Double Plating for Complex Proximal Humeral Fractures: Clinical and Radiological Outcomes
Abstract
1. Introduction
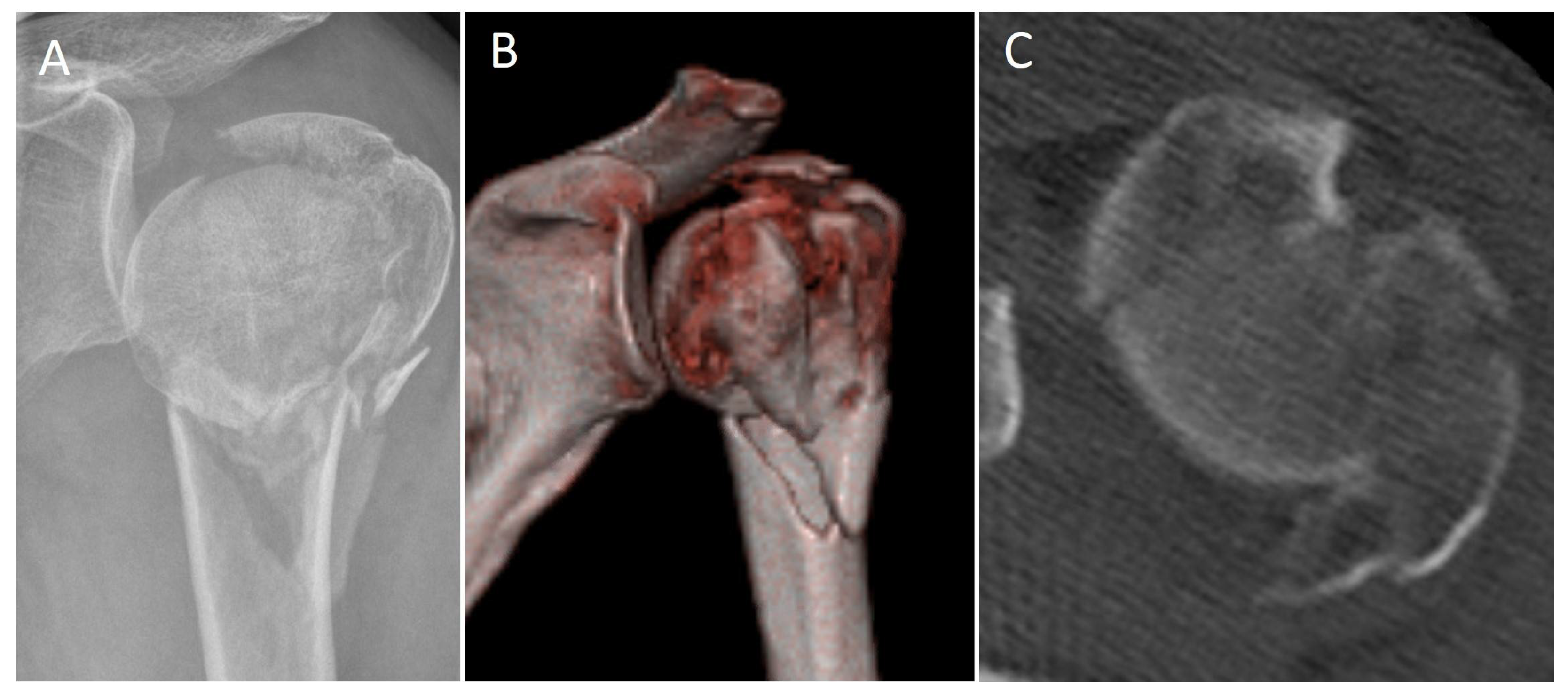
2. Materials and Methods
3. Results
3.1. Recruitment
3.2. Epidemiology
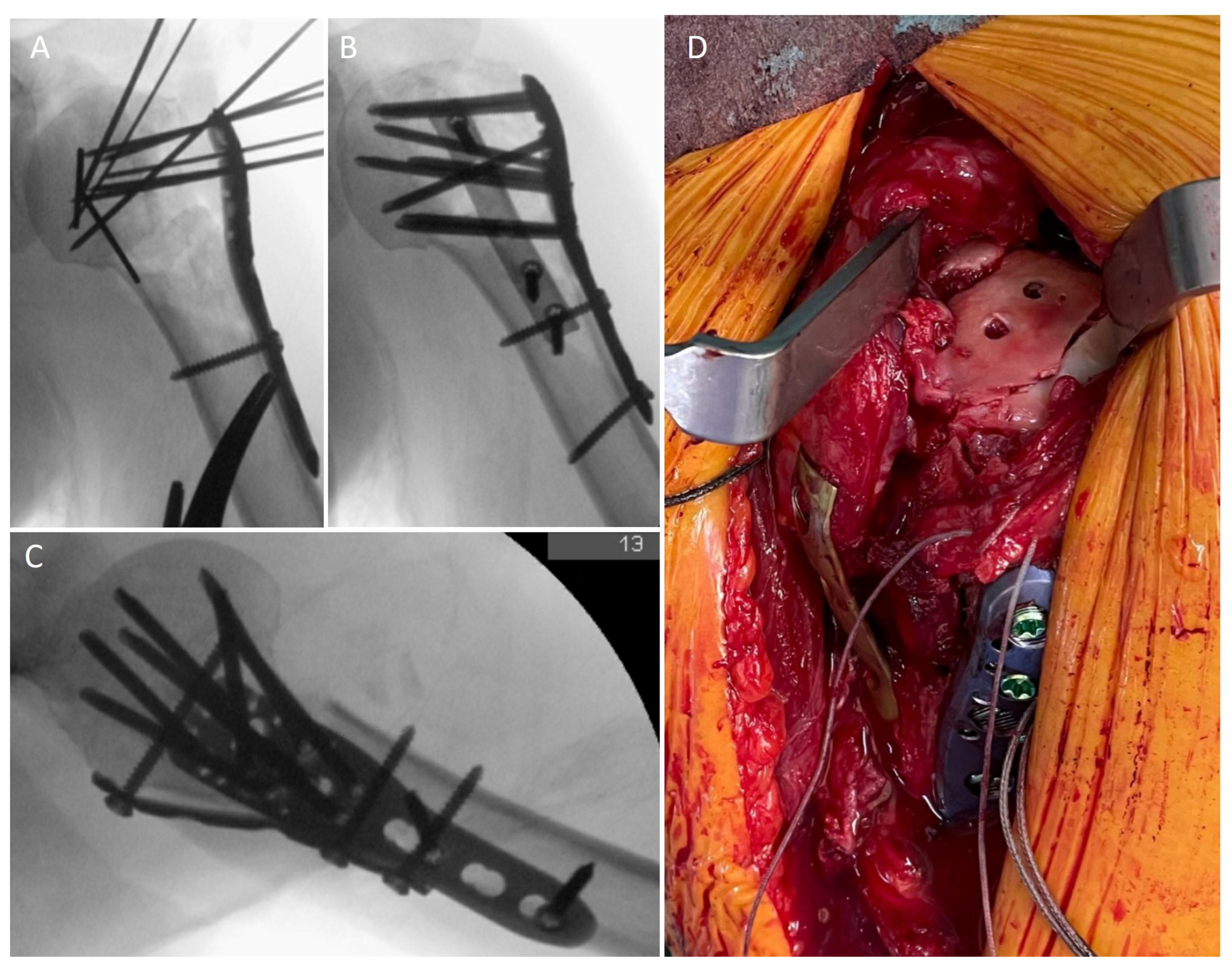
3.3. Surgical Data
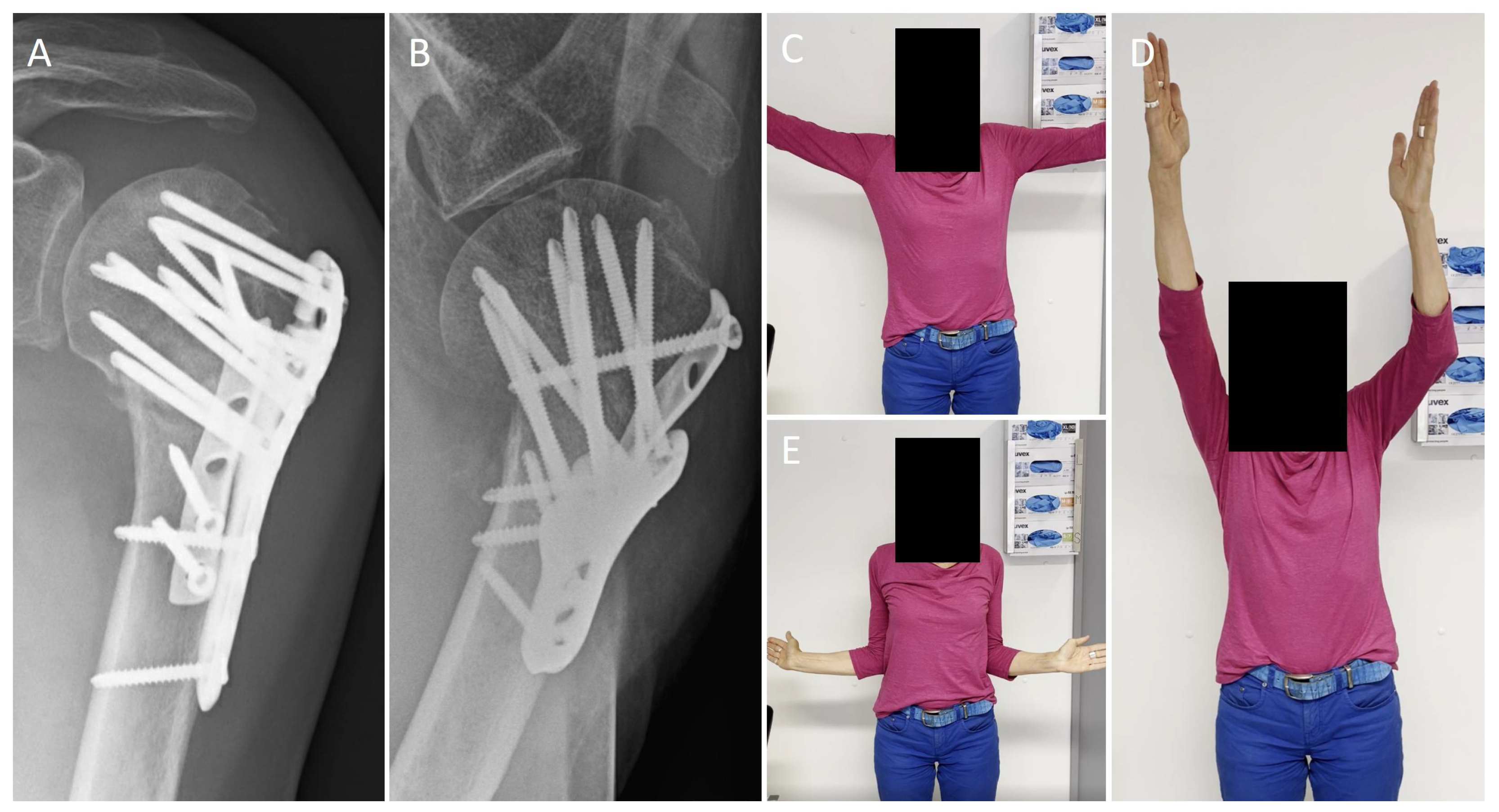
3.4. Funtional Outcome
3.5. Radiological Outcome
3.6. Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Court-Brown, C.M.; Duckworth, A.D.; Clement, N.D.; McQueen, M.M. Fractures in older adults. A view of the future? Injury 2018, 49, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.P.; Gutbrod, J.; Strelzow, J.A.; Maassen, N.H.; Shi, L. Management of Proximal Humerus Fractures in Adults-A Scoping Review. J. Clin. Med. 2022, 11, 6140. [Google Scholar] [CrossRef] [PubMed]
- Brorson, S.; Viberg, B.; Gundtoft, P.; Jalal, B.; Ohrt-Nissen, S. Epidemiology and trends in management of acute proximal humeral fractures in adults: An observational study of 137,436 cases from the Danish National Patient Register, 1996–2018. Acta Orthop. 2022, 93, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Lapner, P.; Sheth, U.; Nam, D.; Schemitsch, E.; Guy, P.; Richards, R.; On behalf of the Canadian Shoulder and Elbow Society (CSES) and the Canadian Orthopedic Trauma Society (COTS). MANAGEMENT OF PROXIMAL HUMERAL FRACTURES IN ADULTS A Systematic Review and Meta-Analysis. J. Orthop. Trauma, 2022; publish ahead of print. [Google Scholar] [CrossRef]
- Alrabaa, R.G.; Ma, G.; Truong, N.M.; Lansdown, D.A.; Feeley, B.T.; Zhang, A.L.; Ma, C.B. Trends in Surgical Treatment of Proximal Humeral Fractures and Analysis of Postoperative Complications over a Decade in 384,158 Patients. JBJS Open Access 2022, 7, e22.00008. [Google Scholar] [CrossRef] [PubMed]
- Cognetti, D.J.; Arana, A.A.; Hoof, M.; Mason, G.; Lin, A.; Sheean, A.J. Short-term Complications for Proximal Humerus Fracture Surgery Have Decreased: An Analysis of the National Surgical Quality Improvement Program Database. Clin. Orthop. Relat. Res. 2022, 480, 2122–2133. [Google Scholar] [CrossRef]
