Assessment of Corneal Angiography Filling Patterns in Corneal Neovascularization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analysis of Images
2.3. Statistical Analysis
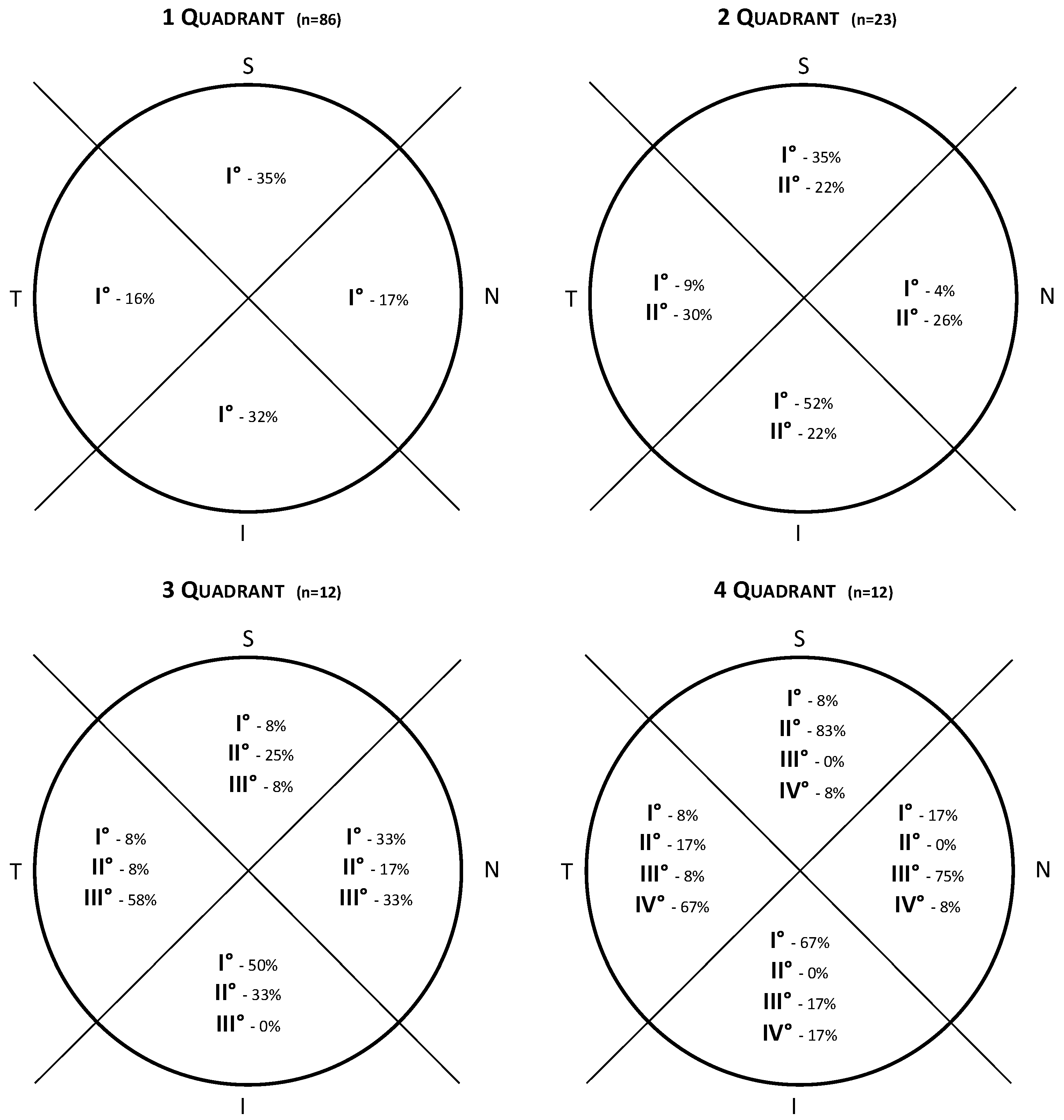
3. Results
3.1. Lesion and CoNV Location
3.2. Order of Filling of CoNV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cursiefen, C.; Schlötzer-Schrehardt, U.; Küchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Investig. Opthalmol. Vis. Sci. 2002, 43, 2127–2135. [Google Scholar]
- Zheng, Y.; Kaye, A.E.; Boker, A.; Stewart, R.K.; Tey, A.; Ahmad, S.; Willoughby, C.; Bron, A.J.; Kaye, S.B. Marginal Corneal Vascular Arcades. Investig. Opthalmol. Vis. Sci. 2013, 54, 7470–7477. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Kheirkhah, A.; Abud, T.B.; Goyal, S.; Dana, R. Management of high-risk corneal transplantation. Surv. Ophthalmol. 2017, 62, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; Dohlman, T.H.; Dana, R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J. Clin. Cell Immunol. 2014, 5, 1000256. [Google Scholar] [CrossRef] [PubMed]
- Palme, C.; Romano, V.; Brunner, M.; Vinciguerra, R.; Kaye, S.B.; Steger, B. Functional Staging of Corneal Neovascularization Using Fluorescein and Indocyanine Green Angiography. Transl. Vis. Sci. Technol. 2018, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Steger, B.; Brunner, M.; Ahmad, S.; Willoughby, C.; Kaye, S.B. Method for Angiographically Guided Fine-Needle Diathermy in the Treatment of Corneal Neovascularization. Cornea 2016, 35, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Steger, B.; Ahmad, M.; Coco, G.; Pagano, L.; Ahmad, S.; Zhao, Y.; Zheng, Y.; Kaye, S.B. Imaging of vascular abnormalities in ocular surface disease. Surv. Ophthalmol. 2022, 67, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Anijeet, D.R.; Zheng, Y.; Tey, A.; Hodson, M.; Sueke, H.; Kaye, S.B. Imaging and Evaluation of Corneal Vascularization Using Fluorescein and Indocyanine Green Angiography. Investig. Opthalmol. Vis. Sci. 2012, 53, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Steger, B.; Kaye, S.B. Fine-Needle Diathermy Guided by Angiography. Cornea 2015, 34, e29–e30. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.P.; Zheng, Y.; Tey, A.; Anijeet, D.; Sueke, H.; Kaye, S.B. Quantifying Changes in Corneal Neovascularization Using Fluorescein and Indocyanine Green Angiography. Am. J. Ophthalmol. 2012, 154, 850–858.e2. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, N.; Romano, V.; Zheng, Y.; Yadav, S.; Dwivedi, R.; Chen, J.; Ahmad, S.; Willoughby, C.E.; Kaye, S.B. Corneal Angiography for Guiding and Evaluating Fine-Needle Diathermy Treatment of Corneal Neovascularization. Ophthalmology 2015, 122, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; Spiteri, N.; Kaye, S.B. Angiographic-Guided Treatment of Corneal Neovascularization. JAMA Ophthalmol. 2015, 133, e143544. [Google Scholar] [CrossRef] [PubMed]
