Correlation between Remote Dielectric Sensing and Chest X-Ray to Assess Pulmonary Congestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
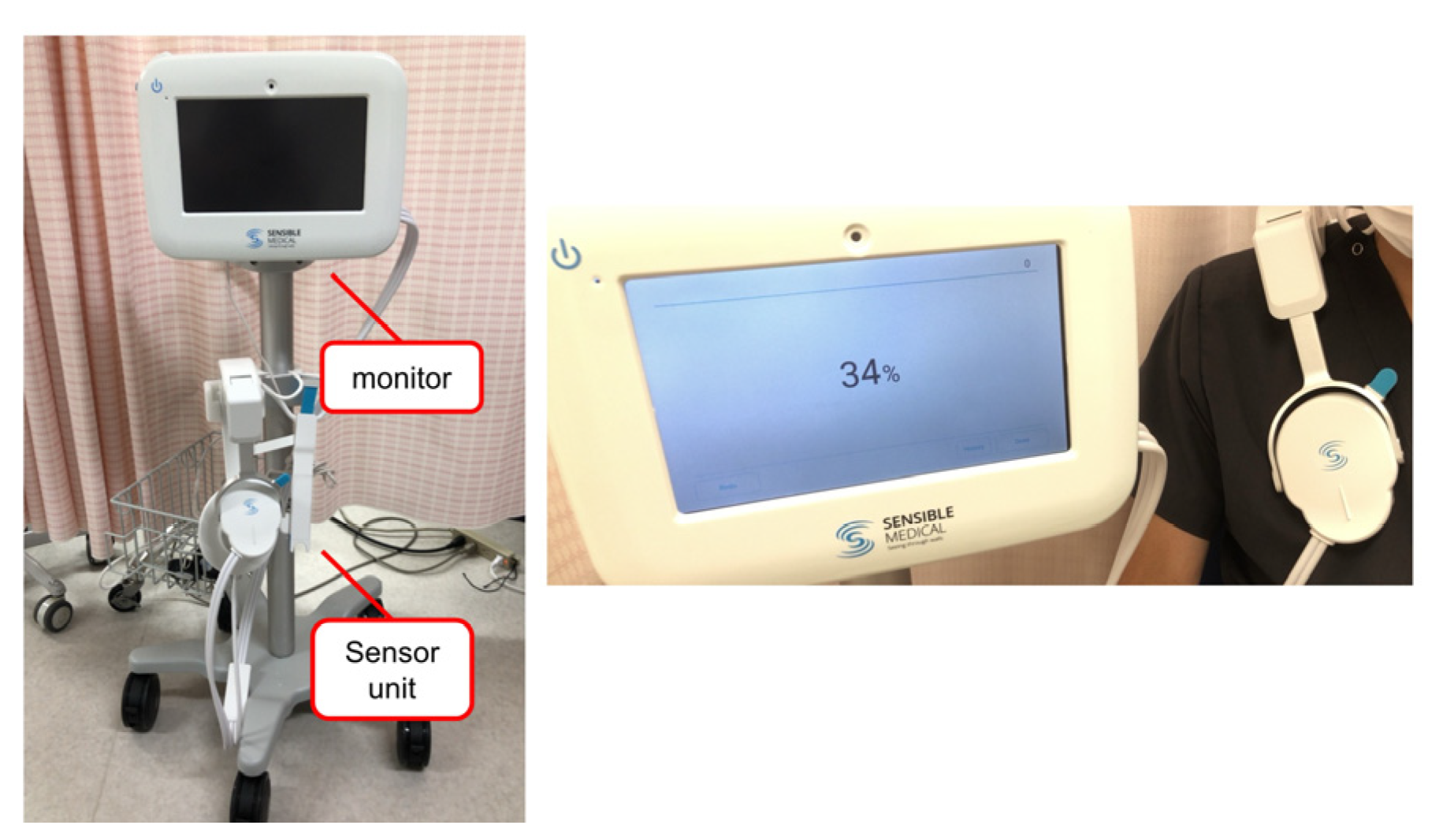
2.2. Measurement of ReDS
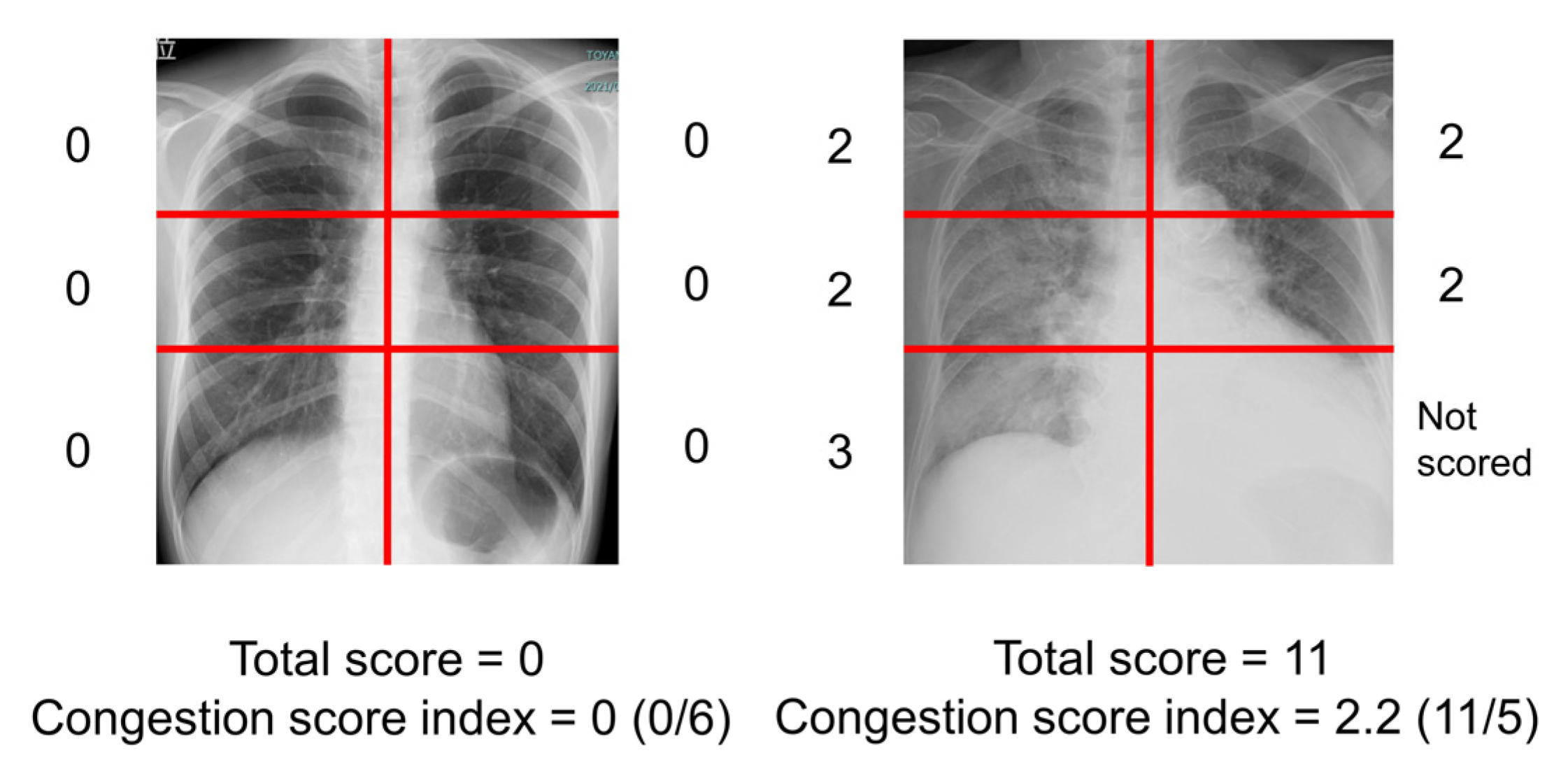
2.3. Measurement of Congestion Score Index of Chest X-Ray
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. ReDS Values and CSI among Those without Lung Diseases
4. Discussion
4.1. ReDS Values and CSIs to Evaluate Pulmonary Congestion
4.2. Clinical Implication of ReDS Measurement
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Patients with Significant Pulmonary Congestion (n = 67) | Patients without Significant Pulmonary Congestion (n = 391) | |
|---|---|---|
| Demographics | ||
| Age, years | 73 (64, 83) | 76 (69, 82) |
| Man | 39 (58%) | 228 (58%) |
| Height, cm | 159 (150, 167) | 159 (151, 166) |
| Body mass index, kg/m2 | 23.8 (21.9, 27.8) | 22.5 (20.2, 24.8) |
| Laboratory data | ||
| Hemoglobin, g/dL | 12.4 (11.0, 13.4) | 12.5 (11.2, 13.8) |
| Serum albumin, g/dL | 3.8 (3.4, 4.1) | 3.9 (3.6, 4.2) |
| Serum creatinine, mg/dL | 0.9 (0.8, 1.5) | 1.0 (0.8, 1.5) |
| Plasma B-type natriuretic peptide, pg/mL | 124 (63, 405) | 89 (30, 233) |
| Echocardiographic data | ||
| Left ventricular ejection fraction, % | 60 (49, 69) | 62 (51, 69) |
| Left ventricular end-diastolic diameter, mm | 49 (45, 56) | 48 (43, 52) |
| Left ventricular end-systolic diameter, mm | 33 (28, 42) | 31 (27, 37) |
| Left atrial diameter, mm | 44 (38, 52) | 40 (34, 46) |
| Past medical history | ||
| Heart failure | 23 (34%) | 107 (27%) |
| Stroke | 14 (21%) | 70 (18%) |
| History of coronary intervention | 17 (25%) | 90 (23%) |
| Hypertension | 47 (70%) | 292 (75%) |
| Dyslipidemia | 35 (52%) | 216 (55%) |
| Diabetes mellitus | 27 (40%) | 136 (35%) |
| Valvular diseases | 28 (42%) | 121 (31%) |
| Chronic kidney diseases | 42 (63%) | 263 (67%) |
| Atrial fibrillation | 30 (45%) | 131 (34%) |
| ReDS, % | 37 (36, 39) | 27 (24, 30) |
| Chest X-ray | ||
| Congestion score index | 0.17 (0.00, 0.73) | 0.00 (0.00, 0.17) |
References
- Platz, E.; Jhund, P.S.; Campbell, R.T.; McMurray, J.J. Assessment and prevalence of pulmonary oedema in contemporary acute heart failure trials: A systematic review. Eur. J. Heart Fail. 2015, 17, 906–916. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Douair, A.; Duarte, K.; Jaeger, D.; Giacomin, G.; Bassand, A.; Jeangeorges, V.; Abensur Vuillaume, L.; P’eud’homme, G.; Huttin, O.; et al. Diagnostic performance of congestion score index evaluated from chest radiography for acute heart failure in the emergency department: A retrospective analysis from the PARADISE cohort. PLoS Med. 2020, 17, e1003419. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Bercker, M.; Huttin, O.; Pierre, S.; Sadoul, N.; Bozec, E.; Chouihed, T.; Ferreira, J.P.; Zannad, F.; Rossignol, P.; et al. Chest X-ray quantification of admission lung congestion as a prognostic factor in patients admitted for worsening heart failure from the ICALOR cohort study. Int. J. Cardiol. 2020, 299, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Douair, A.; Coiro, S.; Giacomin, G.; Bassand, A.; Jaeger, D.; Duarte, K.; Huttin, O.; Zannad, F.; Rossignol, P.; et al. A Combination of Chest Radiography and Estimated Plasma Volume May Predict In-Hospital Mortality in Acute Heart Failure. Front. Cardiovasc. Med. 2022, 8, 752915. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Bensimhon, D.; Pinney, S.P.; Feitell, S.C.; Peacock, W.F.; Amir, O.; Burkhoff, D. Patient monitoring across the spectrum of heart failure disease management 10 years after the CHAMPION trial. ESC Heart Fail. 2021, 8, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Azzam, Z.S.; Gaspar, T.; Faranesh-Abboud, S.; Andria, N.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Validation of remote dielectric sensing (ReDS™) technology for quantification of lung fluid status: Comparison to high resolution chest computed tomography in patients with and without acute heart failure. Int. J. Cardiol. 2016, 221, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Hori, M.; Ueno, Y.; Narang, N.; Onoda, H.; Tanaka, S.; Nakamura, M.; Kataoka, N.; Sobajima, M.; Fukuda, N.; et al. Association between Lung Fluid Levels Estimated by Remote Dielectric Sensing Values and Invasive Hemodynamic Measurements. J. Clin. Med. 2022, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Pang, P.S.; Khan, S.; Konstam, M.A.; Fonarow, G.C.; Traver, B.; Maggioni, A.P.; Cook, T.; Swedberg, K.; Burnett, J.C., Jr.; et al. EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur. Heart J. 2013, 34, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Narang, N.; Kinugawa, K. Clinical implications of remote dielectric sensing system to estimate lung fluid levels. J. Cardiol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Izumida, T.; Imamura, T.; Hori, M.; Kinugawa, K. Correlation Between Plasma B-Type Natriuretic Peptide Levels and Remote Dielectric Sensing in Patients with Heart Failure. Int. Heart J. 2022, 63, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Izumida, T.; Imamura, T.; Kinugawa, K. Remote dielectric sensing and lung ultrasound to assess pulmonary congestion. Heart Vessels. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.; Barghash, M.H.; Giustino, G.; Alvarez-Garcia, J.; Konje, S.; Parikh, A.; