, T.; Baba, A.; Anzai, T.; Mitamura, H.; Ogawa, S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 2001, 37, 418–424. [Google Scholar] [CrossRef]
- Störk, S.; Boivin, V.; Horf, R.; Hein, L.; Lohse, M.J.; Angermann, C.E.; Jahns, R. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am. Heart J. 2006, 152, 697–704. [Google Scholar] [CrossRef]
- Müller, J.; Wallukat, G.; Weng, Y.-G.; Dandel, M.; Spiegelsberger, S.; Semrau, S.; Brandes, K.; Theodoridis, V.; Loebe, M.; Meyer, R.; et al. Weaning From Mechanical Cardiac Support in Patients With Idiopathic Dilated Cardiomyopathy. Circulation 1997, 96, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Drews, T.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation 2008, 118, S94–S105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dandel, M.; Wallukat, G.; Englert, A.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Long-term benefits of immunoadsorption in β(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur. J. Heart. Fail. 2012, 14, 1374–1388. [Google Scholar] [CrossRef]
- Pokrovsky, S.N.; Ezhov, M.V.; Safarova, M.S.; Saidova, M.A.; Shitov, V.N.; Afanasieva, M.I.; Khaustov, A.I.; Adamova, I.Y.; Afanasieva, O.I.; Konovalov, G.A. Ig apheresis for the treatment of severe DCM patients. Atheroscler. Suppl. 2013, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Wollenberger, A.; Morwinski, R.; Pitschner, H.F. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: Mapping of epitopes in the first and second extracellular loops. J. Mol. Cell Cardiol. 1995, 27, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Müller, J.; Podlowski, S.; Nissen, E.; Morwinski, R.; Hetzer, R. Agonistic like beta-adrenergic antibodies in heart failure. Am. J. Cardiol. 1999, 83, 75H–79H. [Google Scholar] [CrossRef] [PubMed]
- Staudt, Y.; Mobini, R.; Fu, M.; Felix, S.B.; Kühn, J.P.; Staudt, A. Beta 1-adrenoceptor autoantibodies induce apoptosis in adult isolated cardiomyocytes. Eur. J. Pharmacol. 2003, 466, 1–6. [Google Scholar] [CrossRef]
- Jane-wit, D.; Altuntas, C.Z.; Johnson, J.M.; Jong, S.; Wicklay, P.J.; Wang, Q.; Popovic, Z.B.; Penn, M.S.; Damron, D.S.; Perez, D.M.; et al. Beta 1-adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation 2007, 116, 399–410. [Google Scholar] [CrossRef]
- Wallukat, G.; Haberland, A.; Berg, S.; Schulz, A.; Freyse, E.J.; Dahmen, C.; Kage, A.; Dandel, M.; Vetter, R.; Salzsieder, E.; et al. The First Aptamer-Apheresis Column Specifically for Clearing Blood of β1-Receptor Autoantibodies–A Successful Proof of Principle Using Autoantibody-Positive SHR Rats. Circ. J. 2012, 76, 2449–2455. [Google Scholar] [CrossRef]
- Haberland, A.; Holtzhauer, M.; Schlichtiger, A.; Bartel, S.; Schimke, I.; Müller, J.; Dandel, M.; Luppa, P.B.; Wallukat, G. Aptamer BC 007—A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur. J. Pharmacol. 2016, 789, 37–45. [Google Scholar] [CrossRef]
- Seferović, P.M.; Polovina, M.M.; Coats, A.J.S. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. Suppl. 2019, 21, M40–M43. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M. The Dilated, Restrictive and Infiltrative Cardiomyopathies. In Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 9th ed.; Bonow, R., Mann, D.L., Zipes, D.P., Libby, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 68; pp. 1561–1581. [Google Scholar]
- Dandel, M.; Javier, M.F.D.M.; Javier Delmo, E.M.D.; Hetzer, R. Accurate assessment of right heart function before and after long-term left ventricular assist device implantation. Expert Rev. Cardiovasc. Ther. 2020, 18, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K.; Halliday, B.P. Myocardial Fibrosis in Dilated Cardiomyopathy: Moving From Stratifying Risk to Improving Outcomes. JACC Cardiovasc. Imaging 2021, 14, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Pathophysiology of Heart Failure. In Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 9th ed.; Bonow, R., Mann, D.L., Zipes, D.P., Libby, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Chapter 25; pp. 487–504. [Google Scholar]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Guihaire, J.; Noly, P.E.; Schrepfer, S.; Mercier, O. Advancing knowledge of right ventricular pathophysiology in chronic pressure overload: Insights from experimental studies. Arch. Cardiovasc. Dis. 2015, 108, 519–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahimi, K.; Bennett, D.; Conrad, N.; Williams, T.M.; Basu, J.; Dwight, J.; Woodword, M.; McMurrai, J.; Patel, A.; MacMahon, S. Risk prediction in patients with heart failure: A systematic review and analysis. JACC Heart Fail. 2014, 2, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Young, M.S.; Wei, J. Prediction of short-term outcome in Chinese patients with ambulatory heart failure for heart transplantation with ejection fraction <25%. Jpn. Heart J. 2000, 41, 349. [Google Scholar] [PubMed]
- Jasaityte, R.; Dandel, M.; Lehmkuhl, H.; Hetzer, R. Prediction of short-term outcomes in patients with idiopathic dilated cardiomyopathy referred for transplantation using standard echocardiography and strain imaging. Transpl. Proc. 2009, 41, 277–280. [Google Scholar] [CrossRef]
- Xu, B.; Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.C.; Hirokawa, M.; Yoshinaga, A.; Watanabe, M. Prognostic Value of a Simple Echocardiographic Parameter, the Right Ventricular Systolic to Diastolic Duration Ratio, in Patients with Advanced Heart Failure with Non-Ischemic Dilated Cardiomyopathy. Int. Heart J. 2018, 59, 968–975. [Google Scholar] [CrossRef]