- Gallinet, D.; Ohl, X.; Decroocq, L.; Dib, C.; Valenti, P.; Boileau, P.; French Society for Orthopaedic, S. Is reverse total shoulder arthroplasty more effective than hemiarthroplasty for treating displaced proximal humerus fractures in older adults? A systematic review and meta-analysis. Orthop. Traumatol. Surg. Res. 2018, 104, 759–766. [Google Scholar] [CrossRef]
- Yahuaca, B.I.; Simon, P.; Christmas, K.N.; Patel, S.; Gorman, R.A., 2nd; Mighell, M.A.; Frankle, M.A. Acute surgical management of proximal humerus fractures: ORIF vs. hemiarthroplasty vs. reverse shoulder arthroplasty. J. Shoulder Elbow Surg. 2020, 29, S32–S40. [Google Scholar] [CrossRef]
- Scheibel, M.; Peters, P.; Moro, F.; Moroder, P. Head-split fractures of the proximal humerus. Obere Extrem. 2019, 14, 93–102. [Google Scholar] [CrossRef]
- Peters, P.M.; Plachel, F.; Danzinger, V.; Novi, M.; Mardian, S.; Scheibel, M.; Moroder, P. Clinical and Radiographic Outcomes After Surgical Treatment of Proximal Humeral Fractures with Head-Split Component. J. Bone Jt. Surg. Am. 2020, 102, 68–75. [Google Scholar] [CrossRef]
- Samborski, S.A.; Haws, B.E.; Karnyski, S.; Soles, G.; Gorczyca, J.T.; Nicandri, G.; Voloshin, I.; Ketz, J.P. Outcomes for type C proximal humerus fractures in the adult population: Comparison of nonoperative treatment, locked plate fixation, and reverse shoulder arthroplasty. JSES Int. 2022, 6, 755–762. [Google Scholar] [CrossRef]
- Südkamp, N.; Bayer, J.; Hepp, P.; Voigt, C.; Oestern, H.; Kaab, M.; Luo, C.; Plecko, M.; Wendt, K.; Kostler, W.; et al. Open Reduction and Internal Fixation of Proximal Humeral Fractures with Use of the Locking Proximal Humerus Plate Results of a Prospective, Multicenter, Observational Study. J. Bone Jt. Surg. Am. 2009, 91, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Stolberg-Stolberg, J.; Koppe, J.; Rischen, R.; Freistuhler, M.; Faldum, A.; Katthagen, C.J.; Raschke, M.J. The Surgical Treatment of Proximal Humeral Fractures in Elderly Patients An Analysis of the Long-Term Course of Locked Plate Fixation and Reverse Total Shoulder Arthroplasty Based on Health Insurance Data. Dtsch. Ärzteblatt Int. 2021, 118, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Brorson, S.; Rasmussen, J.V.; Frich, L.H.; Olsen, B.S.; Hrobjartsson, A. Benefits and harms of locking plate osteosynthesis in intraarticular (OTA Type C) fractures of the proximal humerus: A systematic review. Injury 2012, 43, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Shim, S.B.; Kim, H.M.; Lee, J.H.; Lim, H.S. Factors that Influence Reduction Loss in Proximal Humerus Fracture Surgery. J. Orthop. Trauma 2015, 29, 276–282. [Google Scholar] [CrossRef]
- Krappinger, D.; Bizzotto, N.; Riedmann, S.; Kammerlander, C.; Hengg, C.; Kralinger, F.S. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011, 42, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.P.; Kerzner, B.; Fortier, L.M.; Rea, P.M.; Bodendorfer, B.M.; Chahla, J.; Garrigues, G.E.; Verma, N.N. Improved outcomes for proximal humerus fracture open reduction internal fixation augmented with a fibular allograft in elderly patients: A systematic review and meta-analysis. J. Shoulder Elbow Surg. 2022, 31, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Boraiah, S.; Helfet, D.L.; Lorich, D.G. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J. Orthop. Trauma 2008, 22, 195–200. [Google Scholar] [CrossRef]
- Schliemann, B.; Wahnert, D.; Theisen, C.; Herbort, M.; Kosters, C.; Raschke, M.J.; Weimann, A. How to enhance the stability of locking plate fixation of proximal humerus fractures? An overview of current biomechanical and clinical data. Injury 2015, 46, 1207–1214. [Google Scholar] [CrossRef]
- Varga, P.; Inzana, J.A.; Fletcher, J.W.A.; Hofmann-Fliri, L.; Runer, A.; Sudkamp, N.P.; Windolf, M. Cement augmentation of calcar screws may provide the greatest reduction in predicted screw cut-out risk for proximal humerus plating based on validated parametric computational modelling AUGMENTING PROXIMAL HUMERUS FRACTURE PLATING. Bone Jt. Res. 2020, 9, 534–542. [Google Scholar] [CrossRef]
- Michel, P.A.; Katthagen, J.C.; Raschke, M.J.; Dyrna, F.; Heilmann, L.; Schliemann, B. Doppelplattenosteosynthese bei proximaler Humerusfraktur. Obere Extrem. 2019, 15, 52–54. [Google Scholar] [CrossRef]
