Long Term Outcomes of No-Touch Isolation Principles Applied in Pancreaticoduodenectomy for Treatment of Pancreatic Adenocarcinoma: A Multicenter Retrospective Study with Propensity Score Matching
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Surgical Procedure
2.3. Postoperative Outcomes and Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Outcomes
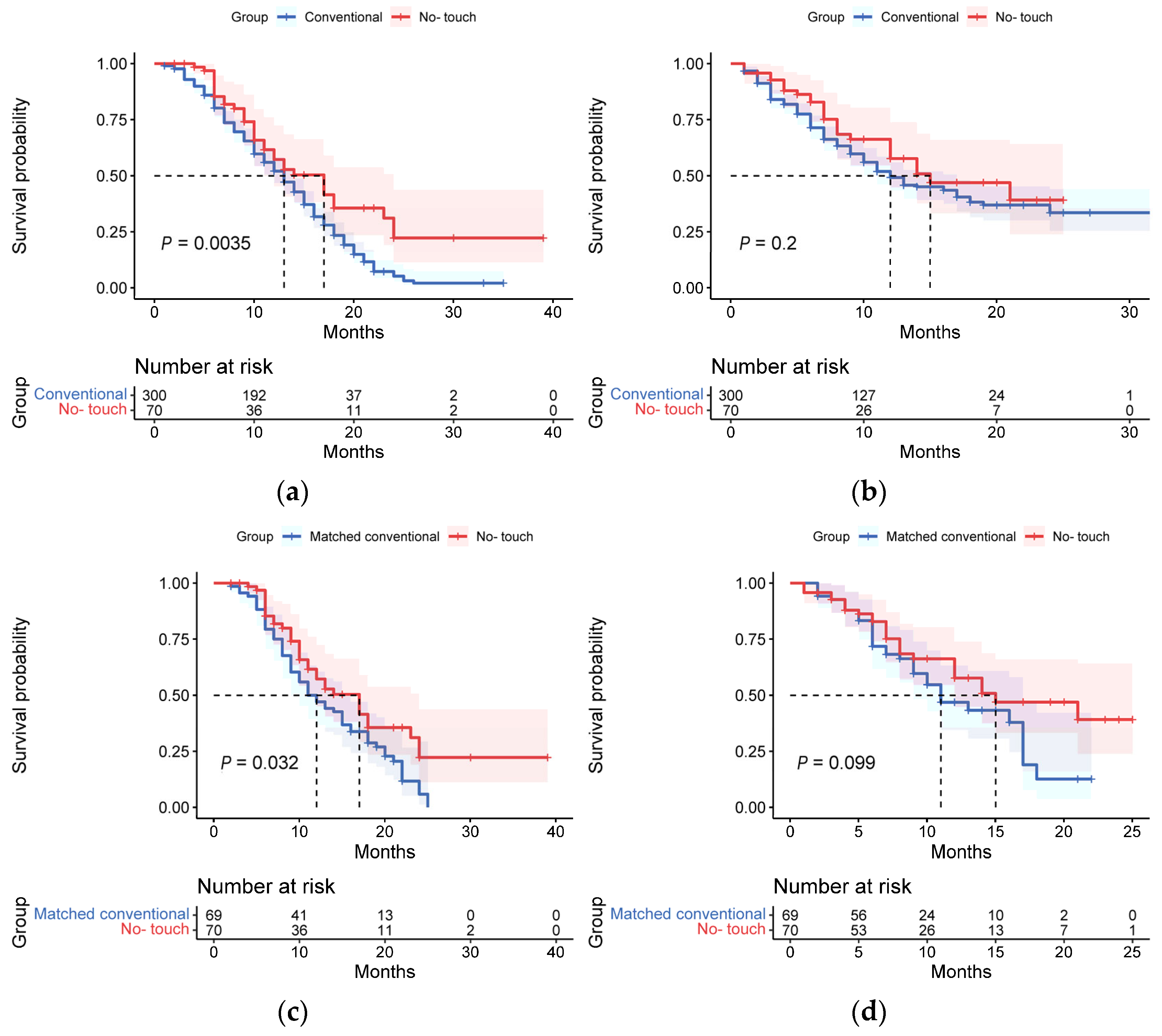
3.3. Long-Term Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, T.; Eguchi, S. No-touch isolation techniques for pancreatic cancer. Surg. Today 2016, 47, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Ogawa, M. No-touch pancreatectomy for invasive ductal carcinoma of the pancreas. JOP J. Pancreas 2014, 15, 243–249. [Google Scholar] [CrossRef]
- Tien, Y.W.; Kuo, H.-C.; Ho, B.-I.; Chang, M.-C.; Chang, Y.-T.; Cheng, M.-F.; Chen, H.-L.; Liang, T.-Y.; Wang, C.-F.; Huang, C.-Y.; et al. A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer. Medicine 2016, 95, e3407. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Lepage, C.; Jouve, J.-L.; Jooste, V.; Chauvenet, M.; Faivre, J.; Bouvier, A.-M. Management and prognosis of pancreatic cancer over a 30-year period. Br. J. Cancer 2009, 101, 215–218. [Google Scholar] [CrossRef]
- Inoue, K.; Hiraoka, T.; Kanemitsu, K.; Takamori, H.; Tsuji, T.; Kawasuji, M. Onset of liver metastasis after histologically curative resection of pancreatic cancer. Surg. Today 2006, 36, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Hayashibe, A.; Kameyama, M.; Shinbo, M.; Makimoto, S. Clinical results on intra-arterial adjuvant chemotherapy for prevention of liver metastasis following curative resection of pancreatic cancer. Ann. Surg. Oncol. 2006, 14, 190–194. [Google Scholar] [CrossRef]
- Katopodis, O.; Souglakos, J.; Stathopoulos, E.; Christopoulou, A.; Kontopodis, E.; Kotsakis, A.; Kalbakis, K.; Kentepozidis, N.; Polyzos, A.; Hatzidaki, D.; et al. Frontline treatment with gemcitabine, oxaliplatin and erlotinib for the treatment of advanced or metastatic pancreatic cancer: A multicenter phase II study of the hellenic oncology research group (HORG). Cancer Chemother. Pharmacol. 2014, 74, 333–340. [Google Scholar] [CrossRef]
- Hess, K.R.; Varadhachary, G.R.; Taylor, S.H.; Wei, W.; Raber, M.N.; Lenzi, R.; Abbruzzese, J.L. Metastatic patterns in adenocarcinoma. Cancer 2006, 106, 1624–1633. [Google Scholar] [CrossRef]
