Viral Infections May Be Associated with Henoch–Schönlein Purpura
Abstract
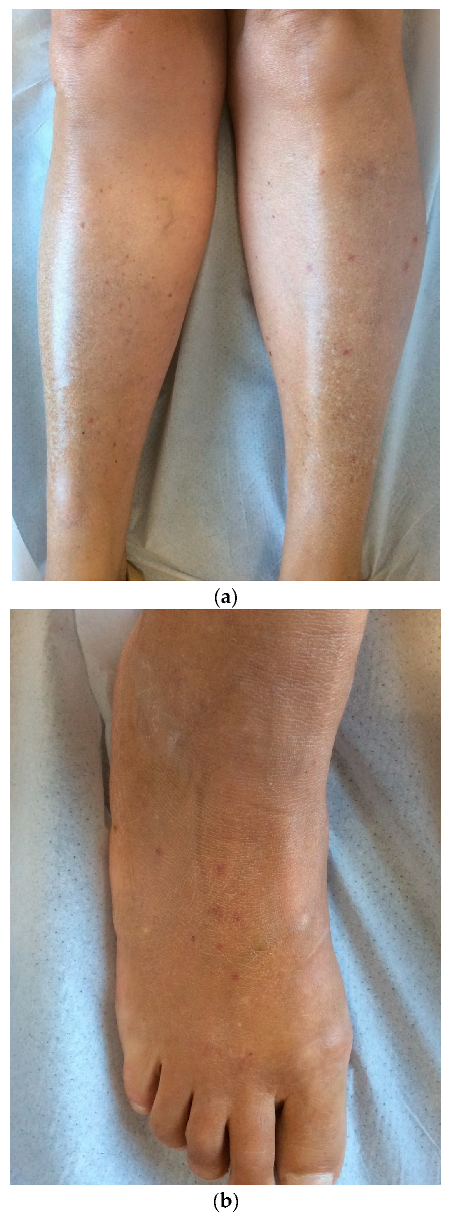
1. Introduction
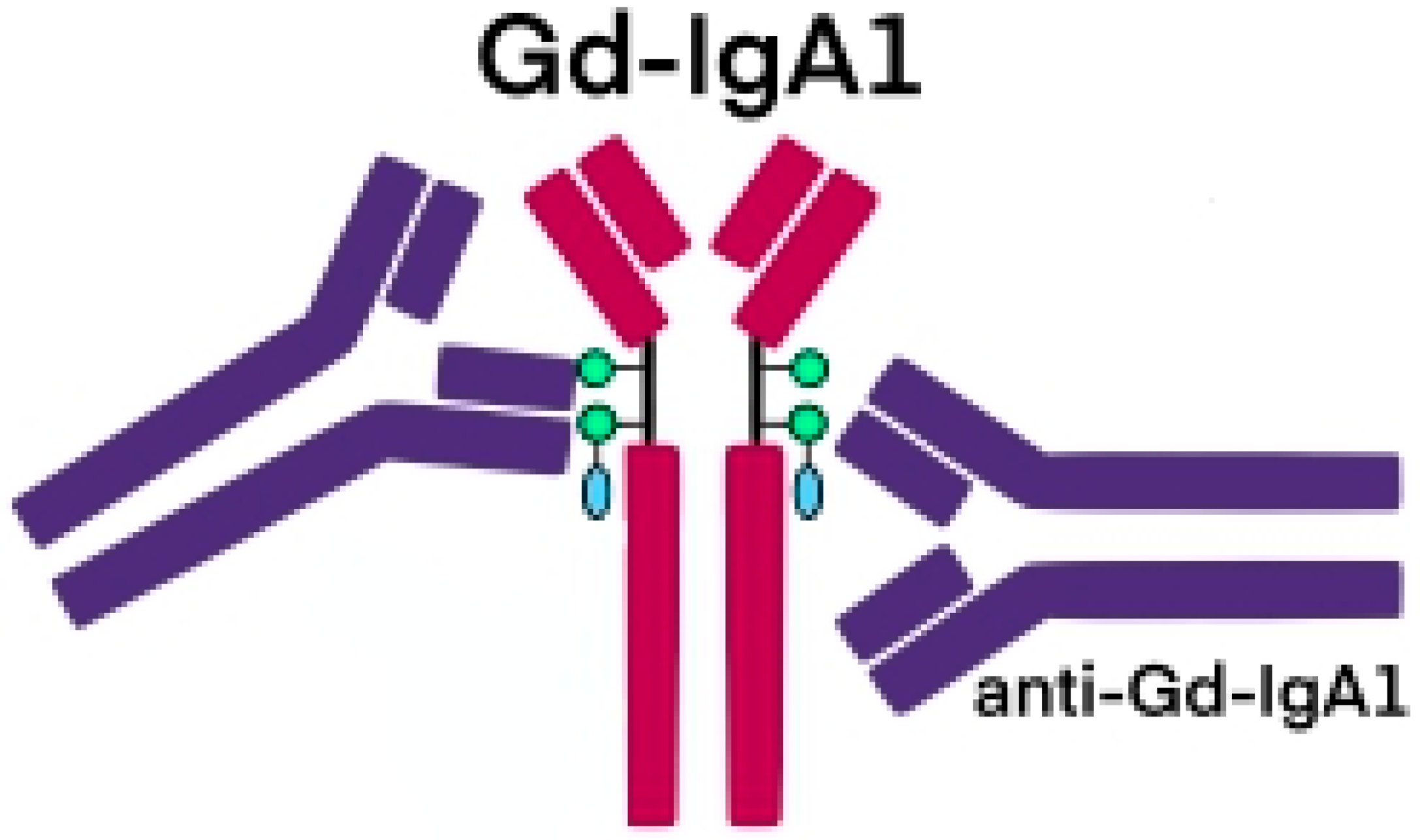
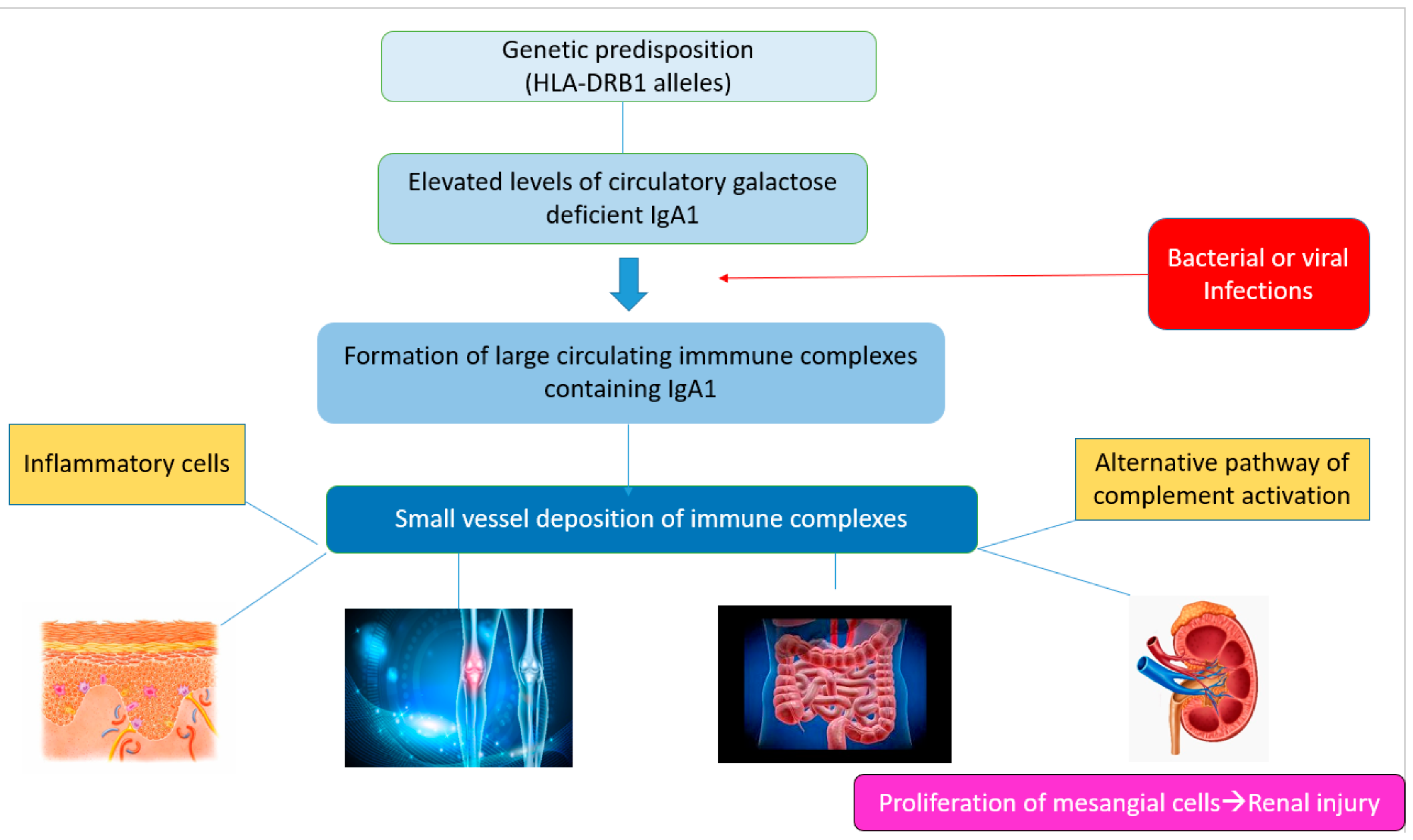
2. Pathogenesis
3. Environment, Microbial, and Virus Infections
3.1. Viruses
3.1.1. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
3.1.2. SARS-CoV-2 Vaccines
3.1.3. Common Respiratory Tract Viruses
3.1.4. Cytomegalovirus
3.1.5. Epstein–Barr Virus
3.1.6. Hepatitis A, B, C Viruses
3.1.7. Measles Virus
3.1.8. Parvovirus B19
3.1.9. Varicella Zoster Virus
4. Treatment Modalities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner-Medwin, J.M.; Dolezalova, P.; Cummins, C.; Southwood, T.R. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 2002, 360, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen, C. Approach to cutaneous vasculitides with special emphasis on small vessel vasculitis: Histopathology and direct immunofluorescence. Curr. Opin. Rheumatol. 2017, 29, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hetland, L.; Susrud, K.; Lindahl, K.; Bygum, A. Henoch-Schönlein Purpura: A Literature Review. Acta Derm.-Venereol. 2017, 97, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Rigante, D.; Castellazzi, L.; Bosco, A.; Esposito, S. Is there a crossroad between infections, genetics, and Henoch–Schönlein purpura? Autoimmun. Rev. 2013, 12, 1016–1021. [Google Scholar] [CrossRef]
- Monteiro, R.C. Role of IgA and IgA Fc Receptors in Inflammation. J. Clin. Immunol. 2009, 30, 1–9. [Google Scholar] [CrossRef]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Person, T.; King, R.G.; Rizk, D.V.; Novak, J.; Green, T.J.; Reily, C. Cytokines and Production of Aberrantly O-Glycosylated IgA1, the Main Autoantigen in IgA Nephropathy. J. Interf. Cytokine Res. 2022, 42, 301–315. [Google Scholar] [CrossRef]
- Kiryluk, K.; Moldoveanu, Z.; Sanders, J.T.; Eison, T.M.; Suzuki, H.; Julian, B.A.; Novak, J.; Gharavi, A.G.; Wyatt, R. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch–Schönlein purpura nephritis. Kidney Int. 2011, 80, 79–87. [Google Scholar] [CrossRef]
- Trnka, P. Henoch-Schönlein purpura in children. J. Paediatr. Child Health 2013, 49, 995–1003. [Google Scholar] [CrossRef]
- Novak, J.; Moldoveanu, Z.; Renfrow, M.B.; Yanagihara, T.; Suzuki, H.; Raska, M.; Hall, S.; Brown, R.; Huang, W.-Q.; Goepfert, A.; et al. IgA Nephropathy and Henoch-Schoenlein Purpura Nephritis: Aberrant Glycosylation of IgA1, Formation of IgA1-Containing Immune Complexes, and Activation of Mesangial Cells. IgA Nephrop. Today 2007, 157, 134–138. [Google Scholar] [CrossRef]
- Boyd, J.K.; Barratt, J. Inherited IgA glycosylation pattern in IgA nephropathy and HSP nephritis: Where do we go next? Kidney Int. 2011, 80, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Julian, B.A.; Tomana, M.; Mestecky, J. IgA Glycosylation and IgA Immune Complexes in the Pathogenesis of IgA Nephropathy. Semin. Nephrol. 2008, 28, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Chuang, Y.-H.; Wang, L.-C.; Huang, H.-Y.; Gershwin, M.E.; Chiang, B.-L. The immunobiology of Henoch–Schönlein purpura. Autoimmun. Rev. 2008, 7, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Heineke, M.H.; Ballering, A.V.; Jamin, A.; Mkaddem, S.B.; Monteiro, R.C.; Van Egmond, M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun. Rev. 2017, 16, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Saulsbury, F.T. Clinical update: Henoch-Schönlein purpura. Lancet 2007, 369, 976–978. [Google Scholar] [CrossRef]
- Allez, M.; Denis, B.; Bouaziz, J.; Battistella, M.; Zagdanski, A.; Bayart, J.; Lazaridou, I.; Gatey, C.; Pillebout, E.; Baudier, M.C.; et al. COVID-19–Related IgA Vasculitis. Arthritis Rheumatol. 2020, 72, 1952–1953. [Google Scholar] [CrossRef]
- Suso, A.S.; Mon, C.; Oñate Alonso, I.; Galindo Romo, K.; Juarez, R.C.; Ramírez, C.L.; Sánchez, M.S.; Valdivia, V.M.; Librero, M.O.; Pala, A.O.; et al. IgA Vasculitis With Nephritis (Henoch−Schönlein Purpura) in a COVID-19 Patient. Kidney Int. Rep. 2020, 5, 2074–2078. [Google Scholar] [CrossRef]
- Sandhu, S.; Chand, S.; Bhatnagar, A.; Dabas, R.; Bhat, S.; Kumar, H.; Dixit, P.K. Possible association between IgA vasculitis and COVID-19. Dermatol. Ther. 2020, 34, e14551. [Google Scholar] [CrossRef]
