Unexpected and Rare Sites of Metastasis in Oncologic Patients
Abstract
1. Introduction
2. Rare and Exclusive Cases Description
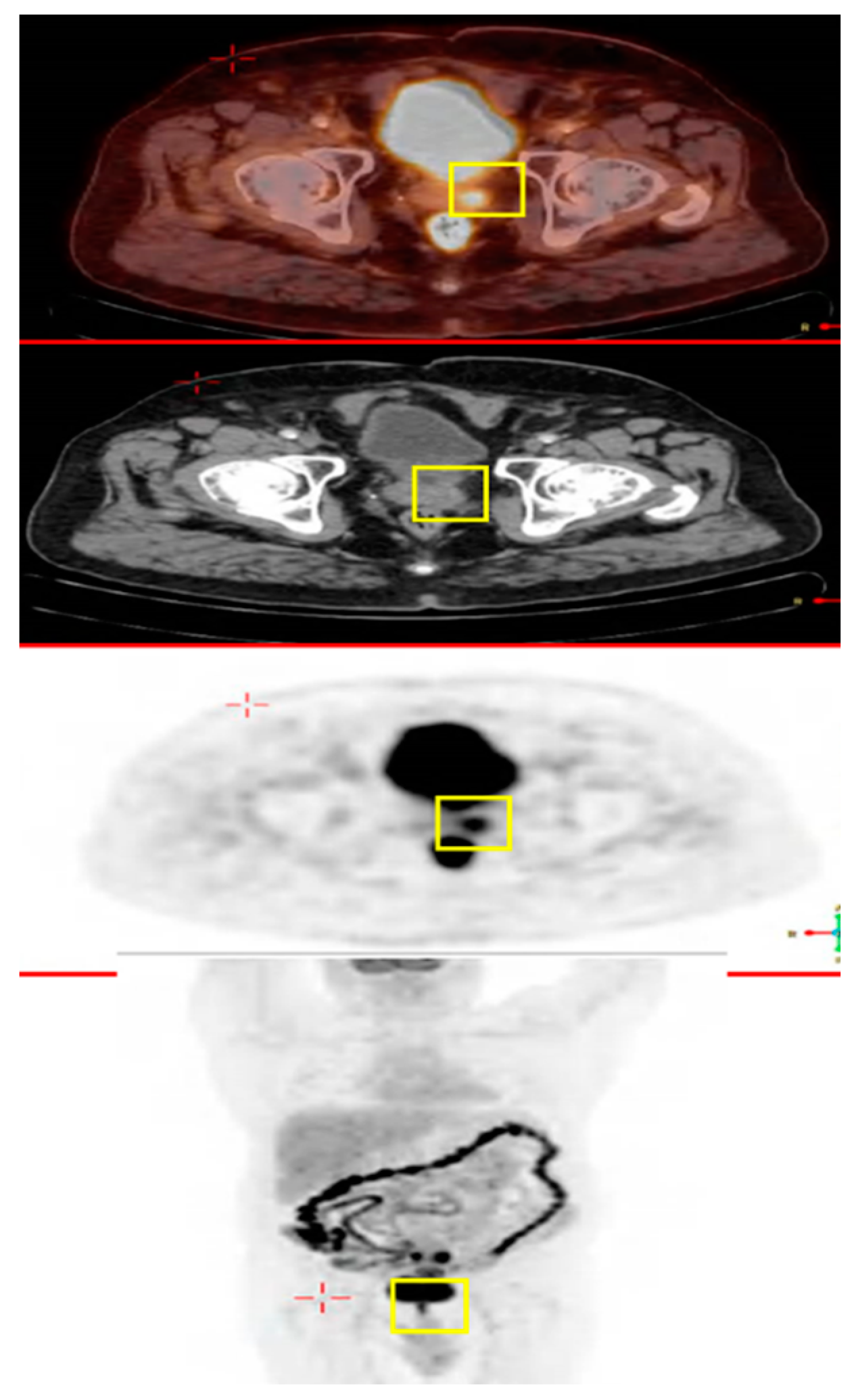
2.1. A 69 Year Old Male with Colon Cancer Metastasized to the Prostate Gland
2.2. A Male with Colon Cancer Metastasized to the Prostate Gland
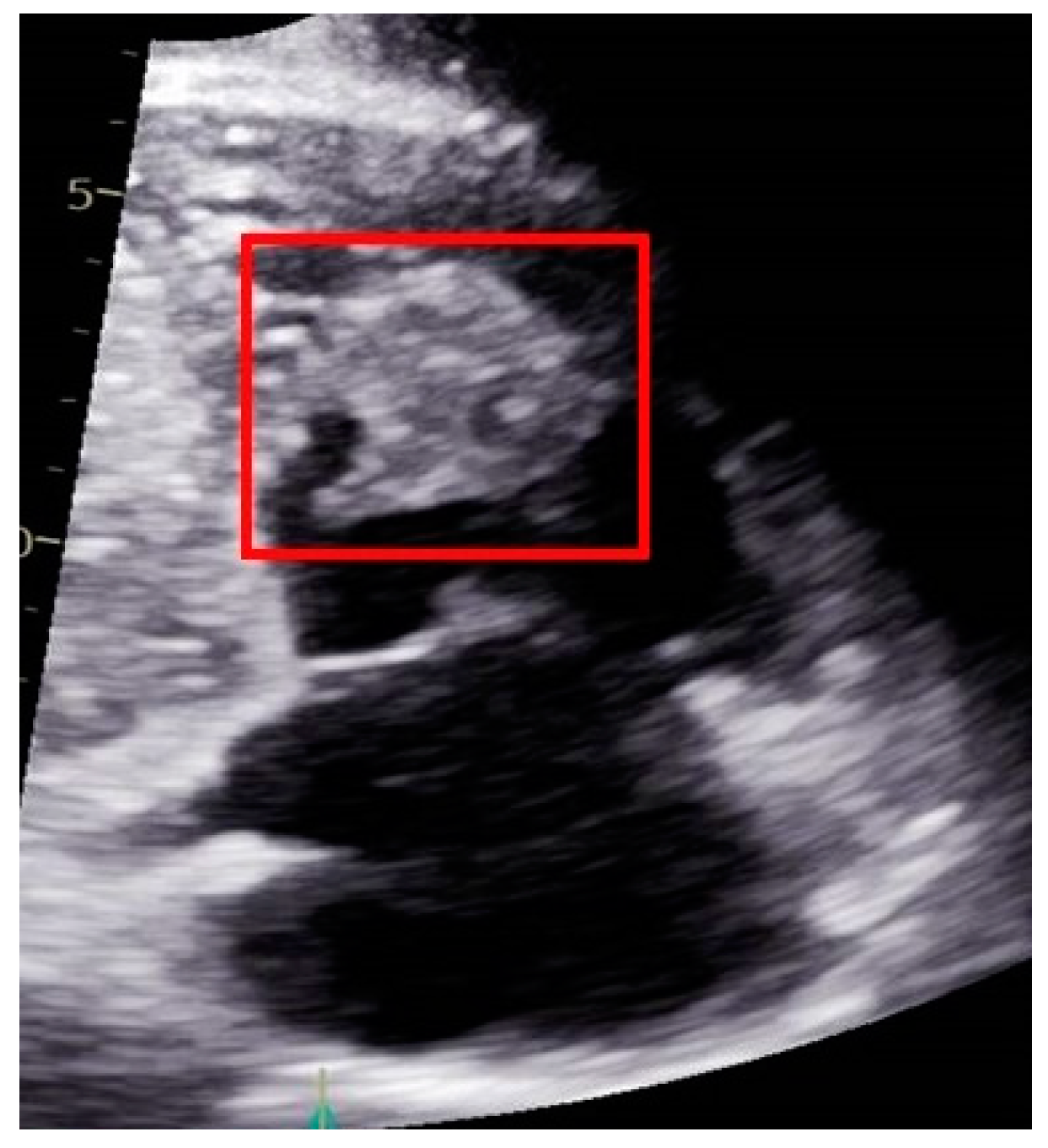
2.3. A Male with Pancreatic Cancer Metastasized to the Right Ventricle of the Heart