- Perl, L.; Kheifets, M.; Guido, A.; Agricola, E.; Denti, P.; Wild, M.G.; Praz, F.; Rubbio, A.P.; Bedogni, F.; De Marco, F.; et al. Acute Reduction in Left Ventricular Function Following Transcatheter Mitral Edge-to-Edge Repair. J. Am. Heart Assoc. 2023, 4, e029735. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Smith, R.L., 2nd. Left Ventricular Ejection Fraction in Mitral Regurgitation Because of Flail Leaflet. Circ. Cardiovasc. Imaging 2014, 7, 220–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witkowski, T.G.; Thomas, J.D.; Debonnaire, P.J.M.R.; Delgado, V.; Hoke, U.; Ewe, S.H.; Versteegh, M.I.; Holan, E.R.; Schalij, M.J.; Bax, J.J.; et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Benz, A.; Mereles, D.; Unnebrink, K.; Kücherer, H.; Haass, M.; Kübler, W.; Katus, H.A. Prognostic value of serial cardiac assessment and familial screening in patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2023, 5, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Makar, M.; Kar, S.; Koseki, K.; Oakley, L.; Sekhon, l.; Patel, D.; Chakravarty, T.; Nakamura, M.; Hamilton, M.; et al. Impact of Left Ventricular Global Longitudinal Strain on Outcomes After Transcatheter Edge-to-Edge Repair in Secondary Mitral Regurgitation. Am. J. Cardiol. 2022, 182, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Meyer, T.E. Left Ventricular Response to Mitral Regurgitation Implications for Management. Circulation 2008, 118, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Sveric, K.M.; Ulbrich, S.; Rady, M. Three-dimensional left ventricular torsion in patients with dilated cardiomyopathy: A marker of disease severity. Circ. J. 2017, 81, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.; Ulbrich, S.; Heidrich, F.; Jellinhaus, S.; Ibrahim, K.; Linke, A.; Sferic, K.M. Left Ventricular Torsion—A New Echocardiographic Prognosticator in Patients with Non-Ischemic Dilated Cardiomyopathy. Circ. J. 2019, 83, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Melillo, F.; Liberale, L.; Montecucco, F.; Agricola, E. Right ventricle dysfunction assessment for transcatheter tricuspid valve repair: A matter of debate. Eur. J. Clin. Investig. 2021, 51, e13653. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.K.; Wang, W.C.; Yang, S.H.; Gin, P.L.; Liu, C.P. Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2006, 19, 902–910. [Google Scholar] [CrossRef]
- Kawata, T.; Daimon, M.; Kimura, K.; Nakao, T.; Lee, S.L.; Hirokawa, M.; Watanabe, M.; Yatomi, Y.; Komuro, I. Echocardiographic assessment of right ventricular function in routine practice: Which parameters are useful to predict one-year outcome in advanced heart failure patients with dilated cardiomyopathy? J. Cardiol. 2017, 70, 316–322. [Google Scholar] [CrossRef]
- Wu, K.C.; Weiss, R.G.; Thiemann, D.R.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.A.; Gerstenblith, G.; Marban, E.; et al. Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance Heralds an Adverse Prognosis in Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Mandawat, A.; Chattranukulchai, P.; Mandawat, A.; Blood, A.J.; Ambati, S.; Hayes, B.; Rehwald, W.; Kim, H.W.; Heitner, J.F.; Shah, D.J.; et al. Progression of Myocardial Fibrosis in Nonischemic DCM and Association With Mortality and Heart Failure Outcomes. ACC Cardiovasc. Imaging 2021, 14, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Evaluation of the Right Ventricle by Echocardiography: Particularities and Major Challenges. Expert Rev. Cardiovasc. Ther. 2018, 16, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Guglin, M.J. Patient selection for ventricular assist devices: A moving target. Am. Coll. Cardiol. 2013, 61, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, F.; Rogers, J.G. Left ventricular assist device therapy in advanced heart failure: Patient selection and outcomes. Eur. J. Heart Fail. 2017, 19, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, N.; Rao, V. Left ventricular assist device as destination therapy for end stage heart failure: The right time for the right patients. Curr. Opin. Cardiol. 2018, 33, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.G.; Goldstein, D.J.; Alturi, P.; Arabia, F.A.; Cheung, A.; Holman, W.; Hoopes, C.; Jeevanandam, V.; John, R.; et al. Adult: Mechanical Circulatory Support: AATS/ISHLT Guidelines On Selected Topics In Mechanical Circulatory Support. American Association for Thoracic Surgery/International Society for Heart and Lung Transplantation guidelines on selected topics in mechanical circulatory support. J. Thorac. Cardiovasc. Surg. 2020, 159, 865–896. [Google Scholar] [PubMed]
- Schramm, R.; Morshuis, M.; Schoenbrodt, M.; Boergermann, J.; Hakim-Maibodi, K.; Hata, M.; Gummert, J.F. Current perspectives on mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 55, i31–i37. [Google Scholar] [CrossRef]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Lehmkuhl, H.B.; Hetzer, R. Long-term results in patients with idiopathic dilated cardiomyopathy after weaning from assist devices. Circulation 2005, 112, 37–50. [Google Scholar] [CrossRef]
- Birks, E.J.; Tansley, P.D.; Hardy, J.; George, R.S.; Bowles, C.T.; Banner, N.R.; Khaghani, A.; Yacoub, M.H. Left ventricular assist device and drug therapy for the reversal of heart failure. N. Engl. J. Med. 2006, 355, 1873–1884. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.P.; Kapelios, C.J.; Stauder, E.L.; Taleb, I.; Hamouche, R.; Sideris, K.; Koliopoulou, A.G.; Bonios, M.J.; Drakos, S.G. LVAD as a Bridge to Remission from Advanced Heart Failure: Current Data and Opportunities for Improvement. J. Clin. Med. 2022, 11, 3542. [Google Scholar] [CrossRef]
- Teuteberg, J.J.; Cleveland, J.C., Jr.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons INTERMACS 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef]
- Mikus, E.; Stepanenko, A.; Krabatsch, T.; Dandel, M.; Lehmkuhl, H.B.; Loforte, A.; Hetzer, R.; Potapov, E. Left ventricular assist device or heart transplantation: Impact of transpulmonary gradient and pulmonary vascular resistance on decision making. Eur. J. Cardiothorac. Surg. 2011, 39, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.J.; van der Lingen, A.C.J.; Wubben, M.; van de Ven, P.M.; van Rossum, A.C.; Cornel, J.H.; Allaart, C.P.; Germans, T. Characteristics and prognostic value of right ventricular (dys)function in patients with non-ischaemic dilated cardiomyopathy assessed with cardiac magnetic resonance imaging. ESC Heart Fail. 2021, 8, 1055–1063. [Google Scholar] [CrossRef]
- Stepanenko, A.; Potapov, E.V.; Jurmann, B.; Lehmkuhl, H.B.; Dandel, M.; Siniawski, H.; Drews, T.; Hennig, E.; Kaufmann, F.; Jurmann, M.J.; et al. Outcomes of elective versus emergent permanent mechanical circulatory support in the elderly: A single-center experience. Heart Lung Transplant. 2010, 29, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Dranishnikov, N.; Stepanenko, A.; Potapov, E.V.; Dandel, M.; Siniawski, H.; Mladenow, A.; Hübler, M.; Grauhan, O.; Weng, Y.; Krabatsch, T.; et al. Simultaneous aortic valve replacement in left ventricular assist device recipients: Single-center experience. Int. J. Artif. Organs 2012, 35, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.S.; Farr, M.; Schulze, P.C.; Maurer, M.; Shahzad, K.; Iwata, S.; Homma, S.; Jorde, U.; Takayama, H.; Naka, Y.; et al. Usefulness of two-dimensional echocardiographic parameters of left side of the heart to predict right ventricular failure after left ventricular assist device implantation. Am. J. Cardiol. 2012, 109, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Temporary Assist Device Support for the Right Ventricle: Pre-Implant and Post-Implant Challenges. Heart Fail. Rev. 2018, 23, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Alnsasra, H.; Asleh, R.; Schettle, S.D.; Pereira, N.L.; Frantz, R.P.; Edwards, B.S.; Clavell, A.L.; Maltais, S.; Daly, R.C.; Stulak, J.M.; et al. Diastolic Pulmonary Gradient as a Predictor of Right Ventricular Failure After Left Ventricular Assist Device Implantation. J. Am. Heart Assoc. 2019, 8, e012073. [Google Scholar] [CrossRef]
- Mikus, E.; Stepanenko, A.; Krabatsch, T.; Loforte, A.; Dandel, M.; Lehmkuhl, H.B.; Hetzer, R.; Potapov, E.V. Reversibility of fixe pulmonary hypertension in left ventricular assist device support recipients. Eur. J. Cardiothorac. Surg. 2011, 40, 971–977. [Google Scholar]
- Hennig, F.; Stepanenko, A.; Lehmkuhl, H.B.; Kukucka, M.; Dandel, M.; Krabatsch, T.; Hetzer, R.; Potapov, E.V. Neurohumoral and inflammatory markers for prediction of right ventricular failure after implantation of a left ventricular assist device. Gen. Thorac. Cardiovasc. Surg. 2011, 59, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Knosalla, C.; Hetzer, R. Contribution of left ventricular assist devices to the recovery of failing hearts: A review and the Berlin Heart Center experience. Eur. J. Heart Fail. 2014, 16, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Barras, N.; Jeanrenaud, X.; Regamey, J.; Yerly, P.; Roumy, A.; Kirsch, M. Echocardiographic predictors of post-implantation right ventricular failure. Right ventricular function before LVAD implantation. Cardiovasc. Med. 2017, 20, 69–71. [Google Scholar]
- Charisopoulou, D.; Banner, N.R.; Demetrescu, C.; Simon, A.R.; Rahman Haley, S. Right atrial and ventricular echocardiographic strain analysis predicts requirement for right ventricular support after left ventricular assist device implantation. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Zuchi, C.; Coiro, S.; Riccini, C.; Mengoni, A.; D’ Antonio, A.; Ambrosio, G. Prognostic Value of Right Ventricular Dysfunction in Heart Failure With Reduced Ejection Fraction: Superiority of Longitudinal Strain Over Tricuspid Annular Plane Systolic Excursion. Circ. Cardiovasc. Imaging 2018, 11, e006894. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.S.; Jiang, J.; Schulze, P.C.; Jorde, U.; Uriel, S.; Takayama, H.; Naka, Y.; Mancini, D.; Gillam, L.; Homma, S.; et al. Serial Echocardiography Using Tissue Doppler and Speckle Tracking Imaging to Monitor Right Ventricular Failure Before and After Left Ventricular Assist Device Surgery. JACC Heart Fail. 2013, 1, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Vachiery, J.L.; Yerly, P.; Vanderpool, R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur. Respir. J. 2013, 41, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Role of echocardiography in selection, implantation, and management of left ventricular assist device therapy. In Encyclopedia of Cardiovascular Research and Medicine; Sawyer, D.B., Vasan, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 4, pp. 327–344. [Google Scholar]
- Cameli, M.; Bernazzali, S.; Lisi, M.; Tsioulpas, C.; Croccia, M.G.; Lisi, G.; Maccherini, M.; Mondillo, S. Right ventricular longitudinal strain and right ventricular stroke work index in patients with severe heart failure: Left ventricular assist device suitability for transplant candidates. Transplant Proc. 2012, 44, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Paruchuri, V.; Denofrio, D.; Kapur, N.K. Pulmonary Artery Pulsatility Index Is Associated With Right Ventricular Failure After Left Ventricular Assist Device Surgery. J. Card. Fail. 2016, 22, 110–116. [Google Scholar] [CrossRef]
- Amsallem, M.; Mercier, O.; Kobayashi, Y.; Moneghetti, K.; Haddad, F. Forgotten No More: A Focused Update on the Right Ventricle in Cardiovascular Disease. JACC Heart Fail. 2018, 6, 891–903. [Google Scholar] [CrossRef]