- Barbetta, L.; Filocamo, G.; Passoni, E.; Boggio, F.; Folli, C.; Monzani, V. Henoch-Schönlein purpura with renal and gastrointestinal involvement in course of COVID-19: A case report. Ann. Rheum. Dis. 2021, 39, 191–192. [Google Scholar] [CrossRef]
- Li, N.L.; Papini, A.B.; Shao, T.; Girard, L. Immunoglobulin-A Vasculitis with Renal Involvement in a Patient with COVID-19: A Case Report and Review of Acute Kidney Injury Related to SARS-CoV-2. Can. J. Kidney Health Dis. 2021, 8, 2054358121991684. [Google Scholar] [CrossRef]
- Oñate, I.; Ortiz, M.; Suso, A.; Mon, C.; Galindo, K.; Lentisco, C.; Camacho, R.; Sánchez, M.; Oliet, A.; Ortega, O.; et al. IgA vasculitis with nephritis (Henoch-Schonelin Purpura) after COVID-19: A case series and review of the literature. Nefrologia 2022, 42, 481–489. [Google Scholar] [CrossRef]
- Valero, C.; Baldivieso-Achá, J.P.; Uriarte, M.; Vicente-Rabaneda, E.F.; Castañeda, S.; García-Vicuña, R. Vasculitis flare after COVID-19: Report of two cases in patients with preexistent controlled IgA vasculitis and review of the literature. Rheumatol. Int. 2022, 42, 1643–1652. [Google Scholar] [CrossRef]
- Messova, A.; Pivina, L.; Muzdubayeva, Z.; Sanbayev, D.; Urazalina, Z.; Adams, A. COVID-19 and New Onset IgA Vasculitis: A Systematic Review of Case Reports. J. Emerg. Nurs. 2022, 48, 348–365. [Google Scholar] [CrossRef]
- Jacobi, M.; Lancrei, H.M.; Brosh-Nissimov, T.; Yeshayahu, Y. Purpurona: A Novel Report of COVID-19-Related Henoch-Schonlein Purpura in a Child. Pediatr. Infect. Dis. J. 2020, 40, e93–e94. [Google Scholar] [CrossRef]
- Hoskins, B.; Keeven, N.; Dang, M.; Keller, E.; Nagpal, R. A Child with COVID-19 and Immunoglobulin A Vasculitis. Pediatr. Ann. 2021, 50, e44–e48. [Google Scholar] [CrossRef]
- Riscassi, S.; Kalapurackal, M.A.; Battisti, L.; Eisendle, K.; Raffeiner, B.; Mercolini, F. Vasculitis in a child with COVID-19: A novel presentation of Henoch-Schonlein purpura. Klin. Pediatr. 2021, 234, 116–118. [Google Scholar] [CrossRef]
- Borocco, C.; Lafay, C.; Plantard, I.; Gottlieb, J.; Koné-Paut, I.; Galeotti, C. SARS-CoV-2 associated Henoch-Schonlein purpura in a 13-year-old girl. Arch. Pediatr. 2021, 28, 573–575. [Google Scholar] [CrossRef] [PubMed]
- AlGhoozi, D.A.; Alkhayyat, H.M. A child with Henoch-Schonlein purpura secondary to a COVID-19 infection. BMJ Case Rep. 2021, 14, e239910. [Google Scholar] [CrossRef]
- El Hasbani, G.; Taher, A.T.; Jawad, A.S.M.; Uthman, I.; Taher, A.T.; Uthman, M.I. Henoch-Schönlein purpura: Another COVID-19 complication. Pediatr. Dermatol. 2021, 38, 1359–1360. [Google Scholar] [CrossRef]
- Ziyara, R.; Thompson, A.; Liu, B. Henoch-Schönlein Purpura in a COVID-19-Positive Child With Abdominal Pain and PIMS-TS. Clin. Pediatr. 2021, 61, 5–8. [Google Scholar] [CrossRef]
- Serafinelli, J.; Mastrangelo, A.; Morello, W.; Cerioni, V.F.; Salim, A.; Nebuloni, M.; Montini, G. Kidney involvement and histological findings in two pediatric COVID-19 patients. Pediatr. Nephrol. 2021, 36, 3789–3793. [Google Scholar] [CrossRef]
- Kaya Akca, U.; Atalay, E.; Cuceoglu, M.K.; Balik, Z.; Sener, S.; Ozsurekci, Y.; Basaran, O.; Batu, E.D.; Bilginer, Y.; Ozen, S. Impact of the COVID-19 pandemic on the frequency of the pediatric rheumatic diseases. Rheumatol. Int. 2022, 42, 51–57. [Google Scholar] [CrossRef]
- Batu, E.D.; Sener, S.; Ozen, S. COVID-19 associated pediatric vasculitis: A systematic review and detailed analysis of the pathogenesis. Semin. Arthritis Rheum. 2022, 55, 152047. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Sciacovelli, L.; Basso, D.; Negrini, D.; Zuin, S.; Cosma, C.; Faggian, D.; Matricardi, P.; Plebani, M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin. Chim. Acta. 2020, 507, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Farooq, H.; Rehman, M.A.U.; Asmar, A.; Asif, S.; Mushtaq, A.; Qureshi, M.A. The pathogenesis of COVID-19-induced IgA nephropathy and IgA vasculitis: A systematic review. J. Taibah Univ. Med Sci. 2021, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.; Alzahrani, F.; Alshehri, S.; AbdelQadir, Y.H. New-Onset Henoch–Schonlein Purpura after COVID-19 Infection: A Case Report and Review of the Literature. Case Rep. Pediatr. 2022, 2022, 1712651. [Google Scholar] [CrossRef]
- Ito, C.; Odajima, K.; Niimura, Y.; Fujii, M.; Sone, M.; Asakawa, S.; Arai, S.; Yamazaki, O.; Tamura, Y.; Saito, K.; et al. IgA vasculitis with transient glomerular hematuria, diarrhea, and pericarditis following COVID-19 mRNA vaccination in a young patient with possible pre-existing ulcerative colitis. CEN Case Rep. 2022, 4, 1–7. [Google Scholar] [CrossRef]
