Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
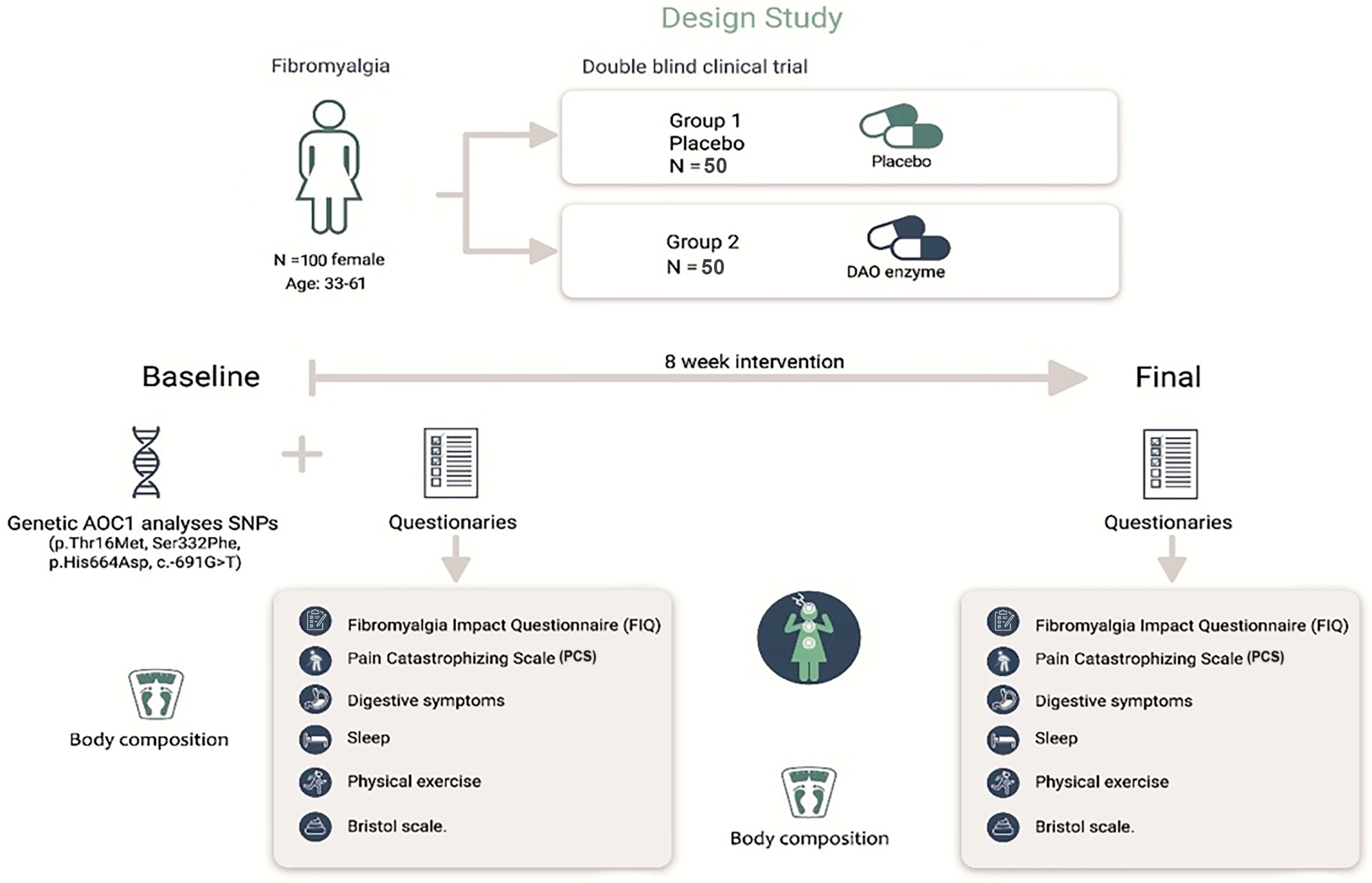
2.1. Design
2.2. Sample
2.3. Intervention
2.4. Fibromyalgia Impact Questionnaire (FIQ)
2.5. Pain Catastrophizing Scale (PCS)
2.6. Clinical Symptoms
2.7. Statistical Analysis
3. Results
3.1. Impact of Fibromyalgia and Pain Catastrophizing
3.2. Clinical Symptoms
3.3. Responders
3.4. Concomitant Medication
3.5. Stepwise Therapeutic Regimen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerdle, B.; Björk, J.; Cöster, L.; Henriksson, K.; Henriksson, C.; Bengtsson, A. Prevalence of widespread pain and associations with work status: A population study. BMC Musculoskelet. Disord. 2008, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet. Disord. 2007, 8, 27. [Google Scholar] [CrossRef]
- Wolfe, F.; Ross, K.; Anderson, J.; Russell, I.J.; Hebert, L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995, 38, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Tsilioni, I.; Bawazeer, M. Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome. Front. Cell. Neurosci. 2019, 13, 353. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Taylor, S.J.; Steer, M.; Ashe, S.C.; Furness, P.J.; Haywood-Small, S.; Lawson, K. Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia. Scand. J. Pain 2019, 19, 167–181. [Google Scholar] [CrossRef]
- Gomez-Arguelles, J.M.; Caceres, O.; Blanco, M.; Maestu, C.; Martin, F. Improvement of digestive symptoms in fibromyalgia patients following a diet modification according to histamine release test—An observational study. Reumatologia 2022, 60, 209–212. [Google Scholar] [CrossRef]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’mahony, L. Histamine and gut mucosal immune regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef]