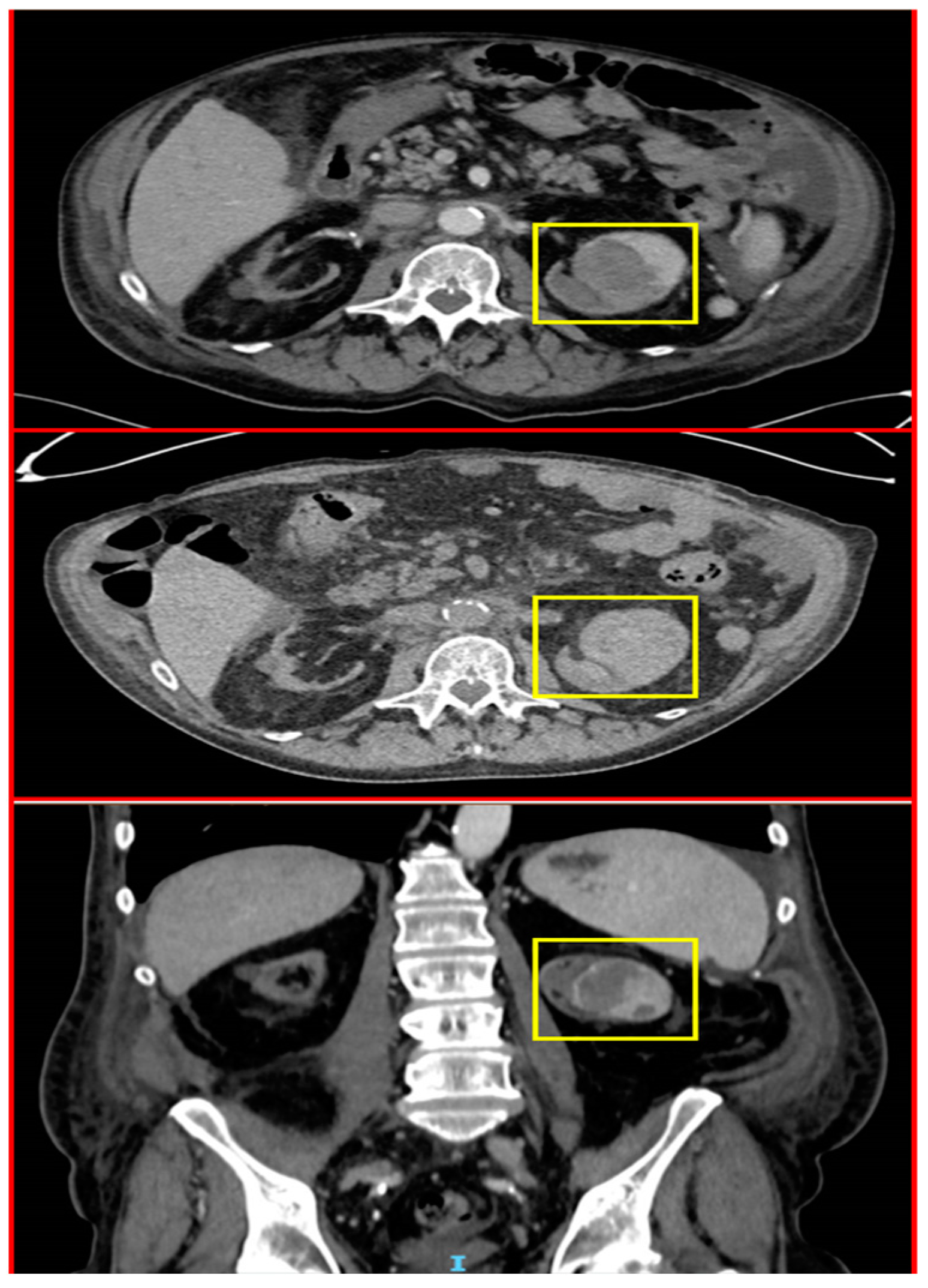
2.4. A Male with Melanoma Metastasized to the Pancreas
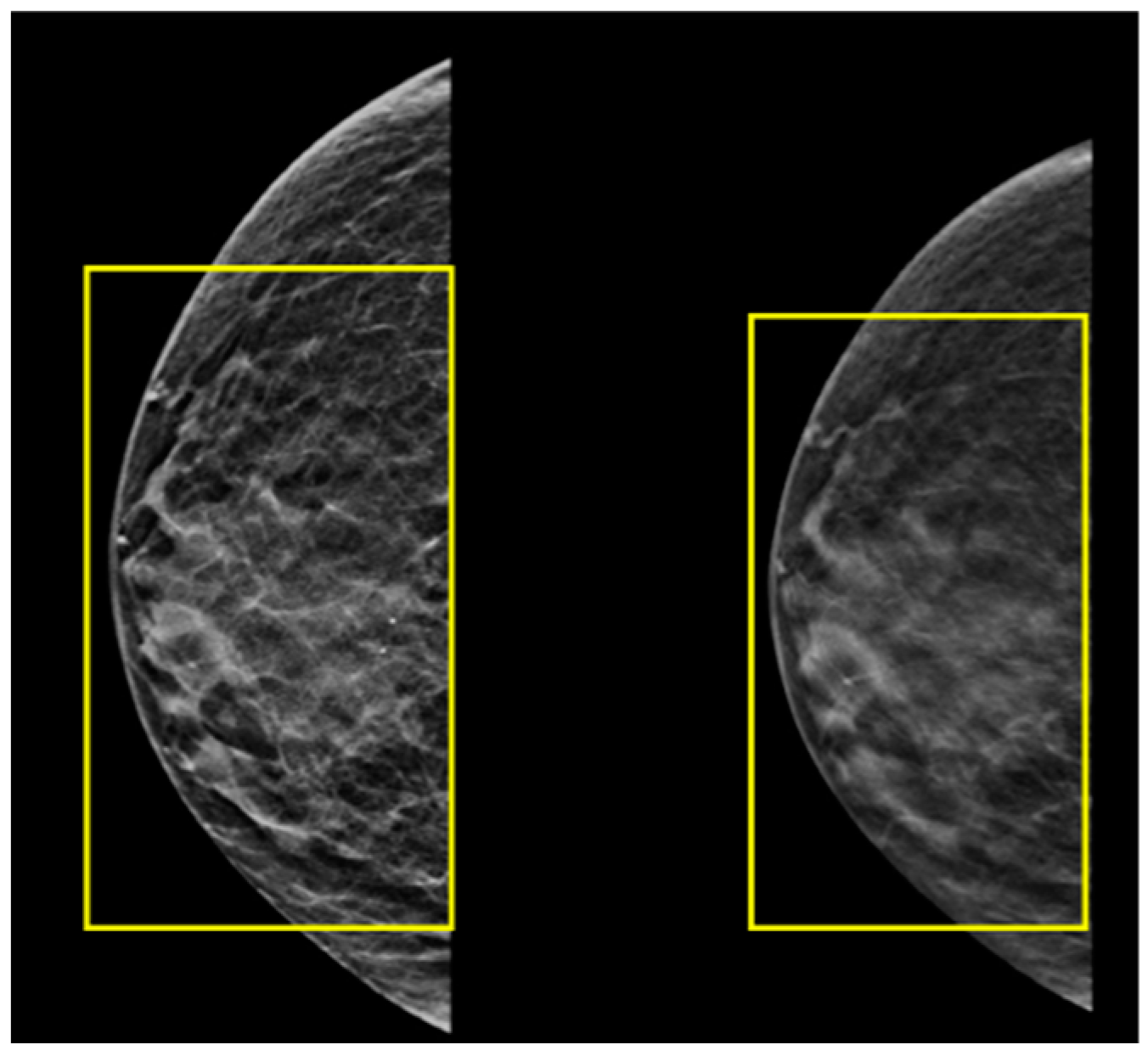
2.5. A Male with Breast Cancer Metastasized to the Left Kidney
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality, worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Moot, A.R.; Polglase, A.; Giles, G.G.; Garson, O.M.; Thursfield, V.; Gunter, D. Men with colorectal cancer are predisposed to prostate cancer. ANZ J. Surg. 2003, 73, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Choi, Y.J.; Shin, D.W.; Han, K.D.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Secondary Primary Prostate Cancer after Colorectal Cancer: A Nationwide Population-based Cohort Study in Korea. J. Cancer Prev. 2017, 22, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Stein, U.; Schlag, P.M. Clinical, Biological, and Molecular Aspects of Metastasis in Colorectal Cancer. In Targeted Therapies in Cancer. Recent Results in Cancer Research; Dietel, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 176. [Google Scholar] [CrossRef]
- CancerCare. Treatment Update: Metastatic Prostate Cancer–Connect Booklet. Available online: https://www.cancercare.org/publications/180-treatment_update_metastatic_prostate_cancer (accessed on 1 March 2023).
- Routh, J.C.; Leibovich, B.C. Adenocarcinoma of the prostate: Epidemiological trends, screening, diagnosis, and surgical management of localized disease. Mayo Clin. Proc. 2005, 80, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Dulskas, A.; Cereska, V.; Zurauskas, E.; Stratilatovas, E.; Jankevicius, F. Prostate cancer solitary metastasis to anal canal: Case report and review of literature. BMC Cancer 2019, 19, 374. [Google Scholar] [CrossRef]
- Patel, H.; Kumar, A.; Shaaban, H.; Nguyen, N.; Baddoura, W.; Maroules, M.; Shaikh, S. Synchronous metastasis of prostate adenocarcinoma to the stomach and colon: A case report. N. Am. J. Med. Sci. 2014, 6, 152–154. [Google Scholar] [CrossRef]
- Berman, J.R.; Nunnemann, R.G.; Broshears, J.R.; Berman, I.R. Sigmoid colon carcinoma metastatic to prostate. Urology 1993, 41, 150–152. [Google Scholar] [CrossRef]
- Kang, A.; Lee, J.H.; Lin, E.; Westerhoff, M. Metastatic colon carcinoma to the prostate gland. J. Comput. Assist. Tomogr. 2013, 37, 463–465. [Google Scholar] [CrossRef]
- Youssef, F.R.; Hunt, L.; Meiring, P.D.; Taraporewalla, D.R.; Gupta, R.; James, M.J. Metastasis of a cecal adenocarcinoma to the prostate five years after a right hemicolectomy: A case report. J. Med. Case Rep. 2011, 5, 223. [Google Scholar] [CrossRef]
- Gupta, T.; Laskar, S.G.; Thakur, M.; Desai, S.; Shrivastava, S.K.; Dinshaw, K.A.; Agarwal, J.P. Isolated prostatic metastasis from primary sigmoid colon carcinoma. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2004, 23, 114–115. [Google Scholar]
- Schips, L.; Zigeuner, R.E.; Langner, C.; Mayer, R.; Pummer, K.; Hubmer, G. Metastasis of an ascending colon carcinoma in the prostate 10 years after hemicolectomy. J. Urol. 2002, 168, 641–642. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Boulay, B.R.; Parepally, M. Managing malignant biliary obstruction in pancreas cancer: Choosing the appropriate strategy. World J. Gastroenterol. 2014, 28, 9345–9353. [Google Scholar] [CrossRef]
- Ouyang, H.; Wang, P.; Meng, Z.; Chen, Z.; Yu, E.; Jin, H.; Chang, D.Z.; Liao, Z.; Cohen, L.; Liu, L. Multimodality treatment of pancreatic cancer with liver metastases using chemotherapy, radiation therapy, and/or Chinese herbal medicine. Pancreas 2011, 40, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Cham, J.; Puglisi, L.; De Shadarevian, M.; Hermel, D.J.; Spierling Bagsic, S.R.; Sigal, D. Do patients with metastatic pancreatic adenocarcinoma to the lung have improved survival? Cancer Med. 2023, 12, 10243–10253. [Google Scholar] [CrossRef]
- Turner, K.M.; Delman, A.M.; Kharofa, J.R.; Smith, M.T.; Choe, K.A.; Olowokure, O.; Wilson, G.C.; Patel, S.H.; Sohal, D.; Ahmad, S.A. Radiation therapy in borderline resectable pancreatic cancer: A review. Surgery 2011, 172, 284–290. [Google Scholar] [CrossRef]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Sahin, I.H.; Elias, H.; Chou, J.F.; Capanu, M.; O’Reilly, E.M. Pancreatic adenocarcinoma: Insights into patterns of recurrence and disease behavior. BMC Cancer 2018, 18, 769. [Google Scholar] [CrossRef]
- Choi, C.W.; Choi, I.K.; Seo, J.H.; Kim, B.S.; Kim, J.S.; Kim, C.D.; Um, S.H.; Kim, J.S.; Kim, Y.H. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am. J. Clin. Oncol. 2000, 23, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Laufman, L.R.; Krzeczowski, K.A.; Roach, R.; Segal, M. Leucovorin plus 5-fluorouracil: An effective treatment for metastatic colon cancer. J. Clin. Oncol. 1987, 5, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Atkins, M.B.; Koon, H.B.; Dummer, R.; Ascierto, P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer 2020, 2, e000948. [Google Scholar] [CrossRef] [PubMed]