- Steger, B.; Romano, V.; Kaye, S.B. Corneal Indocyanine Green Angiography to Guide Medical and Surgical Management of Corneal Neovascularization. Cornea 2016, 35, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Romano, V.; Steger, B.; Kaye, S.B.; Hamill, K.J.; Willoughby, C.E. Gene-based antiangiogenic applications for corneal neovascularization. Surv. Ophthalmol. 2018, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Romano, V.; Steger, B.; Vinciguerra, R.; Lawman, S.; Williams, B.; Hicks, N.; Czanner, G.; Zheng, Y.; Willoughby, C.E.; et al. Imaging of Corneal Neovascularization: Optical Coherence Tomography Angiography and Fluorescence Angiography. Investig. Opthalmol. Vis. Sci. 2018, 59, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Tey, K.Y.; Ke, M.; Tan, B.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. A pilot study investigating anterior segment optical coherence tomography angiography as a non-invasive tool in evaluating corneal vascularisation. Sci. Rep. 2021, 11, 1212. [Google Scholar] [CrossRef] [PubMed]


| Cause | CoNV Quadrants Involvement (n) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Keratitis (n = 76) | 47 (62%) | 16 (21%) | 8 (10%) | 5 (7%) |
| Ocular surface disease (n = 35) | 22 (63%) | 6 (17%) | 2 (6%) | 5 (14%) |
| Previous graft (n = 11) | 10 (91%) | - | 1 (9%) | - |
| Location | CoNV Quadrants Involvement (n) | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Central (n = 38) | 55% (21) | 18% (7) | 16% (6) | 10% (4) |
| Peripheral (n = 95) | 68% (65) | 17% (16) | 6% (6) | 8% (8) |
| Inferior | Superior | Nasal | Temporal | Total | p-Value | ||
|---|---|---|---|---|---|---|---|
| 1st quadrant | Yes | 15 | 11 | 7 | 5 | 38 | p = 0.041 |
| No | 23 | 27 | 31 | 33 | 114 | ||
| 2nd quadrant | Yes | 3 | 10 | 5 | 2 | 20 | p = 0.017 |
| No | 17 | 10 | 15 | 18 | 60 | ||
| 3rd quadrant | Yes | 1 | 1 | 5 | 2 | 9 | p = 0.09 (NS) |
| No | 8 | 8 | 4 | 7 | 27 | ||
| 4th quadrant | Yes | 1 | 0 | 0 | 3 | 4 | p = 0.046 |
| No | 3 | 4 | 4 | 1 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, L.; Shah, H.; Gadhvi, K.; Ahmad, M.; Menassa, N.; Coco, G.; Kaye, S.; Romano, V. Assessment of Corneal Angiography Filling Patterns in Corneal Neovascularization. J. Clin. Med. 2023, 12, 633. https://doi.org/10.3390/jcm12020633
Pagano L, Shah H, Gadhvi K, Ahmad M, Menassa N, Coco G, Kaye S, Romano V. Assessment of Corneal Angiography Filling Patterns in Corneal Neovascularization. Journal of Clinical Medicine. 2023; 12(2):633. https://doi.org/10.3390/jcm12020633
Chicago/Turabian StylePagano, Luca, Haider Shah, Kunal Gadhvi, Mohammad Ahmad, Nardine Menassa, Giulia Coco, Stephen Kaye, and Vito Romano. 2023. "Assessment of Corneal Angiography Filling Patterns in Corneal Neovascularization" Journal of Clinical Medicine 12, no. 2: 633. https://doi.org/10.3390/jcm12020633
APA StylePagano, L., Shah, H., Gadhvi, K., Ahmad, M., Menassa, N., Coco, G., Kaye, S., & Romano, V. (2023). Assessment of Corneal Angiography Filling Patterns in Corneal Neovascularization. Journal of Clinical Medicine, 12(2), 633. https://doi.org/10.3390/jcm12020633