- Jarisch, R.; Wantke, F. Wine and headache. Int. Arch. Allergy Immunol. 1996, 110, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kovacova-Hanuskova, E.; Buday, T.; Gavliakova, S.; Plevkova, J. Histamine, histamine intoxication and intolerance. Allergol. Immunopathol. 2015, 43, 498–506. [Google Scholar] [CrossRef]
- Song, W.; Lv, Y.; Zhang, Z.; Li, Y.; Xiao, L.; Yu, X.; Wang, Y.; Ji, H.; Ma, L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J. Gastroenterol. WJG 2009, 15, 3916. [Google Scholar] [CrossRef]
- DR Healthcare. Enzyme Diamine Oxidase (DAO): A New Biomarker for Fibromyalgia, Chronic Fatigue and Generalized Muscle Pain [Internet]. 2021. Available online: https://dr-healthcare.com/en/portfolio/fibromyalgia-chronic-fatigue-and-muscle-pain-produced-by-dao-deficiency/ (accessed on 22 March 2023).
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Okutan, G.; Ruiz Casares, E.; Perucho Alcalde, T.; Sánchez Niño, G.M.; Penadés, B.F.; Terrén Lora, A.; Torrente Estríngana, L.; López Oliva, S.; San Mauro Martín, I. Prevalence of Genetic Diamine Oxidase (DAO) Deficiency in Femalepatients with Fibromyalgia in Spain. Biomedicines 2023, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Schooner, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Yacoub, M.R.; Ramirez, G.A.; Berti, A.; Mercurio, G.; Breda, D.; Saporiti, N.; Burastero, S.; Dagna, L.; Colombo, G. Diamine Oxidase Supplementation in Chronic Spontaneous Urticaria: A Randomized, Double-Blind Placebo-Controlled Study. Int. Arch. Allergy Immunol. 2018, 176, 268–271. [Google Scholar] [CrossRef]
- Monterde, S.; Salvat, I.; Montull, S.; Fernández-Ballart, J. Validación de la versión española del Fibromyalgia Impact Questionnaire. Rev. Esp. Reumatol. 2004, 31, 507–513. [Google Scholar]