- Frea, S.; Bovolo, V.; Bergerone, S.; D’Ascenzo, F.; Antolini, M.; Capriolo, M.; Canavosio, F.G.; Morello, M.; Gaita, F. Echocardiographic evaluation of right ventricular stroke work index in advanced heart failure: A new index? J. Card. Fail. 2012, 18, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Nishimura, R.A.; Oh, J.K.; McGoon, M.D. The prognostic value of pulmonary vascular capacitance determined by Doppler echocardiography in patients with pulmonary arterial hypertension. J. Am. Soc. Echocardiogr. 2006, 19, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ohtani, T.; Hidetaka Kioka, H.; Onishi, T.; Tsukamoto, Y.; Nakamoto, K.; Taniguchi, T.; Nakatani, S.; Hirayama, A.; Sakata, Y. Clinical Significance of Pulmonary Arterial Capacitance Calculated by Echocardiography in Patients with Advanced Heart Failure. Circ. J. 2017, 81, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Potapov, E.; Krabatsch, T.; Stepanenko, A.; Löw, A.; Vierecke, J.; Knosalla, C.; Hetzer, R. Load Dependency of Right Ventricular Performance is a Major Factor to be Considered in Decision Making Prior to Ventricular Assist Device Implantation. Circulation 2013, 128, S14–S23. [Google Scholar] [CrossRef]
- Grant, A.D.M.; Smedira, N.G.; Starling, R.C.; Marwick, T.H. Independent and Incremental Role of Quantitative Right Ventricular Evaluation for the Prediction of Right Ventricular Failure After Left Ventricular Assist Device Implantation. J. Am. Coll. Cardiol. 2012, 60, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Knosalla, C.; Kemper, D.; Stein, J.; Hetzer, R. Assessment of right ventricular adaptability to loading conditions can improve the timing of listing to transplantation in patients with pulmonary arterial hypertension. J. Heart Lung Transplant. 2015, 34, 319–328. [Google Scholar] [CrossRef]
- Amsallem, M.; Aymami, M.; Hiesinger, W.; Zeigler, S.; Monegetti, K.; Marques, M.; Teuteberg, J.; Ha, R.; Banerjee, D.; Haddad, F. Right ventricular load adaptability metrics in patients undergoing left ventricular assist device implantation. J. Thorac. Cardiovasc. Surg. 2019, 157, 1023–1033. [Google Scholar] [CrossRef]
- Raina, A.; Seetha Rammohan, H.R.; Gertz, Z.M.; Rame, J.E.; Woo, Y.J.; Kirkpatrick, J.N. Postoperative right ventricular failure after left ventricular assist device placement is predicted by preoperative echocardiographic structural, hemodynamic, and functional parameters. J. Card. Fail. 2013, 19, 16–24. [Google Scholar] [CrossRef]
- Matthews, J.C.; Koelling, T.M.; Pagani, F.D.; Aaronson, K.D. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J. Am. Coll. Cardiol. 2008, 51, 2163–2172. [Google Scholar] [CrossRef]
- Kormos, R.L.; Teuteberg, J.J.; Pagani, F.D.; Russell, S.D.; John, R.; Miller, L.W.; Massey, T.; Milano, C.A.; Moazami, N.; Sundareswaran, K.S.; et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: Incidence, risk factors, and effect on outcomes. J. Thorac. Cardiovasc. Surg. 2010, 139, 1316–1324. [Google Scholar] [CrossRef]
- Shiga, T.; Kinugawa, K.; Imamura, T.; Kato, N.; Endo, M.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Nishumura, T.; et al. Combination evaluation of preoperative risk indices predicts requirement of biventricular assist device. Circ. J. 2012, 76, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Drakos, S.G.; Janicki, L.; Horne, B.D.; Kfoury, A.G.; Reid, B.B.; Clayson, S.; Horton, K.; Haddad, F.; Li, D.Y.; Fisher, P.W.; et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am. J. Cardiol. 2010, 105, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.R., 3rd; Frederick, J.R.; Hsu, V.M.; Kozin, E.D.; O’Hara, M.L.; Howell, E.; Dougherty, D.; McCormick, R.C.; Laporte, C.M.; Cohen, J.E.; et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J. Heart Lung Transplant. 2008, 27, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Landra, F.; Sciaccaluga, C.; Pastore, M.C.; Gallone, G.; Barilli, M.; Fusi, C.; Focardi, M.; Cavigli, L.; D’Ascenzi, F.; Natali, B.M.; et al. Right ventricular myocardial work for the prediction of early right heart failure and long-term mortality after left ventricular assist device implant. Eur. Heart J. Cardiovasc. Imaging 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Atluri, P.; Goldstone, A.B.; Fairman, A.S.; McArthur, J.W.; Shudo, Y.; Cohen, J.E.; Acker, A.L.; Hiesinger, W.; Howard, J.L.; Acker, M.A.; et al. Predicting right ventricular failure in the modern, continuous flow left ventricular assist device era. Ann. Thorac. Surg. 2013, 96, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.; Ha, R.; Banerjee, D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation. J. Heart Lung Transplant. 2016, 35, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cacioli, G.; Polizzi, V.; Ciabatti, M.; Cristiano, E.; Pergolini, A.; Distefano, G.; Della Monica, P.L.; Comisso, M.; Piazza, V.; Sbaraglia, F.; et al. Prediction of right ventricular failure after left ventricular assist device implantation: Role of vasodilator challenge. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Myocardial recovery during mechanical circulatory support: Long-term outcome and elective ventricular assist device implantation to promote recovery as a treatment goal. Heart Lung Vessel. 2015, 7, 289–296. [Google Scholar]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; van der Bijl, P.; van der Velde, E.T.; Marsan, A.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef]
- Saito, S.; Matsumiya, G.; Sakaguchi, T.; Miyagawa, S.; Yamauchi, T.; Kuratani, T.; Sawa, Y. Cardiac fibrosis and cellular hypertrophy decrease the degree of reverse remodeling and improvement in cardiac function during left ventricular assist. J. Heart Lung Transplant. 2010, 29, 672–679. [Google Scholar] [CrossRef]