- Katthagen, J.C.; Huber, M.; Grabowski, S.; Ellwein, A.; Jensen, G.; Lill, H. Failure and revision rates of proximal humeral fracture treatment with the use of a standardized treatment algorithm at a level-1 trauma center. J. Orthop. Traumatol. 2017, 18, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Yeh, C.Y.; Wen, P.C.; Yeh, W.L.; Lin, S.J. The effect of medial calcar support on proximal humeral fractures treated with locking plates. J. Orthop. Surg. Res. 2022, 17, 467. [Google Scholar] [CrossRef]
- Katthagen, J.C.; Schwarze, M.; Meyer-Kobbe, J.; Voigt, C.; Hurschler, C.; Lill, H. Biomechanical effects of calcar screws and bone block augmentation on medial support in locked plating of proximal humeral fractures. Clin. Biomech. 2014, 29, 735–741. [Google Scholar] [CrossRef]
- Michel, P.A.; Katthagen, J.C.; Heilmann, L.F.; Dyrna, F.; Schliemann, B.; Raschke, M.J. Biomechanics of Upper Extremity Double Plating. Z Orthop. Unf. 2020, 158, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Katthagen, J.C.; Schliemann, B.; Michel, P.A.; Heilmann, L.F.; Dyrna, F.; Raschke, M.J. Clinical Application and Outcomes of Upper Extremity Double Plating. Z Orthop. Unf. 2020, 158, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Theopold, J.; Schleifenbaum, S.; Muller, M.; Werner, M.; Hammer, N.; Josten, C.; Hepp, P. Biomechanical evaluation of hybrid double plate osteosynthesis using a locking plate and an inverted third tubular plate for the treatment of proximal humeral fractures. PLoS ONE 2018, 13, e0206349. [Google Scholar] [CrossRef]
- Katthagen, J.C.; Michel, P.; Raschke, M.J.; Sussiek, J.; Frank, A.; Wermers, J.; Dyrna, F.; Schliemann, B. The forgotten fragment: Additional lesser tuberosity fixation of 4-part proximal humeral fractures-a biomechanical investigation. J. Shoulder Elbow Surg. 2021, 30, 2852–2861. [Google Scholar] [CrossRef]
- Schnetzke, M.; Bockmeyer, J.; Porschke, F.; Studier-Fischer, S.; Grutzner, P.A.; Guehring, T. Quality of Reduction Influences Outcome After Locked-Plate Fixation of Proximal Humeral Type-C Fractures. J. Bone Jt. Surg Am. 2016, 98, 1777–1785. [Google Scholar] [CrossRef]