- Campayo, J.G.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clin. 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Dailey, D.L.; Frey Law, L.A.; Vance, C.G.; Rakel, B.A.; Merriwether, E.N.; Darghosian, L.; Golchha, M.; Geasland, K.M.; Spitz, R.; Crofford, L.J.; et al. Perceived function and physical performance are associated with pain and fatigue in women with fibromyalgia. Arthritis Res. Ther. 2016, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Mace, R.A.; Popok, P.J.; Kulich, R.J.; Patel, K.V.; Burns, J.W.; Somers, T.J.; Keefe, F.J.; Schatman, M.E.; Vranceanu, A.M. Psychosocial Correlates of Objective, Performance-Based, and Patient-Reported Physical Function Among Patients with Heterogeneous Chronic Pain. J. Pain Res. 2020, 13, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
- Estévez-López, F.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; Soriano-Maldonado, A.; Borges-Cosic, M.; Pulido-Martos, M.; Aparicio, V.A.; Carbonell-Baeza, A.; Delgado-Fernández, M.; Geenen, R. The discordance between subjectively and objectively measured physical function in women with fibromyalgia: Association with catastrophizing and self-efficacy cognitions. Al-Ándalus Proj. Disabil. Rehabil. 2018, 40, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Sarzi-Puttini, P.; Tölle, T.R.; Wolfe, F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: Systematic review and meta-analysis. Clin. Exp. Rheumatol. 2012, 30 (Suppl. S74), 78–87. [Google Scholar]
- Maintz, L.; Benfadal, S.; Allam, J.; Hagemann, T.; Fimmers, R.; Novak, N. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J. Allergy Clin. Immunol. 2006, 117, 1106–1112. [Google Scholar] [CrossRef]
- Maintz, L.; Yu, C.F.; Rodríguez, E.; Baurecht, H.; Bieber, T.; Illig, T.; Weidinger, S.; Novak, N. Association of single nucleotide polymorphisms in the diamine oxidase gene with diamine oxidase serum activities. Allergy 2011, 66, 893–902. [Google Scholar] [CrossRef]