- A Maye, J.; Chong, H.P.; Rajagopal, V.; Petchey, W. Reactivation of IgA vasculitis following COVID-19 vaccination. BMJ Case Rep. 2021, 14, e247188. [Google Scholar] [CrossRef]
- Nishimura, N.; Shiomichi, Y.; Takeuchi, S.; Akamine, S.; Yoneda, R.; Yoshizawa, S. IgA vasculitis following COVID-19 vaccination. Mod. Rheumatol. Case Rep. 2022, 7, 122–126. [Google Scholar] [CrossRef]
- Wu, H.H.L.; Kalra, P.A.; Chinnadurai, R. New-Onset and Relapsed Kidney Histopathology Following COVID-19 Vaccination: A Systematic Review. Vaccines 2021, 9, 1252. [Google Scholar] [CrossRef]
- Hines, A.M.; Murphy, N.; Mullin, C.; Barillas, J.; Barrientos, J.C. Henoch-Schönlein purpura presenting post COVID-19 vaccination. Vaccine 2021, 39, 4571–4572. [Google Scholar] [CrossRef]
- Badier, L.; Toledano, A.; Porel, T.; Dumond, S.; Jouglen, J.; Sailler, L.; Bagheri, H.; Moulis, G.; Lafaurie, M. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV-19. Autoimmun. Rev. 2021, 20, 102951. [Google Scholar] [CrossRef]
- Hočevar, A.; Tomšič, M. Immune mediated events timely associated with COVID-19 vaccine. A comment on article by Badier; et al.: “IgA vasculitis in adult patients following vaccination by ChadOx1 nCoV-19”. Autoimmun. Rev. 2021, 21, 102989. [Google Scholar] [CrossRef]
- Iwata, H.; Kamiya, K.; Kado, S.; Nakaya, T.; Kawata, H.; Komine, M.; Ohtsuki, M. Case of immunoglobulin A vasculitis following coronavirus disease 2019 vaccination. J. Dermatol. 2021, 48, e598–e599. [Google Scholar] [CrossRef]
- Obeid, M.; Fenwick, C.; Pantaleo, G. Reactivation of IgA vasculitis after COVID-19 vaccination. Lancet Rheumatol. 2021, 3, e617. [Google Scholar] [CrossRef]
- Sugita, K.; Kaneko, S.; Hisada, R.; Harano, M.; Anno, E.; Hagiwara, S.; Imai, E.; Nagata, M.; Tsukamoto, Y. Development of IgA vasculitis with severe glomerulonephritis after COVID-19 vaccination: A case report and literature review. CEN Case Rep. 2022, 11, 436–441. [Google Scholar] [CrossRef]
- Hashizume, H.; Ajima, S.; Ishikawa, Y. Immunoglobulin A vasculitis post-severe acute respiratory syndrome coronavirus 2 vaccination and review of reported cases. J Dermatol. 2022, 49, 560–563. [Google Scholar] [CrossRef]
- Grossman, M.E.; Appel, G.; Little, A.J.; Ko, C.J.; E Grossman, F.M. Post-COVID-19 vaccination IgA vasculitis in an adult. J. Cutan. Pathol. 2021, 49, 385–387. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, Y.; Liu, F.F.; Wu, Y.; Samadli, S.; Wu, Y.F.; Luo, H.H.; Zhang, D.D.; Hu, P. Association of the infectious triggers with childhood Henoch–Schonlein purpura in Anhui province, China. J. Infect. Public Health 2020, 13, 110–117. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Dong, L.; Feng, S.; Wang, Z. Parainfluenza infection is associated with Henoch-Schönlein purpura in children. Pediatr. Infect. Dis. 2016, 8, 110–114. [Google Scholar] [CrossRef]
- Watanabe, T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1). Pediatr. Nephrol. 2010, 26, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Damjanov, J.; Amato, J.A. Progression of renal disease in Henoch-Schönlein purpura after influenza vaccination. JAMA 1979, 242, 2555–2556. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Komeda, Y.; Watanabe, T.; Kudo, M. Purpura-free small intestinal IgA vasculitis complicated by cytomegalovirus reactivation. BMJ Case Rep. 2020, 13, e235042. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Wasiak, M.; Winiarska, M.; Nogal, K.; Cichoń-Kawa, K.; Pańczyk-Tomaszewska, M.; Małdyk, J. IgA Vasculitis Complicated by Both CMV Reactivation and Tuberculosis. Pediatr. Rep. 2021, 13, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.B.; Wu, J.G.; Cheng, Y.; Li, J.J. Epidemiology and Clinical Characteristics of Henoch-Schönlein Purpura Associated with Epstein-Barr Virus Infection. Mediterr. J. Hematol. Infect. Dis. 2021, 13, e2021064. [Google Scholar] [CrossRef]
- Mohan, N.; Karkra, S. Henoch schonlein purpura as an extra hepatic manifestation of hepatitis A. Indian Pediatr. 2010, 47, 448. [Google Scholar]
- Jariwala, S.; Vernon, N.; Shliozberg, J. Henoch-Schönlein purpura after hepatitis A vaccination. Ann. Allergy Asthma Immunol. 2011, 107, 180–181. [Google Scholar] [CrossRef]