- Boehm, D.; Wollmerstedt, N.; Doesch, M.; Handwerker, M.; Mehling, E.; Gohlke, F. Development of a questionnaire based on the Constant-Murley-Score for self-evaluation of shoulder function by patients. Unfallchirurg 2004, 107, 397–402. [Google Scholar] [CrossRef]
- Resch, H.; Tauber, M.; Neviaser, R.J.; Neviaser, A.S.; Majed, A.; Halsey, T.; Hirzinger, C.; Al-Yassari, G.; Zyto, K.; Moroder, P. Classification of proximal humeral fractures based on a pathomorphologic analysis. J. Shoulder Elbow Surg. 2016, 25, 455–462. [Google Scholar] [CrossRef]
- Pastor, M.F.; Kraemer, M.; Hurschler, C.; Claassen, L.; Wellmann, M.; Smith, T. Transfer of the long head of biceps to the conjoint tendon. A biomechanical study. Clin. Biomech. 2016, 32, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Katolik, L.I.; Romeo, A.A.; Cole, B.J.; Verma, N.N.; Hayden, J.K.; Bach, B.R. Normalization of the Constant score. J. Shoulder Elbow Surg. 2005, 14, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 160–164. [Google Scholar] [CrossRef]
- Katthagen, J.C.; Hennecke, D.; Jensen, G.; Ellwein, A.; Voigt, C.; Lill, H. Arthroscopy after locked plating of proximal humeral fractures: Implant removal, capsular release, and intra-articular findings. Arthroscopy 2014, 30, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hessmann, M.H.; Korner, J.; Hofmann, A.; Sternstein, W.; Rommens, P.M. Angle-fixed plate fixation or double-plate osteosynthesis in fractures of the proximal humerus: A biomechanical study. Biomed. Tech. 2008, 53, 130–137. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Y.S.; Wang, Y.; Zhou, D.S.; Wang, F. Biomechanical evaluation of a novel dualplate fixation method for proximal humeral fractures without medial support. J. Orthop. Surg. Res. 2017, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Fu, Z.G.; Wang, T.B.; Chen, J.H.; Zhang, P.X.; Zhang, D.Y.; Jiang, B.G. Radiological evaluation of reduction loss in unstable proximal humeral fractures treated with locking plates. Orthop. Traumatol. Surg. Res. 2014, 100, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Katthagen, J.C.; Ellwein, A.; Lutz, O.; Voigt, C.; Lill, H. Outcomes of proximal humeral fracture fixation with locked CFR-PEEK plating. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Katthagen, J.C.; Lutz, O.; Voigt, C.; Lill, H.; Ellwein, A. Cement augmentation of humeral head screws reduces early implant-related complications after locked plating of proximal humeral fractures. Obere Extrem. 2018, 13, 123–129. [Google Scholar] [CrossRef]
- Warnhoff, M.; Jensen, G.; Dey Hazra, R.O.; Theruvath, P.; Lill, H.; Ellwein, A. Double plating—Surgical technique and good clinical results in complex and highly unstable proximal humeral fractures. Injury 2021, 52, 2285–2291. [Google Scholar] [CrossRef]
- Pizzo, R.A.; Gianakos, A.L.; Haring, R.S.; Gage, M.J.; Stevens, N.M.; Liporace, F.A.; Yoon, R.S. Are Arthroplasty Procedures Really Better in the Treatment of Complex Proximal Humerus Fractures? A Comprehensive Meta-Analysis and Systematic Review. J. Orthop. Trauma 2021, 35, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Solberg, B.D.; Moon, C.N.; Franco, D.P.; Paiement, G.D. Surgical treatment of three and four-part proximal humeral fractures. J. Bone Jt. Surg Am. 2009, 91, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Stirling, P.H.C.; Goudie, E.B.; MacDonald, D.J.; Strelzow, J.A. Complications and Long-Term Outcomes of Open Reduction and Plate Fixation of Proximal Humeral Fractures. J. Bone Jt. Surg Am. 2019, 101, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Hernigou, J.; Scarlat, M. Shoulder Osteonecrosis: Pathogenesis, Causes, Clinical Evaluation, Imaging, and Classification. Orthop. Surg. 2020, 12, 1340–1349. [Google Scholar] [CrossRef]
- Miltenberg, B.; Masood, R.; Katsiaunis, A.; Moverman, M.A.; Puzzitiello, R.N.; Pagani, N.R.; Menendez, M.E.; Salzler, M.J.; Drager, J. Fracture dislocations of the proximal humerus treated with open reduction and internal fixation: A systematic review. J. Shoulder Elbow Surg. 2022, 31, e480–e489. [Google Scholar] [CrossRef] [PubMed]