- Mušič, E.; Korošec, P.; Šilar, M.; Adamič, K.; Košnik, M.; Rijavec, M. Serum diamine oxidase activity as a diagnostic test for histamine intolerance. Wien. Klin. Wochenschr. 2013, 125, 239–243. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Lackner, S.; Enko, D.; Schenk, M.; Holasek, S.J.; Mangge, H. Evaluation of symptoms and symptom combinations in histamine intolerance. Intest. Res. 2019, 17, 427–433. [Google Scholar] [CrossRef]
- Gutiérrez Cabano, C.A. Histamina y secreción ácida gástrica [Histamine and gastric acid secretion]. Acta Gastroenterol. Latinoam. 1980, 10, 77–84. [Google Scholar]
- Yamada, S.; Guo, X.; Wang, K.Y.; Tanimoto, A.; Sasaguri, Y. Novel function of histamine signaling via histamine receptors in cholesterol and bile acid metabolism: Histamine H2 receptor protects against nonalcoholic fatty liver disease. Pathol. Int. 2016, 66, 376–385. [Google Scholar] [CrossRef][Green Version]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet. Disord. 2020, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef] [PubMed]
- Nosková, E.; Vochosková, K.; Knop, V.; Stopková, P.; Kopeček, M. Histamine intolerance and anxiety disorders: Pilot cross-sectional study of histamine intolerance prevalence in cohort of patients with anxiety disorders. Eur. Psychiatry. 2022, 65 (Suppl. S1), S387–S388. [Google Scholar] [CrossRef]
- Tao, R.; Fu, Z.; Xiao, L. Chronic Food Antigen-specific IgG-mediated Hypersensitivity Reaction as A Risk Factor for Adolescent Depressive Disorder. Genom. Proteom. Bioinform. 2019, 17, 183–189. [Google Scholar] [CrossRef]

| DAO (n = 50) | Placebo (n = 50) | Total | |
|---|---|---|---|
| Age | 48.48 ± 7.17 | 48.48 ± 7.60 | 48.48 ± 7.35 |
| BMI (Kg/m2) | 27.59 ± 5.08 | 27.65 ± 5.77 | 27.62 ± 5.41 |
| Nationality, n (%) | |||
| Spanish | 45 (90) | 48 (96) | 93 (93) |
| Romanian | 2 (4) | 0 (0) | 2 (2) |