- Yamaue, H.; Tani, M.; Onishi, H.; Kinoshita, H.; Nakamori, M.; Yokoyama, S.; Iwahashi, M.; Uchiyama, K. Locoregional chemotherapy for patients with pancreatic cancer intra-arterial adjuvant chemotherapy after pancreatectomy with portal vein resection. Pancreas 2002, 25, 366–372. [Google Scholar] [CrossRef]
- Beger, H.G.; Rau, B.; Gansauge, F.; Poch, B.; Link, K.-H. Treatment of pancreatic cancer: Challenge of the facts. World J. Surg. 2003, 27, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Fujii, T.; Sugimoto, H.; Kanazumi, N.; Nomoto, S.; Kodera, Y.; Inoue, S.; Takeda, S. Oncological problems in pancreatic cancer surgery. World J. Gastroenterol. 2006, 12, 4466–4472. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.F.; Bockhorn, M.; König, A.; Izbicki, J.R.; Cataldegirmen, G. Surgery for advanced and metastatic pancreatic cancer—Current state and trends. Anticancer. Res. 2012, 32, 1999–2002. [Google Scholar] [PubMed]
- Takii, Y.; Mizusawa, J.; Kanemitsu, Y.; Komori, K.; Shiozawa, M.; Ohue, M.; Ikeda, S.; Takiguchi, N.; Kobatake, T.; Ike, H.; et al. The Conventional Technique Versus the No-touch isolation technique for primary tumor resection in patients with colon cancer (JCOG1006): A Multicenter, open-label, randomized, phase III trial. Ann. Surg. 2022, 275, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Takagi, H. Isolated pancreatectomy for pancreatic head carcinoma using catheter bypass of the portal vein. Hepato-Gastroenterology 1993, 40, 426–429. [Google Scholar] [PubMed]
- Kobayashi, S.; Asano, T.; Ochiai, T. A proposal of no-touch isolation technique in pancreatoduodenectomy for periampullary carcinomas. Hepato-Gastroenterology 2001, 48, 372–374. [Google Scholar]
- Hirota, M.; Kanemitsu, K.; Takamori, H.; Chikamoto, A.; Tanaka, H.; Sugita, H.; Sand, J.; Nordback, I.; Baba, H. Pancreatoduodenectomy using a no-touch isolation technique. Am. J. Surg. 2010, 199, e65–e68. [Google Scholar] [CrossRef]
- Sanjay, P.; Takaori, K.; Govil, S.; Shrikhande, S.V.; Windsor, J.A. ‘Artery-first’ approaches to pancreatoduodenectomy. Br. J. Surg. 2012, 99, 1027–1035. [Google Scholar] [CrossRef]
- Hirota, M.; Shimada, S.; Yamamoto, K.; Tanaka, E.; Sugita, H.; Egami, H.; Ogawa, M. Pancreatectomy using the no-touch isolation technique followed by extensive intraoperative peritoneal lavage to prevent cancer cell dissemination: A pilot study. JOP J. Pancreas 2005, 6, 143–151. [Google Scholar]
- Gall, T.M.H.; Jacob, J.; Frampton, A.E.; Krell, J.; Kyriakides, C.; Castellano, L.; Stebbing, J.; Jiao, L.R. Reduced dissemination of circulating tumor cells with no-touch isolation surgical technique in patients with pancreatic cancer. JAMA Surg. 2014, 149, 482–485. [Google Scholar] [CrossRef]
- Herfarth, C. Surgical strategies and minimal residual disease detection. Semin. Surg. Oncol. 2001, 20, 329–333. [Google Scholar] [CrossRef]
- Tsumura, H.; Satoh, T.; Ishiyama, H.; Tabata, K.-I.; Takenaka, K.; Sekiguchi, A.; Nakamura, M.; Kitano, M.; Hayakawa, K.; Iwamura, M. Perioperative search for circulating tumor cells in patients undergoing prostate brachytherapy for clinically nonmetastatic prostate cancer. Int. J. Mol. Sci. 2017, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tanaka, F.; Yoneda, K.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Kondo, N.; Tsubota, N.; Tsujimura, T.; Tabata, C.; et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Slade, M.J.; Payne, R.; Riethdorf, S.; Ward, B.; Zaidi, S.A.A.; Stebbing, J.; Palmieri, C.; Sinnett, H.D.; Kulinskaya, E.; Pitfield, T.; et al. Comparison of bone marrow, disseminated tumour cells and blood-circulating tumour cells in breast cancer patients after primary treatment. Br. J. Cancer 2008, 100, 160–166. [Google Scholar] [CrossRef]
- Ren, C.; Han, C.; Zhang, J.; He, P.; Wang, D.; Wang, B.; Zhao, P.; Zhao, X. Detection of apoptotic circulating tumor cells in advanced pancreatic cancer following 5-fluorouracil chemotherapy. Cancer Biol. Ther. 2011, 12, 700–706. [Google Scholar] [CrossRef]
- Jiao, L.R.; Apostolopoulos, C.; Jacob, J.; Szydlo, R.; Johnson, N.; Tsim, N.; Habib, N.A.; Coombes, R.C.; Stebbing, J. Unique localization of circulating tumor cells in patients with hepatic metastases. J. Clin. Oncol. 2009, 27, 6160–6165. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 2010, 138, 1714–1726. [Google Scholar] [CrossRef]
- Kanno, K.; Andou, M.; Yanai, S.; Toeda, M.; Nimura, R.; Ichikawa, F.; Teishikata, Y.; Shirane, T.; Sakate, S.; Kihira, T.; et al. Long-term oncological outcomes of minimally invasive radical hysterectomy for early-stage cervical cancer: A retrospective, single-institutional study in the wake of the LACC trial. J. Obstet. Gynaecol. Res. 2019, 45, 2425–2434. [Google Scholar] [CrossRef]
- Yasukawa, M.; Sawabata, N.; Kawaguchi, T.; Taniguchi, S. Wedge resection of tumor before lobectomy for lung cancer could be a no-touch isolation technique. Vivo 2020, 34, 779–785. [Google Scholar] [CrossRef]

| No-Touch PD Group (n = 70) | Conventional PD Group (n = 300) | Matched Conventional PD Group (n = 70) | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 59.93 ± 9.35 | 60.24 ± 10.70 | 61.22 ± 9.37 | 0.829 | 0.418 |
| Sex | 0.560 | 0.324 | |||
| Male | 41 (58.6%) | 187 (62.3%) | 46 (66.7%) | ||
| Female | 29 (41.4%) | 113 (37.7%) | 23 (23.3%) | ||
| BMI (kg/m2) | 22.11 ± 3.80 | 21.88 ± 3.36 | 21.90 ± 3.52 | 0.583 | 0.732 |
| Adjuvant therapy | 23 (32.9%) | 117 (39.0%) | 17 (24.6%) | 0.340 | 0.285 |
| Total bilirubin (mol/L) | 0.580 | 0.571 | |||
| <28 | 22 (31.4%) | 111 (37.0%) | 25 (35.7%) | ||
| 28–34 | 4 (5.7%) | 5 (1.7%) | 1 (1.4%) | ||
| 34–171 | 22 (31.4%) | 96 (32.0%) | 21 (30.4%) | ||
| >171 | 22 (31.4%) | 88 (29.3%) | 22 (31.9%) | ||
| Comorbidities, n (%) | |||||
| Hypertension | 15 (21.4%) | 59 (19.7%) | 13 (18.8%) | 0.426 | 0.704 |
| Diabetes mellitus | 7 (10%) | 43 (14.3%) | 10 (14.3%) | 0.227 | 0.438 |
| Tumor size (cm) | 3.13 ± 1.49 | 3.25 ± 0.98 | 3.25 ± 1.10 | 0.067 | 0.609 |
| Tumor marker | |||||
| CA19-9 | 268.89 ± 332.61 | 389.53 ± 378.54 | 260.27 ± 297.84 | 0.009 | 0.873 |
| CA125 | 27.60 ± 24.56 | 29.02 ± 31.68 | 31.03 ± 30.42 | 0.552 | 0.442 |
| CEA | 5.38 ± 5.58 | 7.15 ± 18.89 | 5.26 ± 6.77 | 0.992 | 0.903 |
| No-Touch PD Group (n = 70) | Conventional PD Group (n = 300) | Matched Conventional PD Group (n = 70) | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Operative information | |||||
| Laparoscopic PD/Open PD | 26/44 | 35/265 | 24/45 | 0.000 | 0.772 |
| Time (min) | 432 ± 110 | 339 ± 104 | 410 ± 88 | 0.000 | 0.178 |
| Blood loss (mL) | 410 ± 329 | 375 ± 367 | 360 ± 353 | 0.140 | 0.390 |
| Blood transfusion, n (%) | 27 (38.6%) | 72 (24.0%) | 23 (33.3%) | 0.013 | 0.520 |
| PV/SMV resection, n (%) | 7 (10.0%) | 47 (15.7%) | 5 (7.2%) | 0.227 | 0.563 |
| Postoperative complications | |||||
| Post-pancreatectomy hemorrhage | 0 (0.0%) | 11 (3.7%) | 2 (2.9%) | 0.030 | 0.151 |
| Gastrointestinal bleeding | 1 (1.4%) | 9 (3.0%) | 4 (5.8%) | 0.432 | 0.167 |
| Pancreatic fistula | 9 (12.9%) | 45 (15%) | 14 (20.3%) | 0.647 | 0.238 |
| Biliary fistula | 2 (2.9%) | 11 (3.7%) | 1 (1.4%) | 0.734 | 0.568 |
| Intestinal obstruction | 2 (2.9%) | 4 (1.3%) | 2 (2.9%) | 0.398 | 0.988 |
| Delayed gastric emptying | 6 (8.6%) | 33 (11%) | 7 (10.1%) | 0.551 | 0.750 |
| Postoperative hospital stay (days) | 17 ± 10 | 14 ± 11 | 16 ± 9 | 0.030 | 0.496 |
| Reoperation | 3 (4.3%) | 10 (3.3%) | 2 (2.9%) | 0.704 | 0.661 |
| No-Touch PD Group (n = 70) | Conventional PD Group (n = 300) | Matched Conventional PD Group (n = 70) | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Pathological results | |||||
| Tumor size, cm | 3.13 ± 1.49 | 3.25 ± 0.98 | 3.25 ± 1.10 | 0.067 | 0.609 |
| Tumor differentiation | 0.000 | 0.138 | |||
| Well | 12 (17.1%) | 5 (1.7%) | 5 (7.2%) | ||
| Moderately | 55 (78.6) | 239 (79.7%) | 58 (84.1%) | ||
| Poorly | 3 (4.3%) | 56 (18.7%) | 6 (8.7%) | ||
| Lymph node metastases | 15 (21.4%) | 124 (41.3%) | 19 (27.5%) | 0.002 | 0.402 |
| Angiolymphatic invasion | 5 (7.1%) | 79 (26.3%) | 8 (11.6%) | 0.001 | 0.367 |
| Perineural invasion | 39 (55.7%) | 234 (78.0%) | 46 (66.7%) | 0.000 | 0.185 |
| AJCC 8th Stage | |||||
| T stage (T1/T2/T3/T4) | 15/40/15/0 | 34/219/45/2 | 10/47/12/0 | 0.040 | 0.389 |
| N stage (N0/N1/N2) | 55/14/1 | 176/99/25 | 50/15/4 | 0.050 | 0.356 |
| M stage (M0/M1) | 70/0 | 296/4 | 69/0 | 0.331 | |
| Stage (I/II/III/IV) | 35/34/1/0 | 149/120/27/4 | 41/24/4/0 | 0.102 | 0.136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, Y.; Song, Y.; Liu, J.; Song, H.; Liu, X.; Li, J.; Ke, N. Long Term Outcomes of No-Touch Isolation Principles Applied in Pancreaticoduodenectomy for Treatment of Pancreatic Adenocarcinoma: A Multicenter Retrospective Study with Propensity Score Matching. J. Clin. Med. 2023, 12, 632. https://doi.org/10.3390/jcm12020632
Mou Y, Song Y, Liu J, Song H, Liu X, Li J, Ke N. Long Term Outcomes of No-Touch Isolation Principles Applied in Pancreaticoduodenectomy for Treatment of Pancreatic Adenocarcinoma: A Multicenter Retrospective Study with Propensity Score Matching. Journal of Clinical Medicine. 2023; 12(2):632. https://doi.org/10.3390/jcm12020632
Chicago/Turabian StyleMou, Yu, Yi Song, Jinheng Liu, Haiyu Song, Xubao Liu, Jiang Li, and Nengwen Ke. 2023. "Long Term Outcomes of No-Touch Isolation Principles Applied in Pancreaticoduodenectomy for Treatment of Pancreatic Adenocarcinoma: A Multicenter Retrospective Study with Propensity Score Matching" Journal of Clinical Medicine 12, no. 2: 632. https://doi.org/10.3390/jcm12020632
APA StyleMou, Y., Song, Y., Liu, J., Song, H., Liu, X., Li, J., & Ke, N. (2023). Long Term Outcomes of No-Touch Isolation Principles Applied in Pancreaticoduodenectomy for Treatment of Pancreatic Adenocarcinoma: A Multicenter Retrospective Study with Propensity Score Matching. Journal of Clinical Medicine, 12(2), 632. https://doi.org/10.3390/jcm12020632