- Mann, D.L.; Barger, M.P.; Burkhoff, D. Myocardial recovery and the failing Heart. J. Am. Coll. Cardiol. 2012, 60, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.J.; Miller, L.W. Myocardial recovery with use of ventricular assist devices. In Mechanical Circulatory Support. A Companion Braunwald’s Heart Disease; Kormos, R.L., Miller, L.W., Eds.; Elsevier: Philadelphia, PA, USA, 2012; pp. 258–271. [Google Scholar]
- Nakatani, T.; Sasako, Y.; Kumon, K.; Sasako, Y.; Kumon, K.; Nagata, S.; Kosakai, Y.; Isobe, F.; Nakano, K.; Kobayashi, J.; et al. Long-term circulatory support to promote recovery from profound heart failure. ASAIO J. 1995, 41, M526–M530. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Weng, Y.; Siniawski, H.; Potapov, E.; Krabatsch, T.; Lehmkuhl, H.B.; Drews, T.; Knodalla, C.; Hetzer, R. Pre-explant stability of unloading-promoted cardiac improvement predicts outcome after weaning from ventricular assist devices. Circulation 2012, 126, S9–S19. [Google Scholar] [CrossRef]
- Birks, E. Molecular changes after left ventricular assist device support for heart failure. Circ. Res. 2013, 113, 777–791. [Google Scholar] [CrossRef]
- Frazier, O.H.; Benedict, C.R.; Radovancevic, B.; Bick, R.J.; Capek, P.; Springer, W.E.; Macris, M.P.; Delgado, R.; Buja, L.M. Improved left ventricular function after chronic left ventricular unloading. Ann. Thorac. Surg. 1996, 62, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Selzman, C.H.; Van-Khue Ton, V.-K.; Miera, O.; Cornwell, W.K., 3rd; Antaki, J.; Drakos, S.; Shah, P. Clinical myocardial recovery in advanced heart failure with long term left ventricular assist device support. J. Heart Lung Transplant. 2022, 41, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Frazier, O.H.; Baldwin, A.C.; Demirozu, Z.T.; Segura, A.M.; Hernandez, R.; Taegtmeyer, H.; Mallidi, H.; Cohn, W.E. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2015, 34, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.J.; George, R.S.; Firouzi, A.; Wright, G.; Bahrami, T.; Yacoub, M.H.; Khaghani, A. Long-term outcomes of patients bridged to recovery versus patients bridged to transplantation. J. Thorac. Cardiovasc. Surg. 2012, 144, 190–196. [Google Scholar] [CrossRef]
- Dandel, M.; Drakos, S.; Birks, E. Left ventricular recovery during LVAD device support. In Mechanical Circulatory Support; Pagani, F., Schueler, S., Uriel, N., Maltais, S., Kirklin, J., Eds.; ISHLT Monography Series; International Society for Heart and Lung Transplantation (ISHLT): Addison, IL, USA, 2021; Volume 14, pp. 313–353. ISBN 9781098385880. [Google Scholar]
- Drakos, S.G.; Pagani, F.D.; Lundberg, M.S.; Baldwin, J.T. Advancing the Science of Myocardial Recovery With Mechanical Circulatory Support: A Working Group of the National, Heart, Lung, and Blood Institute. JACC Basic Transl. Sci. 2017, 2, 335–340. [Google Scholar] [CrossRef]
- Mancini, D.M.; Beniaminovitz, A.; Levin, H.; Catanese, K.; Flannery, M.; DiTullio, M.; Savin, S.; Cordisco, M.E.; Rose, E.; Oz, M. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation 1998, 98, 2383–2389. [Google Scholar] [CrossRef]
- Maybaum, S.; Mancini, D.; Xydas, S.; Starling, R.C.; Aaronson, K.; Pagani, F.D.; Miller, L.M.; Margulies, K.; McRee, S.; Frazier, O.H.; et al. Cardiac improvement during mechanical circulatory support: A prospective multicenter study of the LVAD Working Group. Circulation 2007, 15, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Knierim, J.; Heck, R.; Pieri, M.; Schoenrath, F.; Soltani, S.; Stawowy, P.; Dreysse, S.; Stein, J.; Müller, M.; Mulzer, J.; et al. Outcomes from a recovery protocol for patients with continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 2019, 38, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Maybaum, S.; Kamalakannan, G.; Murthy, S. Cardiac recovery during mechanical assist device support. Semin. Thorac. Cardiovasc. Surg. 2008, 20, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.J.; Drakos, S.G.; Patel, S.R.; Lowes, B.D.; Selzman, C.H.; Starling, R.C.; Trivedi, J.; Slaughter, M.S.; Alturi, P.; Goldstein, D.; et al. Prospective Multicenter Study of Myocardial Recovery Using Left Ventricular Assist Devices (RESTAGE-HF [Remission from Stage D Heart Failure]): Medium-Term and Primary End Point Results. Circulation 2020, 142, 2016–2028. [Google Scholar] [CrossRef] [PubMed]
- Wever-Pinzon, O.; Drakos, S.G.; McKellar, S.H.; Horne, B.D.; Caine, W.T.; Kfouri, A.G.; Li, D.Y.; Fang, J.C.; Stehlik, J.; Selzmann, C.H. Cardiac Recovery During Long-Term LVAD Support. J. Am. Coll. Cardiol. 2016, 68, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Hetzer, R.; Müller, J.H.; Weng, Y.; Meyer, R.; Dandel, M. Bridging to Recovery. Ann. Thorac. Surg. 2001, 71, S109–S1013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Williamitis, C.A.; Slaughter, M.S. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: Is there an advantage to pulsatility? Ann. Cardiothorac. Surg. 2014, 3, 573–581. [Google Scholar] [PubMed]
- Blume, E.D.; Naftel, D.C.; Bastardi, H.J.; Ducan, B.W.; Kirklin, J.K.; Webber, S.A. Pediatric Heart Transplant Study Investigators: Outcomes of children bridged to heart transplantation with ventricular assist devices. Circulation 2006, 113, 2213–2219. [Google Scholar] [CrossRef]
- Hetzer, R.; Potapov, E.V.; Alexi-Meskishvili, V.; Weng, Y.; Miera, O.; Berger, F.; Hennig, E.; Hübler, M. Single-center experience with treatment of cardiogenic shock in children by pediatric assist devices. J. Thorac. Cardiovasc. Surg. 2011, 141, 616–623. [Google Scholar] [CrossRef][Green Version]
- Miera, O.; Germann, M.; Cho, M.Y.; Photiadis, J.; Delmo Walter, E.M.; Hetzer, R.; Berger, F.; Schmitt, K.R.L. Bridge to recovery in children on ventricular assist devices–Protocol, predictors of recovery, and long-term follow-up. J. Heart Lung Transplant. 2018, 37, 1459–1466. [Google Scholar] [CrossRef]