- Pelster, M.S.; Amaria, R.N. Combined targeted therapy and immunotherapy in melanoma: A review of the impact on the tumor microenvironment and outcomes of early clinical trials. Ther. Adv. Med. Oncol. 2019, 11, 1758835919830826. [Google Scholar] [CrossRef]
- Rager, T.; Eckburg, A.; Patel, M.; Qiu, R.; Gantiwala, S.; Dovalovsky, K.; Fan, K.; Lam, K.; Roesler, C.; Rastogi, A.; et al. Treatment of Metastatic Melanoma with a Combination of Immunotherapies and Molecularly Targeted Therapies. Cancers 2022, 14, 3779. [Google Scholar] [CrossRef]
- Shalata, W.; Steckbeck, R.; Polishchuk, I.; Cohen, A.Y.; Rouvinov, K.; Tokar, M.; Abu Jama, A.; Abu Saleh, O.; Sheva, K.; Yakobson, A. Safety and rapid response of dabrafenib and trametinib therapy during hyperbilirubinemia in metastatic melanoma. Front. Oncol. 2023, 13, 1102330. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327, Erratum in: Lancet Oncol. 2018, 19, e509. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Dummer, R. Immunological effects of BRAF+MEK inhibition. Oncoimmunology 2018, 7, e1468955. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. New Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Mukherji, R.; Debnath, D.; Hartley, M.L.; Noel, M.S. The Role of Immunotherapy in Pancreatic Cancer. Curr. Oncol. 2022, 29, 6864–6892. [Google Scholar] [CrossRef]
- Springfeld, C.; Ferrone, C.R.; Katz, M.H.G.; Philip, P.A.; Hong, T.S.; Hackert, T.; Büchler, M.W.; Neoptolemos, J. Neoadjuvant therapy for pancreatic cancer. Nat. Rev. Clin. Oncol. 2023, 20, 318–337. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Peled, N.; Gabizon, I.; Abu Saleh, O.; Kian, W.; Yakobson, A. Associated Myocarditis: A Predictive Factor for Response? Case Rep. Oncol. 2020, 13, 550–557. [Google Scholar] [CrossRef]
- Yousef, A.J.A. Male Breast Cancer: Epidemiology and Risk Factors. Semin. Oncol. 2017, 44, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Burga, A.M.; Fadare, O.; Lininger, R.A.; Tavassoli, F.A. Invasive carcinomas of the male breast: A morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006, 449, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.F.; Jatoi, I.; Tse, J.; Rosenberg, P.S. Male breast cancer: A population-based comparison with female breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 232–239. [Google Scholar] [CrossRef]
- Foerster, R.; Schroeder, L.; Foerster, F.; Wulff, V.; Schubotz, B.; Baaske, D.; Rudlowski, C. Metastatic male breast cancer: A retrospective cohort analysis. Breast Care 2014, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yu, B.; Cheng, Y.; Xu, X.; Shui, R.; Bi, R.; Lu, H.; Tu, X.; Yang, W. Metastases to the breast from non-mammary malignancies: A clinicopathologic study of 28 cases. Chin. J. Pathol. (Zhonghua Bing Li Xue Za Zhi) 2014, 43, 231–235. [Google Scholar]
- Smymiotis, V.; Theodosopoulos, T.; Marinis, A.; Goula, K.; Psychogios, J.; Kondi-Pafiti, A. Metastatic disease in the breast from nonmammary neoplasms. Eur. J. Gynaecol. Oncol. 2005, 26, 547–550. [Google Scholar]
- Mandaliya, H.; Sung, J.; Hill, J.; Samali, R.; George, M. Prostate Cancer: Cases of Rare Presentation and Rare Metastasis. Case Rep. Oncol. 2015, 8, 526–529. [Google Scholar] [CrossRef]
- Satram-Hoang, S.; Ziogas, A.; Anton-Culver, H. Risk of second primary cancer in men with breast cancer. Breast Cancer Res. BCR 2007, 9, R10. [Google Scholar] [CrossRef]