- Akizue, N.; Suzuki, E.; Yokoyama, M.; Inoue, M.; Wakamatsu, T.; Saito, T.; Kusakabe, Y.; Ogasawara, S.; Ooka, Y.; Tawada, A.; et al. Henoch-Schönlein Purpura Complicated by Hepatocellular Carcinoma. Intern. Med. 2017, 56, 3041–3045. [Google Scholar] [CrossRef]
- Helbling, R.; Lava, S.A.; Simonetti, G.D.; Camozzi, P.; Bianchetti, M.G.; Milani, G.P. Gallbladder and Pancreas in Henoch-Schönlein Purpura. J. Craniofacial Surg. 2016, 62, 457–461. [Google Scholar] [CrossRef]
- Da Dalt, L.; Zerbinati, C.; Strafella, M.S.; Renna, S.; Riceputi, L.; Di Pietro, P.; Barabino, P.; Scanferla, S.; Raucci, U. Henoch-Schönlein purpura and drug and vaccine use in childhood: A case-control study. Ital. J. Pediatr. 2016, 42, 60. [Google Scholar] [CrossRef]
- Veraldi, S.; Rizzitelli, G. Henoch–Schönlein purpura and human parvovirus B19. Dermatology 1994, 189, 213–214. [Google Scholar] [CrossRef]
- Veraldi, S.; Mancuso, R.; Rizzitelli, G.; Gianotti, R.; Ferrante, P. Henoch-Schönlein syndrome associated with human Parvovirus B19 primary infection. Eur. J. Dermatol. 1999, 9, 232–233. [Google Scholar]
- Cioc, A.M.; Sedmak, D.D.; Nuovo, G.J.; Dawood, M.R.; Smart, G.; Magro, C.M. Parvovirus B19 associated adult Henoch Schönlein purpura. J. Cutan. Pathol. 2002, 29, 602–607. [Google Scholar] [CrossRef]
- Heegaard, E.D.; Taaning, E.B. Parvovirus B19 and parvovirus V9 are not associated with Henoch-Schönlein purpura in children. Pediatr. Infect. Dis. J. 2002, 21, 31–34. [Google Scholar] [CrossRef]
- Kalman, S.; Aydın, H.I.; Atay, A. Henoch-Schönlein Purpura in a Child Following Varicella. J. Trop. Pediatr. 2005, 51, 240–241. [Google Scholar] [CrossRef]
- Leonardi, S.; Fischer, A.; Arcidiacono, G.; Barone, P.; Ferlito, G.; Musumeci, S. Varicella e porpora di Schönlein-Henoch: Descrizione di un caso con nefropatia. Pediatr. Med. Chir. 1992, 14, 535–537. [Google Scholar]
- Chung, J.-Y.; WookKoo, J.; Kim, S.W. Henoch Schonlein Purpura After Varicella Infection. Pediatr. Infect. Dis. J. 2005, 24, 288. [Google Scholar] [CrossRef]
- Ushigome, Y.; Shiohara, T.; Yamazaki, Y. IgA vasculitis with severe gastrointestinal symptoms may be an unusual manifestation of varicella zoster virus reactivation. Br. J. Dermatol. 2017, 176, 1103–1105. [Google Scholar] [CrossRef]