| Ecuadorian | 1 (2) | 1 (2) | 2 (2) |
| Peruvian | 0 (0) | 1 (2) | 1 (1) |
| Bolivian | 1 (2) | 0 (0) | 1 (1) |
| Japanese | 1 (2) | 0 (0) | 1 (1) |
| Final—Baseline DAO Group | Final—Baseline Placebo Group | ||||
|---|---|---|---|---|---|
| Mean Change (95% CI) | p-Value | Mean Change (95% CI) | p-Value | ||
| FIQ | |||||
| Physical function | −0.22 (−0.78, 0.34) | 0.441 | −0.25 (−0.82, 0.32) | 0.390 | |
| Feel good | −0.77 (−1.25, −0.30) | 0.002 | −0.56 (−1.03, −0.09) | 0.021 | |
| Work missed | 1.13 (−0.23, 2.50) | 0.100 | 0.70 (−0.62, 2.03) | 0.288 | |
| Job ability | −0.24 (−0.74, 0.27) | 0.353 | −0.37 (−0.87, 0.13) | 0.146 | |
| Pain | −0.22 (−0.67, 0.24) | 0.348 | −0.36 (−0.81, 0.09) | 0.118 | |
| Fatigue | −0.71 (−1.23, −0.19) | 0.008 | −0.40 (−0.91, 0.11) | 0.125 | |
| Morning tiredness | −0.43 (−0.95, 0.09) | 0.107 | −0.53 (−1.05, −0.01) | 0.046 | |
| Stiffness | −0.74 (−1.23, −0.26) | 0.003 | −0.55 (−1.03, −0.07) | 0.026 | |
| Anxiety | −0.71 (−1.27, −0.15) | 0.014 | −0.49 (−1.05, 0.74) | 0.088 | |
| Depression | −0.70 (−1.33, −0.07) | 0.030 | −0.42 (−1.05, 0.20) | 0.182 | |
| Total score | −3.97 (−7.07, −0.86) | 0.013 | −4.67 (−7.76, −1.59) | 0.003 | |
| PCS | |||||
| Rumination | −1.49 (−2.41, −0.57) | 0.002 | −1.10 (−2.01, −0.18) | 0.020 | |
| Magnification | −0.68 (−1.30, −0.05) | 0.035 | −0.75 (−1.37, −0.13) | 0.019 | |
| Helplessness | −3.01 (−4.26, −1.75) | <0.0001 | −1.79 (−3.04, −0.55) | 0.005 | |
| Total score | −5.14 (−7.48, −2.79) | <0.0001 | −3.63 (−5.96, −1.30) | 0.003 |
| Final—Baseline DAO Group | Final—Baseline Placebo Group | ||||
|---|---|---|---|---|---|
| Mean Change (95% CI) | p-Value | Mean Change (95% CI) | p-Value | ||
| Sleep | |||||
| Sleep quality | 0.13 (−0.42, 0.68) | 0.646 | 0.45 (−0.09, 1.00) | 0.102 | |
| Atopic dermatitis | |||||
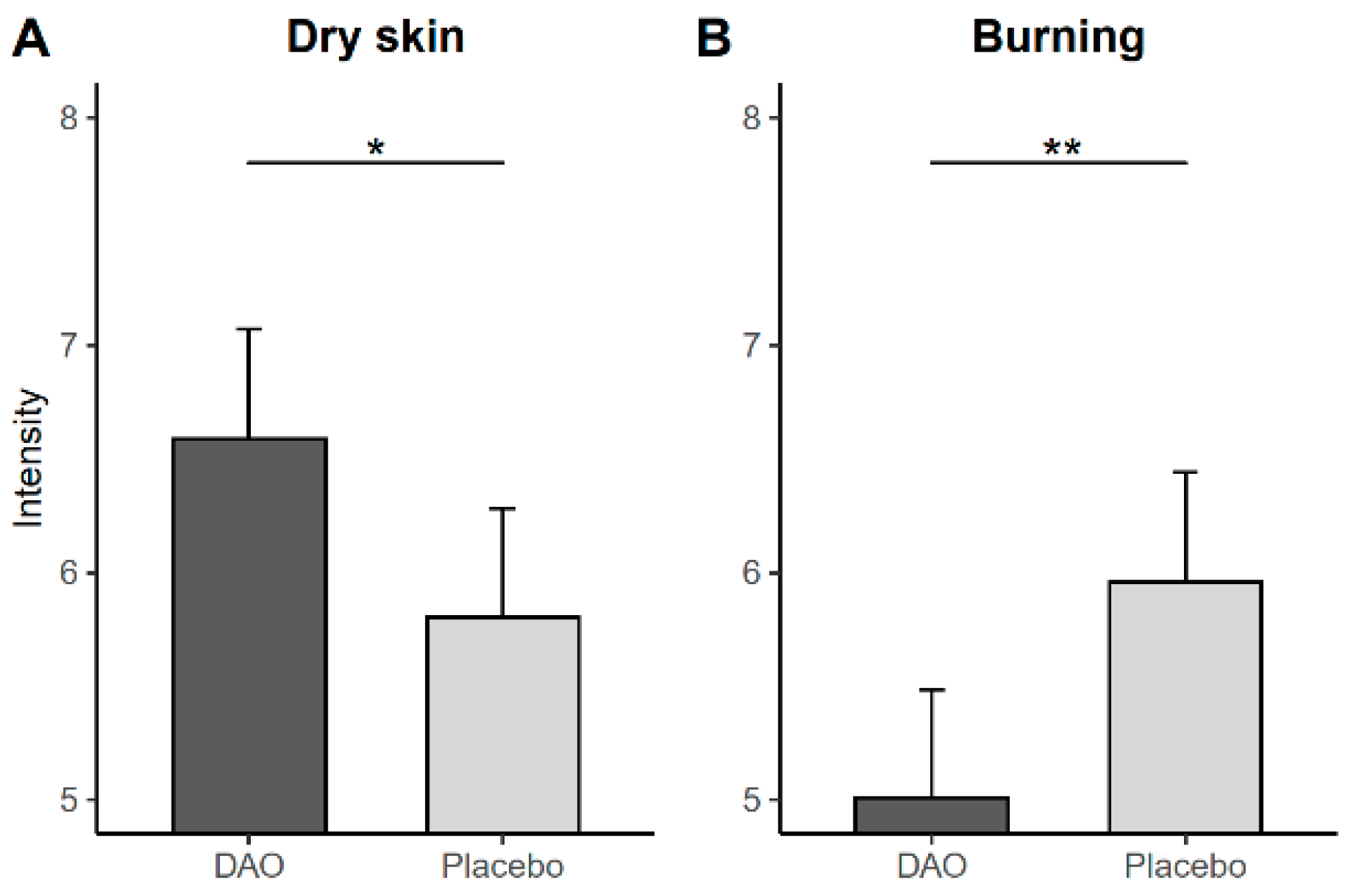