- Javier Delmo, E.M.; Javier, M.F.D.M.; Böthig, D.; Rüffer, A.; Cesnjevar, R.; Dandel, M.; Hetzer, R. Heart failure in the young: Insights into myocardial recovery with ventricular assist device support. Cardiovasc. Diagn. Ther. 2021, 11, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Devore, A.D.; Mentz, R.J.; Patel, C.B. Medical Management of Patients With Continuous-Flow Left Ventricular Assist Devices. Curr. Treat. Options Cariovasc. Med. 2014, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Raghuvir, R.; Eryazicu, P.; Macaluso, G.; Sharma, P.; Blair, C.; Tatooles, A.J.; Pappas, P.S.; Bhat, G. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. Ann. Thorac. Surg. 2013, 95, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Geens, J.; Rega, F.; Burkhoff, D.; Meyns, B. Continuous-flow left ventricular assist devices induce left ventricular reverse remodeling. J. Heart Lung Transplant. 2013, 32, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Feldman, D.; Banayosy, A.E.; Birks, E.; Blume, E.; Cowger, J.; Hayward, C.; Jorde, U.; Kremer, J.; MacGovan, G.; et al. The 2023 International Society for Heart and Lung Transplantation Guidelines for Mechanical Circulatory Support: A 10- Year Update. J. Heart Lung Transplant. 2023, 42, e1–e222. [Google Scholar] [CrossRef] [PubMed]
- Hnat, T.; Veselka, J.; Honek, J. Left ventricular reverse remodelling and its predictors in non-ischaemic cardiomyopathy. ESC Heart Fail. 2022, 9, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Antonidesb, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; de Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardiothorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef]
- Chaggar, P.S.; Williams, S.G.; Yonan, N.; Fildes, J.; Venkatesvaran, R.; Shaw, S.M. Myocardial recovery with mechanical circulatory support. Eur. J. Heart Fail. 2016, 18, 1220–1227. [Google Scholar] [CrossRef]
- Dandel, M.; Weng, Y.; Siniawski, H.; Stepanenko, A.; Krabatsch, T.; Potapov, E.; Lehmkuhl, H.B.; Knosalla, C.; Hetzer, R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: Criteria for weaning from ventricular assist devices. Eur. Heart J. 2011, 32, 1148–1160. [Google Scholar] [CrossRef]
- Simon, M.A.; Primack, B.A.; Teuteberg, J.; Kormos, R.L.; Bermudez, C.; Toyoda, Y.; Shah, H.; Gorcsan, J., 3rd; McNamara, D.M. Left ventricular remodeling and myocardial recovery on mechanical circulatory support. J. Card. Fail 2010, 16, 99–105. [Google Scholar] [CrossRef]
- Birks, E.J.; Selzman, C.H. Facilitating Myocardial recovery. In Mechanical Circulatory Support. A Companion to Braunwald’s Heart Disease; Kirklin, J.K., Rogers, J.G., Eds.; Elsevier: Philadelphia, PA, USA, 2020; pp. 223–241. [Google Scholar]
- Formica, P.; Murthy, S.; Edwards, P.; Goldstein, D.; Maybaum, S. A structured 3-step approach to evaluate cardiac recovery with continuous flow circulatory support. J. Heart Lung Transplant. 2010, 29, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- George, R.S.; Yacoub, M.H.; Tasca, G.; Webb, C.; Bowles, C.T.; Transley, P.; Hardy, J.P.; Dreyfus, G.; Khaghani, A.; Birks, E.J. Heamodynamic and echocardiographic responses to acute interruption of left ventricular assist device support—Relevance to assessment of myocardial recovery. J. Heart Lung Transplant. 2007, 26, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Birks, E.J.; George, R.S.; Hedger, M.; Bahrami, T.; Wilton, P.; Bowles, C.T.; Webb, C.; Bougard, R.; Amrani, M.; Yacoub, M.H.; et al. A Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: A prospective study. Circulation 2011, 123, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.G.; Thomas, J.G.; Freed, B.H.; Rich, J.D.; Sauer, A.J. Echocardiography and continuous flow left ventricular assist devices. JACC Heart Fail. 2015, 3, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Gyoten, T.; Amiya, E.; Kinoshita, O.; Tsuji, M.; Kimura, M.; Hatano, M.; Ono, M. Myocardial recovery evaluation from ventricular assist device in patients with dilated cardiomyopathy. ESC Heart Fail. 2022, 9, 2491–2499. [Google Scholar] [CrossRef]
- Estep, J.D.; Stainback, R.F.; Little, S.H.; Torre, G.; Zoghbi, W.A. The role of echocardiography and other imaging modalities in patients with left ventricular assist devices. JACC Cardiovasc. Imaging 2010, 3, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- George, R.S.; Sabharwal, N.K.; Webb, C.; Yacoub, M.H.; Bowles, C.T.; Hedger, M.; Khaghani, A.; Birks, E.J. Echocardiographic assessment of flow across continuous-flow ventricular assist devices at low speeds. J. Heart Lung Transplant. 2010, 29, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Okerberg, K.; Hernandez, A.; Miller, K.; Myers, T.J.; Radovancevic, B.; Delgado, R.M.; Gregoric, I.; Frazier, O.H. Assessment of myocardial recovery using dobutamin stress echocardiography in LVAD patients. J. Heart Lung Transplant. 2001, 20, 202–203. [Google Scholar] [CrossRef]
- Khan, T.; Delgado, R.M.; Radovancevic, B.; Torre-Amione, G.; Abrams, J.; Miller, K.; Myers, T.; Okerberg, K.; Stetson, S.J.; Gregoric, I.; et al. Dobutamine stress echocardiography predicts myocardial improvement in patients supported by left ventricular assist devices (LVADs): Hemodynamic and histologic evidence of improvement before LVAD explantation. J. Heart Lung Transplant. 2003, 22, 137–146. [Google Scholar] [CrossRef]
- Andersen, M.; Gustafsson, F.; Madsen, P.L.; Brassard, P.; Jensen, H.S.; Secher, N.; Hassager, C.; Nordsborg, R.; Mǿller, J.E. Hemodynamic stress echocardiography in patients supported with a continuous-flow left ventricular assist device. JACC Cardiovasc. Imaging 2010, 3, 854–859. [Google Scholar] [CrossRef]