| Resch Type | Patients | Percentage |
|---|---|---|
| I | 0 | 0% |
| II | 1 | 2.9% |
| III | 11 | 31.4% |
| IV | 19 | 55.3% |
| V 1 | 4 | 11.4% |
| Movement | Operated Side | Non-Operated Side |
|---|---|---|
| Forward flexion | 139 ± 37° | 169 ± 13° |
| Retroversion | 41 ± 16° | 45 ± 13° |
| Abduction | 128 ± 39° | 168 ± 17° |
| Adduction | 34 ± 12° | 42 ± 11° |
| External rotation 1 | 61 ± 24° | 78 ± 13° |
| Internal rotation 1 | 34 ± 24° | 55 ± 19° |
| Complication | Number |
|---|---|
| Humeral head necrosis | 2 (5.7%) |
| Infection | 1 (2.9%) |
| Non-union | 2 (5.7%) |
| Secondary dislocation | 2 (5.7%) |
| Implant failure | 1 (2.9%) |
| Stiffness | 6 (17.1%) |
| Revision Operation | Number |
|---|---|
| Debridement and implant retention | 1 (2.9%) |
| Conversion to RSA | 2 (5.7%) |
| Conversion to hemi-arthroplasty | 1 (2.9%) |
| Revision ORIF | 2 (5.7%) |
| Arthroscopic Arthrolysis + Implant removal | 6 (17.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, P.A.; Raschke, M.J.; Katthagen, J.C.; Schliemann, B.; Reißberg, I.; Riesenbeck, O. Double Plating for Complex Proximal Humeral Fractures: Clinical and Radiological Outcomes. J. Clin. Med. 2023, 12, 696. https://doi.org/10.3390/jcm12020696
Michel PA, Raschke MJ, Katthagen JC, Schliemann B, Reißberg I, Riesenbeck O. Double Plating for Complex Proximal Humeral Fractures: Clinical and Radiological Outcomes. Journal of Clinical Medicine. 2023; 12(2):696. https://doi.org/10.3390/jcm12020696
Chicago/Turabian StyleMichel, Philipp A., Michael J. Raschke, J. Christoph Katthagen, Benedikt Schliemann, Isabelle Reißberg, and Oliver Riesenbeck. 2023. "Double Plating for Complex Proximal Humeral Fractures: Clinical and Radiological Outcomes" Journal of Clinical Medicine 12, no. 2: 696. https://doi.org/10.3390/jcm12020696
APA StyleMichel, P. A., Raschke, M. J., Katthagen, J. C., Schliemann, B., Reißberg, I., & Riesenbeck, O. (2023). Double Plating for Complex Proximal Humeral Fractures: Clinical and Radiological Outcomes. Journal of Clinical Medicine, 12(2), 696. https://doi.org/10.3390/jcm12020696