| Dry skin | 0.13 (−0.58, 0.85) | 0.712 | −0.62 (−1.33, −0.09) | 0.086 | |
| Hives | −0.34 (−1.15, 0.48) | 0.419 | −0.97 (−1.78, −0.16) | 0.019 | |
| Eczema | 0.80 (−0.32, 1.92) | 0.161 | 0.30 (−0.83, 1.43) | 0.597 | |
| Migraine | |||||
| Migraine | −0.24 (−1.04, 0.57) | 0.562 | −0.58 (−1.38, 0.22) | 0.150 | |
| GI disorders | |||||
| Bloating | −0.34 (−0.81, 0.12) | 0.148 | −0.66 (−1.13, −0.20) | 0.005 | |
| Abdominal pain | −0.22 (−0.80, 0.35) | 0.443 | −0.39 (−0.97, 0.19) | 0.182 | |
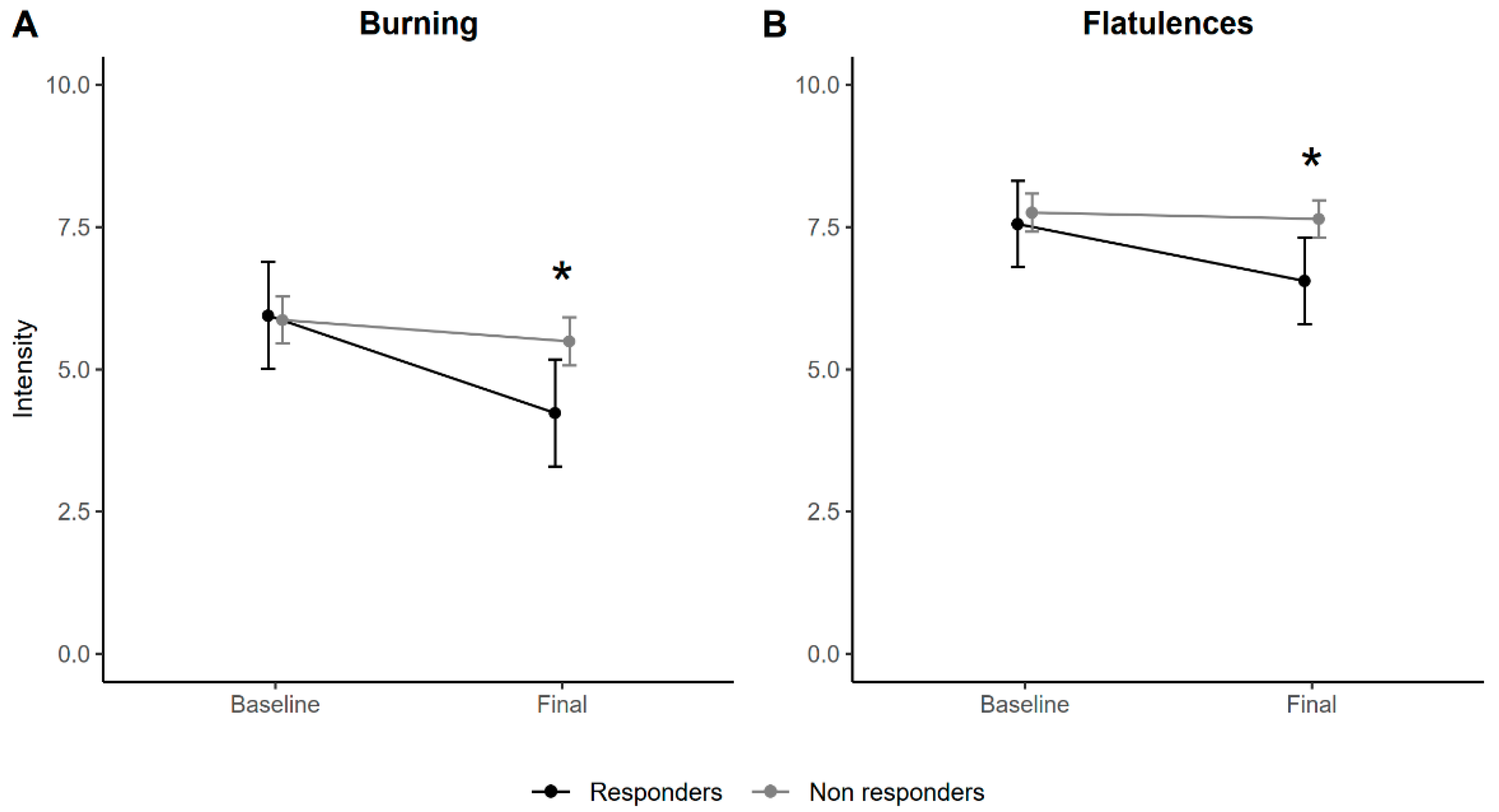
| Burning | −0.58 (−1.24, 0.08) | 0.086 | 0.43 (−0.25, 1.10) | 0.209 | |
| Flatulence | −0.25 (−0.78, 0.29) | 0.366 | −0.60 (−1.14, 0.06) | 0.030 | |
| Bristol scale (1–7) | 0.02 (−0.40, 0.40) | 0.991 | 0.10 (−0.29, 0.50) | 0.606 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okutan, G.; Sánchez Niño, G.M.; Terrén Lora, A.; López Oliva, S.; San Mauro Martín, I. Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial. J. Clin. Med. 2023, 12, 6449. https://doi.org/10.3390/jcm12206449
Okutan G, Sánchez Niño GM, Terrén Lora A, López Oliva S, San Mauro Martín I. Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial. Journal of Clinical Medicine. 2023; 12(20):6449. https://doi.org/10.3390/jcm12206449
Chicago/Turabian StyleOkutan, Gülşah, Guerthy Melissa Sánchez Niño, Ana Terrén Lora, Sara López Oliva, and Ismael San Mauro Martín. 2023. "Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial" Journal of Clinical Medicine 12, no. 20: 6449. https://doi.org/10.3390/jcm12206449
APA StyleOkutan, G., Sánchez Niño, G. M., Terrén Lora, A., López Oliva, S., & San Mauro Martín, I. (2023). Exogenous Supplementation with DAO Enzyme in Women with Fibromyalgia: A Double-Blind Placebo-Controlled Clinical Trial. Journal of Clinical Medicine, 12(20), 6449. https://doi.org/10.3390/jcm12206449






