Factors Associated with Periodontitis in Younger Individuals: A Scoping Review
Abstract
:1. Introduction
2. Methods
3. Results
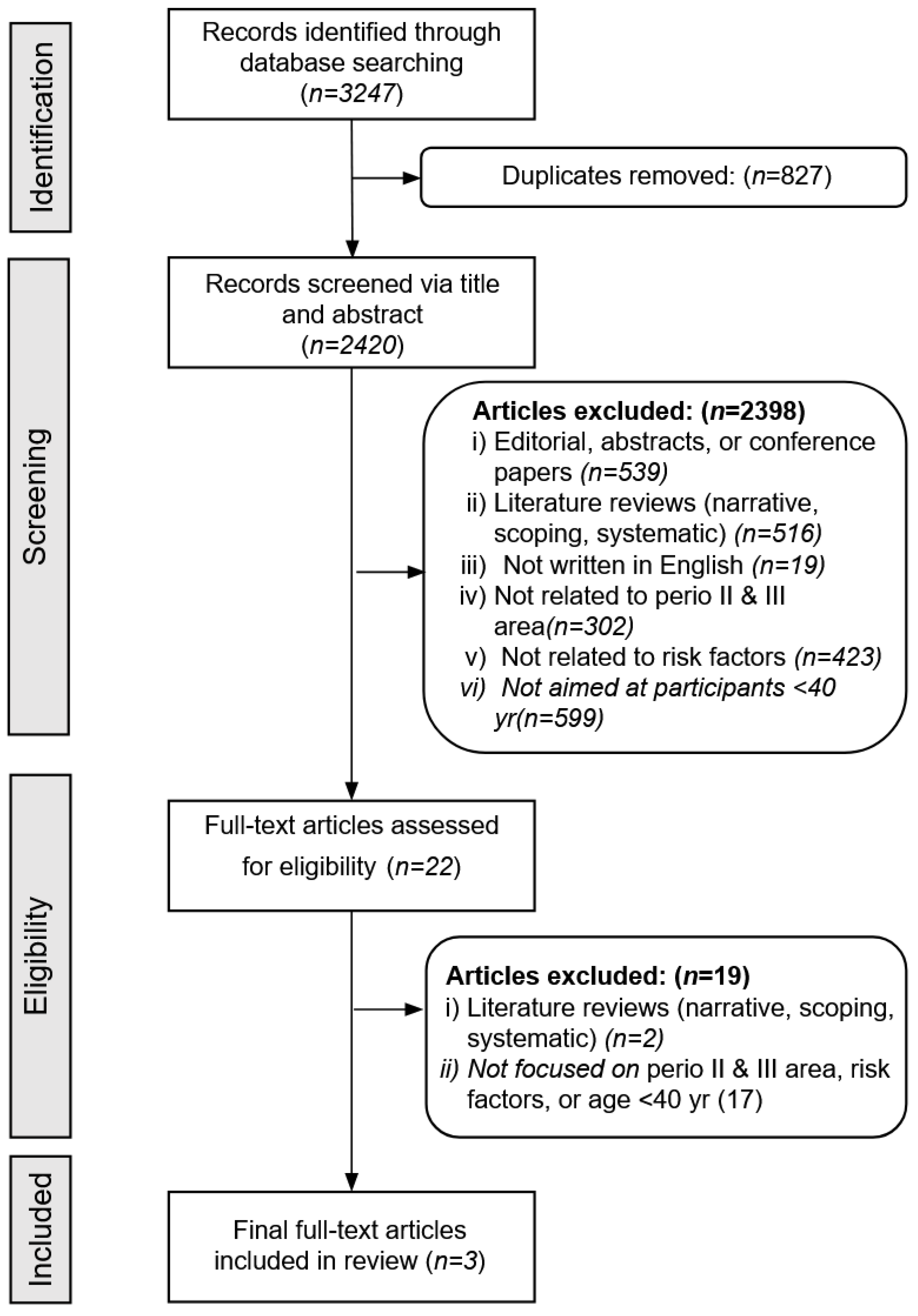
3.1. Study Selection
3.2. Study Features
3.3. Findings
| Author (Year) | Country | Sample Size | Age Range (Years) | Study Aims | Study Design | Outcomes |
|---|---|---|---|---|---|---|
| Da Silva et al., 2022 [39] | Brazil | 2022 | 26–40 | Investigated the influence of smoking on clinical, microbiological, and immunological parameters in young adults with stage III-IV, Grade C periodontitis | Longitudinal, case-control study | Young smokers with stage III-IV grade disease responded less favorably to treatment for all study parameters at both 3 and 6 months |
| Jiang et al., 2016 [38] | China | 987 | 18–40 | Examined the prevalence and risk factors of periodontal disease among Chinese women preconception | Cross-sectional | Women with bleeding during brushing are at increased risk of periodontal disease. Smoking and obesity were not significant factors |
| Costa et al., 2021 [37] | Brazil | 2032 | 18–19 | Investigated the association between low bone mineral density (BMD) and severe periodontitis | Cross-sectional design | Low BMD is associated with both the severity and extent of periodontitis in adolescents. Secondary results found education and income inversely related to severe disease |
4. Discussion
4.1. Limitations
4.2. Implications
4.3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eke, P.; Dye, B.; Wei, L.; Thornton-Evans, G.O.; Genco, R.J. Prevalence of Periodontitis in Adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. [Google Scholar] [CrossRef]
- Eke, P.I.; Wei, L.; Borgnakke, W.S.; Thornton-Evans, G.; Zhang, X.; Lu, H.; McGuire, L.C.; Genco, R.J. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontology 2000 2016, 72, 76–95. [Google Scholar] [CrossRef]
- Ray, R.R. Periodontitis: An Oral Disease with Severe Consequences. Appl. Biochem. Biotechnol. 2022, 195, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Sheilesh, D. Risk factors for periodontitis. J. Int. Acad. Periodontol. 2005, 7, 3–7. [Google Scholar] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 2013, 62, 95–162. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontology 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Bartold, P.M. Clinical relevance of the host responses of periodontitis. Periodontology 2000 2007, 43, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Liljenberg, B.; Lindhe, J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J. Clin. Periodontol. 