- David, M.K.; Leslie, S.W. Prostate Specific Antigen. 2022 Nov 10. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Y.; Harada, M.; Yano, H.; Ogasawara, S.; Tanaka, M.; Yamada, A.; Itoh, K. Prostate-specific antigen-reactive cytotoxic T lymphocyte precursors in colon cancer patients. Oncol. Rep. 2006, 15, 317–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; Elizondo-Riojas, M.Á. Prostate-specific antigen (PSA) as a possible biomarker in non-prostatic cancer: A review. Cancer Epidemiol. 2018, 54, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Batra, S.K. Pancreatic cancer metastasis: Are we being pre-EMTed? Curr. Pharm. Des. 2015, 21, 1249–1255. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Speers, C.; McGahan, C.E.; Renouf, D.J.; Schaeffer, D.F.; Kennecke, H.F. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015, 4, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Nunnery, S.; Bottinor, W.; Das, S. Cardiac Masses in a Patient with Pancreatic Adenocarcinoma and a History of Breast Carcinoma. JAMA Oncol. 2020, 6, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Shalata, W.; Massalha, I.; Ishay, S.Y.; Chernomordikova, E.; Jama, A.A.; Rouvinov, K.; Dudnik, Y.; Yakobson, A. Radiotherapy-Induced Atrial Myxoma: A Case Report and Literature Review. Life 2023, 13, 1585. [Google Scholar] [CrossRef]
- Patel, J.K.; Didolkar, M.S.; Pickren, J.W.; Moore, R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef]
- Larsen, A.K.; Krag, C.; Geertsen, P.; Jakobsen, L.P. Isolated malignant melanoma metastasis to the pancreas. Plast. Reconstr. Surg. Glob. Open 2013, 1, e74. [Google Scholar] [CrossRef]
- Shalata, W.; Levin, D.; Fridman, J.; Makarov, V.; Iraqi, M.; Golosky, M.; Rouvinov, K.; Kian, W.; Yakobson, A. Metabolic Activity Assessment by 18F-Fluorodeoxyglucose Positron Emission Tomography in Patients after COVID-19 Vaccination. Curr. Oncol. 2022, 29, 989–1000. [Google Scholar] [CrossRef]





| Patient No. | Age at Diagnosis | Gender | Diagnosis | Site of Rare Metastasis | Diagnostic Method | Treatment * |
|---|---|---|---|---|---|---|
| 1 | 69 | M | Colorectal Adenocarcinoma | Prostate | Histopathology | FOLFOX |
| 2 | 73 | M | Colorectal Adenocarcinoma | Prostate | Histopathology | FOLFOX |
| 3 | 63 | M | Pancreatic Adenocarcinoma | Heart | PET-CT Histopathology | FOLFIRINOX |
| 4 | 68 | M | Metastatic Melanoma | Pancreas | CT Histopathology | Ipilimumab/Nivolumab |
| 5 | 73 | M | Breast Carcinoma | Left Kidney | CT Histopathology | Resection Tamoxifen |
| Date of Publication | Age (Years) | Stage at Presentation | Reference No. |
|---|---|---|---|
| February 1993 | N/A * | N/A | [17] |
| August 2002 | N/A | N/A | [18] |
| May 2004 | 38 | IIIC | [19] |
| June 2011 | 54 | IIIC | [20] |
| June 2013 | 70 | IV | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalata, W.; Abu Jama, A.; Abu Salman, A.; Golosky, M.; Solomon, A.; Abu Saleh, O.; Michlin, R.; Shalata, S.; Agbarya, A.; Yakobson, A. Unexpected and Rare Sites of Metastasis in Oncologic Patients. J. Clin. Med. 2023, 12, 6447. https://doi.org/10.3390/jcm12206447
Shalata W, Abu Jama A, Abu Salman A, Golosky M, Solomon A, Abu Saleh O, Michlin R, Shalata S, Agbarya A, Yakobson A. Unexpected and Rare Sites of Metastasis in Oncologic Patients. Journal of Clinical Medicine. 2023; 12(20):6447. https://doi.org/10.3390/jcm12206447
Chicago/Turabian StyleShalata, Walid, Ashraf Abu Jama, Amjad Abu Salman, Mitchell Golosky, Adam Solomon, Omar Abu Saleh, Regina Michlin, Sondos Shalata, Abed Agbarya, and Alexander Yakobson. 2023. "Unexpected and Rare Sites of Metastasis in Oncologic Patients" Journal of Clinical Medicine 12, no. 20: 6447. https://doi.org/10.3390/jcm12206447
APA StyleShalata, W., Abu Jama, A., Abu Salman, A., Golosky, M., Solomon, A., Abu Saleh, O., Michlin, R., Shalata, S., Agbarya, A., & Yakobson, A. (2023). Unexpected and Rare Sites of Metastasis in Oncologic Patients. Journal of Clinical Medicine, 12(20), 6447. https://doi.org/10.3390/jcm12206447