- Ozen, S.; Marks, S.D.; Brogan, P.; Groot, N.; de Graeff, N.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis—The SHARE initiative. Rheumatology 2019, 58, 1607–1616. [Google Scholar] [CrossRef]
- Lopalco, G.; Rigante, D.; Venerito, V.; Emmi, G.; Anelli, M.G.; Lapadula, G.; Iannone, F.; Cantarini, L. Management of Small Vessel Vasculitides. Curr. Rheumatol. Rep. 2016, 18, 36. [Google Scholar] [CrossRef]
- Hahn, D.; Hodson, E.M.; Willis, N.S.; Craig, J.C. Interventions for preventing and treating kidney disease in Henoch-Schönlein Purpura (HSP). Cochrane Database Syst. Rev. 2015, 2015, CD005128. [Google Scholar] [CrossRef] [PubMed]
- Batu, E.D.; Sener, S.; Baykal, G.O.; Aydin, E.A.; Özdel, S.; Gagro, A.; Esen, E.; Heshin-Bekenstein, M.; Tekgöz, N.A.; Demirkan, F.G.; et al. The characteristics of patients with COVID-19-associated pediatric vasculitis: An international, multicenter study. Arthritis Rheumatol. 2022, 1. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Fujimoto, S.; Maruyama, S.; Mukoyama, M.; Sugiyama, H.; Tsuruya, K.; Sato, H.; Soma, J.; Yano, J.; Itano, S.; et al. Distinct characteristics and outcomes in elderly-onset IgA vasculitis (Henoch-Schönlein purpura) with nephritis: Nationwide cohort study of data from the Japan Renal Biopsy Registry (J-RBR). PLoS ONE 2018, 13, e0196955. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaishvili, M.; Pazhava, A.; Di Lernia, V. Viral Infections May Be Associated with Henoch–Schönlein Purpura. J. Clin. Med. 2023, 12, 697. https://doi.org/10.3390/jcm12020697
Nikolaishvili M, Pazhava A, Di Lernia V. Viral Infections May Be Associated with Henoch–Schönlein Purpura. Journal of Clinical Medicine. 2023; 12(2):697. https://doi.org/10.3390/jcm12020697
Chicago/Turabian StyleNikolaishvili, Mariam, Ani Pazhava, and Vito Di Lernia. 2023. "Viral Infections May Be Associated with Henoch–Schönlein Purpura" Journal of Clinical Medicine 12, no. 2: 697. https://doi.org/10.3390/jcm12020697
APA StyleNikolaishvili, M., Pazhava, A., & Di Lernia, V. (2023). Viral Infections May Be Associated with Henoch–Schönlein Purpura. Journal of Clinical Medicine, 12(2), 697. https://doi.org/10.3390/jcm12020697