- Topkara, V.K.; Chambers, K.T.; Yang, K.C.; Tzeng, H.-P.; Evans, S.; Weinheimer, C.; Kovacs, A.; Robbins, J.; Barger, P.; Mann, D.L. Functional significance of the discordance between transcriptional profile and left ventricular structure/function during reverse remodeling. JCI Insight 2016, 1, e86038. [Google Scholar] [CrossRef]
- Drakos, S.G.; Wever-Pinzon, O.; Selzman, C.H.; Gilbert, E.M.; Alharethi, R.; Reid, B.B.; Saidi, A.; Diakos, N.A.; Stoker, S.; Davis, E.S.; et al. Magnitude and time course of changes induced by continuous-flow left ventricular assist device unloading in chronic heart failure: Insights into cardiac recovery. J. Am. Coll. Cardiol. 2013, 61, 1985–1994. [Google Scholar] [CrossRef]
- Liang, H.; Lin, H.; Weng, Y.; Dandel, M.; Hetzer, R. Prediction of cardiac function after weaning from ventricular assist devices. J. Thorac. Cardiovasc. Surg. 2005, 130, 1555–1560. [Google Scholar] [CrossRef][Green Version]
- Jakovljevic, D.G.; Yacoub, M.H.; Schueler, S.; MacGowan, G.A.; Velicki, L.; Seferovic, P.M.; Hothi, S.; Tzeng, B.H.; Brodie, D.A.; Birks, E.; et al. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J. Am. Coll. Cardiol. 2017, 69, 1924–1933. [Google Scholar] [CrossRef]
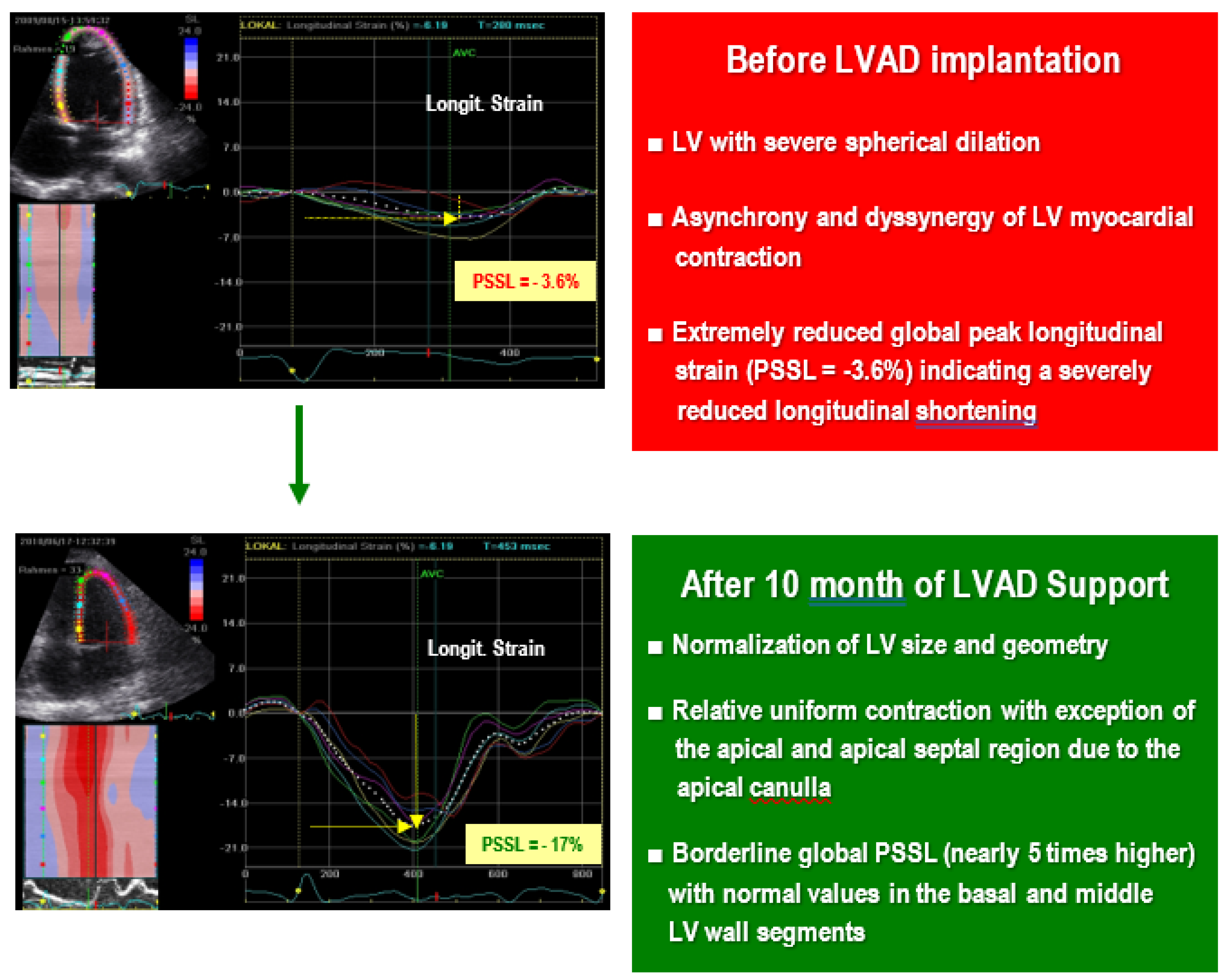
| Combined or Integrative Variables (Units) | Components | AUC (95% CI*) | Cutoff Values | PV (%) for RVF | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| TTE score (points [p]) [101] | FACRV; LAV index; estimated RAP | 0.73 | ≥5p | 75.0 | 64.0 |
| SWIRV (mm Hg • mL/m²) [102,103] | SWIRV = SV • (PAPm − CVP)/BSA | 0.63 | 450 | 42.6 | 75.8 |
| (0.52–0.72) | 300 | 72.2 | 54.9 | ||
| CVP/PCWP [103,104] | Central venous pressure (CVP) Pulmonary capillary wedge pressure (PCWP) | 0.68 (0.68–0.96) 0.82 | 0.63 - | 64.5 - | 53.3 - |
| Michigan RVFRS [p] [102] | |||||
| Vasopressor requirement, Bilirubin ≥ 2 mg/dL, AST ≥80 IU/L, and Crea ≥ 2.3 mg/dL | 0.73 | ≤3p | 70.9 | 79.6 | |
| (0.65–0.81) | |||||
| - | ≥5p | 80.0 | 73.7 | ||
| Michigan RVFRS + RV/LV [p] [102] | Michigan RVFRS components and RV/LV diameter ratio | 0.74 | - | - | - |
| Michigan RVFRS + PSSLRV [p] [98] | Michigan RVFRS components and RV peak systolic longitudinal strain | 0.77 | - | - | - |
| RVF risk score for RVAD need [p] [106] | Crea ≥ 1.9 mg/dL, CI ≤ 2.2L/min/m², SBP ≤ 96 mmHg, SWIRV ≤ 250 mmHg • ml/m², severe RV dysfunction, and previous cardiac surgery | - | <30p | 45.3 | 96.2 |
| - | ≥65p | 89.5 | 71.5 | ||
| RVF risk score [p] [105] | |||||
| PVR, inotrope dependency, obesity, destination therapy, ACE inhibitor and/or AT II receptor blocker therapy, and β-blocker therapy | 0.74 ± 0.04 | ≥12p | 83.3 | 69.3 | |
| - | ≤5p | 56.0 | 88.9 | ||
| Quantitative preoperative risk score (CRITT) [p] [108] | RV dysfunction, CVP, TR, tachycardia, and intubation | 0.80 | ≥4p | 80.0 | - |
| (0.72–0.88) | |||||
| - | <2p | - | 93.0 | ||
| Modified LV-echo-for-RV score [p] [79] | LVEDD, LVEF, LAD/LVEDD, SWIRV, and serum bilirubin and albumin | 0.79 | ≥7p | 93.4 | - |
| ≤3p | - | 97.1 | |||
| RV load-corrected PSSrL (mmHg/s) [89] | Peak systolic longitudinal strain rate (PSSrL) and pressure gradient between RV and RA (∆PRV-RA) | 0.95 | 24 | 97 | 87 |
| (0.93–1.0) | (84–99) | (77–93) | |||
| RV load adaptation index (LAI) (dimensionless) [97] | TR velocity-time integral, RV end-diastolic area, and RV long axis length | 0.97 | 14 | 83 | 97 |
| (0.97–0.99) | (73–88) | (95–99) | |||
| Echo-derived RVGWE [p] [99] | RV global strain, pulmonary pressure, and cardiac cycle timings | 0.92 | 77% | sensitivity 100% specificity 82% | |
| Pulmonary arterial pulsatility index (PAPi) [109,110] | Pulmonary artery systolic pressure, pulmonary artery diastolic pressure, and right atrial pressure (RAP) PAPi = (PAPs − PAPd)/RAP | 0.77 [101] | 2 | sensitivity 67% specificity 74% | |
| 0.94 [102] | 1.85 | sensitivity 94%, specificity 81% | |||
| Post-NTP PAPi combined with FAC and PAPs [p] [110] | Post-sodium nitroprusside (NTP) pulmonary artery pulsatility index, fractional area change (FAC), and pulmonary artery systolic pressure (PAPs) | 0.95 (0.89–1.0) | - | Predictive accuracy 90.7% | |
| ECHO Modes | Relevant Measurements and Parameter Calculations |
|---|---|
| M-mode and 2D [32,68,86,117,123,136,144,146,147,148,150,152,159] | - Left ventricular end-diastolic and end-systolic diameter [LVEDD and LVESD, respectively]; - LV end-diastolic short/long axis ration [LV-S/LED]; - LV end-diastolic relative wall thickness * [RWTED = (septum thickness + posterior wall thickness)/LVEDD]; - LV fractional shortening [FS] and LV ejection fraction [LVEF] measured by biplane Simpson’s method; - Right ventricular [RV] end-diastolic dimensions (measured on parasternal and apical views); - Tricuspid annular plane systolic excursion [TAPSE], measured by placing the M-mode tracer in the tricuspid annulus just against the RV free wall; - RV fractional area change [FAC] and ejection fraction [RVEF]. |
| Pulsed wave (PW) and Color Doppler [32,68,86,117,123,136,144,146,147,148,150,152] | - Doppler indices of LV diastolic function (isovolumetric relaxation time, trans-mitral flow velocities); - LV stroke volume [SV] measured at the LV outflow tract; - Detection and severity grading of aortic, mitral, tricuspid, and pulmonary valve regurgitation. |
| Continuous wave (CW) Doppler [32,68,86,117,123,136,144,146,148,152] | - Pressure gradient between the RV and right atrium [∆PRV-RA] calculated from the mean velocity of the tricuspid valve regurgitation [TR] jet; - TR velocity-time integral [VTITR] for calculation of the RV load adaptation index [LAI]; - Pulmonary artery systolic pressure estimation from the peak velocity of the TR jet; |
| Pulsed Tissue Doppler (PW-TD) [32,86,117,123,150] | - LV systolic wall motion peak velocity [Sm] at the basal posterior wall; - Mitral annulus early diastolic peak velocity [e’] for calculation of the E/e’ ratio; - Tricuspid lateral annulus peak systolic velocity [TAPS’]; |
| Speckle tracking 2D and 3D Strain imaging [59,86,98,117,123,159] | - LV global longitudinal and circumferential strain and strain rate; - Calculation of the afterload-corrected RV global peak systolic longitudinal strain rate [cPSSrL]; - Evaluation of LV synchrony and synergy of contraction. - LV torsion assessed by 3D strain imaging |
| Type of Investigation | Relevant Parameters and Threshold Values for Weaning Decision-Making |
|---|---|
| Electrocardiography [115,117] | - Sinus rhythm, heart rate (HR) <90/min - No more than 25% HR increase during off-pump trials |
| Echocardiography [117,123,144] | - LV end-diastolic diameter ≤55 mm (or ≤30 mm/m² BSA), stable after maximum improvement * - LV ejection fraction ≥45%, stable after maximum improvement * - Stable stroke volume (SV) during the final off-pump trial - Systolic wall motion peak velocity † (Sm) ≥8 cm/s, stable after maximum improvement - No or <grade II mitral and/or aortic valve regurgitation - No RV dilation (RVOT <35mm, end-diastolic short/long axis ratio <0.6) - No or ≤grade II tricuspid and/or pulmonary valve regurgitation |
| Right Heart Catheterization [72,115,117,123] | - Cardiac index (CI) >2.6 L/min/m² BSA - Pulmonary capillary wedge pressure (PCWP) <13 mmHg - Mean right atrial pressure (RAPm) < 10 mmHg - Diastolic pulmonary gradient (DPG) ‡ < 7 mmHg |
| Arterial Pressure [117,123] | - Mean systemic arterial pressure ≥ 65 mmHg during off-pump trials in CF-LVAD recipients |
| Pre-Explant Measurements at Off-Pump Trials | PV for ≥5 Years Post-Weaning Cardiac Stability [30,108,129] | ||
|---|---|---|---|
| Examination | Parameters and Threshold Value | Measurement Details | |
| ECHO | LVEF ≥45% | - measured at the last off-pump trial - stable from maximum improvement to LVAD explantation | 74% 86% |
| LVEF ≥45% plus LVEDD ≤55 mm | - measured at the last off-pump trial - both LVEF and LVEDD stable from maximum improvement to LVAD explantation | 86% 94% | |
| LVEF ≥45% plus RWTED ≥0.38 | - measured at the last off-pump trial | 87% | |
| LVEF ≥45% plus Sm ≥8 cm/s | - both LVEF and Sm stable from maximum improvement to LVAD explantation | 87% | |
| SV (PW Doppler-derived VTI in the LVOT) Absence or ≤ grade 1 AR and/or MR No RV dilation (RVOT <35 mm, S/L <0.6) Absence or ≤ grade 2 TR and/or PR | - stable during the pre-explant off-pump trials - assessed at the last off-pump trial - assessed at the last off-pump trial - assessed at the last off-pump trial | All are required preconditions for successful weaning. Alone, none of these parameters can predict post-weaning cardiac stability. | |
| RHC | Cardiac index (CI) >2.6 L/m² BSA PCWP <13 mmHg RAP <10 mmHg | - measured during the last off-pump trial - measured during the last off-pump trial - measured during the last off-pump trial | RHC alone cannot predict post-weaning cardiac stability, but the limit values are required preconditions for successful weaning. |
| Risk Factors for Heart Failure * (HF) Recurrence after Left Ventricular Assist Device (LVAD) Explantation | PV for HF Recurrence during the First 3 Years after LVAD Explantation [30,108,129,143,145] |
|---|---|
| Pre-implant HF duration >3 years | 78% |
| Left ventricular ejection fraction (LVEF) 35–45% at the final pre-explant off-pump trial | 88% |
| LVEF 35–45% at the final pre-explant off-pump trial in patients with pre-implant HF duration of >5 years | 100% |
| LVEF ≥45% but unstable (i.e., pre-explant alteration of >10% of the best LVEF value achieved until then) | 90% |
| LV end-diastolic diameter (LVEDD) 56–66 mm | 90% |
| No LVEDD normalization plus persistence of LV geometry alterations (RWTED <0.38) despite of optimal LVEF (≥45%) | 89% |
| LVEF ≥45% but unstable geometry (RWTED reduction of >8%, or S/LED increase of >10% at the final off-pump trial) | 87% and 85%, respectively |
| Unstable LVEF ≥45% with either reduced wall motion velocity (Sm < 8 cm/s) or unstable Sm (alteration of >10% during the final off-pump trial) | 83% and 90%, respectively |
| SV reduction (i.e., VTI reduction in the LVOT during the final off-pump trial) | All these parameter abnormalities are validated risk factors for early recurrence of HF after LVAD explantation. Precise information on their predictive value for early recurrence of HF after LVAD removal is currently not available. |
| Relevant LV diastolic stiffness despite optimal LVEF ≥45% | |
| LVEF 45–50% with concomitant MR grade 1–2 which can induce misleading overestimation of LVEF | |
| Systemic APd ≤ 50mmHg (can misleadingly increase the LVEF) | |
| Asynchrony or dyssynergy of LV contraction at the final off-pump trial | |
| RV size and geometry alteration, and/or a reduced LAIRV during the final off-pump trial | |
| TR new appearance or aggravation with or without jet velocity increase during the final off-pump trial. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dandel, M. Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy. J. Clin. Med. 2023, 12, 6451. https://doi.org/10.3390/jcm12206451
Dandel M. Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(20):6451. https://doi.org/10.3390/jcm12206451
Chicago/Turabian StyleDandel, Michael. 2023. "Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy" Journal of Clinical Medicine 12, no. 20: 6451. https://doi.org/10.3390/jcm12206451
APA StyleDandel, M. (2023). Cardiological Challenges Related to Long-Term Mechanical Circulatory Support for Advanced Heart Failure in Patients with Chronic Non-Ischemic Cardiomyopathy. Journal of Clinical Medicine, 12(20), 6451. https://doi.org/10.3390/jcm12206451