2002, 29, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Delima, A.J.; Van Dyke, T.E. Origin and function of the cellular components in gingival crevice fluid. Periodontology 2000 2003, 31, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, B.S.; Diehl, S.R.; Gunsolley, J.C.; Sparks, B.S.; Brooks, C.N.; Koertge, T.E.; Califano, J.V.; Burmeister, J.A.; Schenkein, H.A. Evidence of a Substantial Genetic Basis for Risk of Adult Periodontitis. J. Periodontol. 2000, 71, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Thomason, J.M.; Ellis, J. The pathogenesis of drug-induced gingival overgrowth. J. Clin. Periodontol. 1996, 23 Pt 1, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Tinoco, E.M.B. Global epidemiology of periodontal diseases in children and young persons. Periodontology 2000 2002, 29, 153–176. [Google Scholar] [CrossRef]
- Skeie, M.S.; Raadal, M.; Strand, G.V.; Espelid, I. The relationship between caries in the primary dentition at 5 years of age and permanent dentition at 10 years of age—A longitudinal study. Int. J. Paediatr. Dent. 2006, 16, 152–160. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and Its Relationship to Initiation of Localized Aggressive Periodontitis: Longitudinal Cohort Study of Initially Healthy Adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef]
- Moutsopoulos, N.M.; Zerbe, C.S.; Wild, T.; Dutzan, N.; Brenchley, L.; DiPasquale, G.; Uzel, G.; Axelrod, K.C.; Lisco, A.; Hajishengallis, G.; et al. Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1. N. Engl. J. Med. 2017, 376, 1141–1146. [Google Scholar] [CrossRef]
- Uy, S.N.M.R.; Deng, K.; Fok, C.T.C.; Fok, M.R.; Pelekos, G.; Tonetti, M.S. Food intake, masticatory function, tooth mobility, loss of posterior support, and diminished quality of life are associated with more advanced periodontitis stage diagnosis. J. Clin. Periodontol. 2022, 49, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Van Der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-De-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Periodontology. 2017 Classification of Periodontal and Peri-Implant Diseases and Conditions. 31 August 2022. Available online: https://www.perio.org/research-science/2017-classification-of-periodontal-and-peri-implant-diseases-and-conditions (accessed on 1 September 2023).
- American Academy of Periodontology. Staging and Grading Periodontitis. Available online: https://sites.perio.org/wp-content/uploads/2019/08/Staging-and-Grading-Periodontitis.pdf (accessed on 1 September 2023).
- Arigbede, A.; Babatope, B.; Bamidele, M. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Kotronia, E.; Ramsay, S.E. Frailty, aging, and periodontal disease: Basic biologic considerations. Periodontology 2000 2021, 87, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Ameet, M.M.; Avneesh, H.T.; Babita, R.P.; Pramod, P.M. The Relationship Between Periodontitis and Systemic Diseases—Hype or Hope? J. Clin. Diagn. Res. 2013, 7, 758–762. [Google Scholar] [CrossRef]
- Page, R.C.; Altman, L.C.; Ebersole, J.L.; Vandesteen, G.E.; Dahlberg, W.H.; Williams, B.L.; Osterberg, S.K. Rapidly Progressive Periodontitis: A Distinct Clinical Condition. J. Periodontol. 1983, 54, 197–209. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Gum Disease Risk Factors. 7 February 2020. Available online: https://www.perio.org/for-patients/gum-disease-information/gum-disease-risk-factors (accessed on 1 September 2023).
- Albandar, J.M. Aggressive periodontitis: Case definition and diagnostic criteria. Periodontology 2000 2014, 65, 13–26. [Google Scholar] [CrossRef]
- Demmer, R.T.; Papapanou, P.N. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontology 2000 2010, 53, 28–44. [Google Scholar] [CrossRef]
- Huang, Q.; Dong, X. Prevalence of periodontal disease in middle-aged and elderly patients and its influencing factors. Am. J. Transl. Res. 2022, 14, 5677–5684. [Google Scholar]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- PRISMA for Scoping Reviews. PRISMA. Available online: http://www.prisma-statement.org/Extensions/ScopingReviews (accessed on 1 September 2023).
- Costa, S.A.; Ribeiro, C.C.C.; de Oliveira, K.R.; Alves, C.M.C.; Thomaz, E.B.A.F.; Casarin, R.C.V.; Souza, S.d.F.C. Low bone mineral density is associated with severe periodontitis at the end of the second decade of life: A population-based study. J. Clin. Periodontol. 2021, 48, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Su, Y.; Xiong, X.; Harville, E.; Wu, H.; Jiang, Z.; Qian, X. Prevalence and risk factors of periodontal disease among pre-conception Chinese women. Reprod. Health 2016, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.V.C.; Rangel, T.P.; Corrêa, M.G.; Monteiro, M.d.F.; Casati, M.Z.; Ruiz, K.G.; Sallum, E.A.; Casarin, R.C.V.; Sallum, A.W. Smoking negatively impacts the clinical, microbiological, and immunological treatment response of young adults with Grade C periodontitis. J. Periodontal Res. 2022, 57, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Williams, S.M.; Fletcher, D.J.; Cameron, C.M.; Broadbent, J.M.; Shearer, D.M.; Thomson, W.M. Reexamining the Association Between Smoking and Periodontitis in the Dunedin Study With an Enhanced Analytical Approach. J. Periodontol. 2014, 85, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Asma, S. Smoking-Attributable Periodontitis in the United States: Findings From NHANES III. J. Periodontol. 2000, 71, 743–751. [Google Scholar] [CrossRef]
- Bergström, J.; Eliasson, S.; Dock, J. A 10-Year Prospective Study of Tobacco Smoking and Periodontal Health. J. Periodontol. 2000, 71, 1338–1347. [Google Scholar] [CrossRef]
- Johnson, G.K.; Hill, M. Cigarette Smoking and the Periodontal Patient. J. Periodontol. 2004, 75, 196–209. [Google Scholar] [CrossRef]
- Mavropoulos, A.; Aars, H.; Brodin, P. Hyperaemic response to cigarette smoking in healthy gingiva. J. Clin. Periodontol. 2003, 30, 214–221. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2013, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Microbiology of Dental Decay and Periodontal Disease. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Ferrante, A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 34–38. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From Basic Science to Medicine—40 Years in Immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Genco, R.J.; Grossi, S.G.; Ho, A.; Nishimura, F.; Murayama, Y. A Proposed Model Linking Inflammation to Obesity, Diabetes, and Periodontal Infections. J. Periodontol. 2005, 76, 2075–2084. [Google Scholar] [CrossRef]
- Pischon, N.; Heng, N.; Bernimoulin, J.-P.; Kleber, B.-M.; Willich, S.; Pischon, T. Obesity, Inflammation, and Periodontal Disease. J. Dent. Res. 2007, 86, 400–409. [Google Scholar] [CrossRef]
- Suvan, J.; D’Aiuto, F.; Moles, D.R.; Petrie, A.; Donos, N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes. Rev. 2011, 12, e381–e404. [Google Scholar] [CrossRef]
- Chaffee, B.W.; Weston, S.J. Association Between Chronic Periodontal Disease and Obesity: A Systematic Review and Meta-Analysis. J. Periodontol. 2010, 81, 1708–1724. [Google Scholar] [CrossRef]
- Cheng, H.L.; Medlow, S.; Steinbeck, K. The Health Consequences of Obesity in Young Adulthood. Curr. Obes. Rep. 2016, 5, 30–37. [Google Scholar] [CrossRef]
- Jagannathachary, S.; Kamaraj, D. Obesity and periodontal disease. J. Indian Soc. Periodontol. 2010, 14, 96–100. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Databases | Search Strategies | Number of Articles Found |
|---|---|---|
| PubMed | ((“risk factors”[Title/Abstract] OR “causal”[Title/Abstract]) AND (“periodontal disease”[Title/Abstract] OR “periodontitis”[Title/Abstract] OR “Aggressive Periodontitis”[Title/Abstract] OR “Periapical Periodontitis”[Title/Abstract] OR “Chronic Periodontitis”[Title/Abstract] OR “Early-Onset periodontitis”[Title/Abstract] OR “Juvenile Periodontitis”[Title/Abstract])) AND ((humans[Filter]) AND (2013:2023[pdat])) | 1326 |
| Web of Science | (TS = (“risk factors” OR causal)) AND TS = (“periodontal disease” OR periodontitis OR “Aggressive Periodontitis” OR “Periapical Periodontitis” OR “Chronic Periodontitis” OR “Early-Onset periodontitis” OR “Juvenile Periodontitis”) AND ((ALL = ((“population groups” not “animal models”))) OR ALL = (men OR women OR patient OR female OR male OR subjects OR adult)) NOT ALL = (“animal models”) | 1716 |
| Cochrane | #1 (“risk factors” OR causal):ti,ab,kw AND (“periodontal disease” OR periodontitis OR “Aggressive Periodontitis” OR “Periapical Periodontitis” OR “Chronic Periodontitis” OR “Early-Onset periodontitis” OR “Juvenile Periodontitis”):ti,ab,kw (Word variations have been searched) #2 (men OR women OR patient OR female OR male OR subjects OR adult NOT animal):ti,ab,kw #3 #1 AND #2 | 282 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, M.; Kelly, R.; Mohajeri, A.; Reese, L.; Badawi, S.; Frost, C.; Sevathas, T.; Lipsky, M.S. Factors Associated with Periodontitis in Younger Individuals: A Scoping Review. J. Clin. Med. 2023, 12, 6442. https://doi.org/10.3390/jcm12206442
Hung M, Kelly R, Mohajeri A, Reese L, Badawi S, Frost C, Sevathas T, Lipsky MS. Factors Associated with Periodontitis in Younger Individuals: A Scoping Review. Journal of Clinical Medicine. 2023; 12(20):6442. https://doi.org/10.3390/jcm12206442
Chicago/Turabian StyleHung, Man, Roah Kelly, Amir Mohajeri, Logan Reese, Sarah Badawi, Cole Frost, Taroniar Sevathas, and Martin S. Lipsky. 2023. "Factors Associated with Periodontitis in Younger Individuals: A Scoping Review" Journal of Clinical Medicine 12, no. 20: 6442. https://doi.org/10.3390/jcm12206442
APA StyleHung, M., Kelly, R., Mohajeri, A., Reese, L., Badawi, S., Frost, C., Sevathas, T., & Lipsky, M. S. (2023). Factors Associated with Periodontitis in Younger Individuals: A Scoping Review. Journal of Clinical Medicine, 12(20), 6442. https://doi.org/10.3390/jcm12206442







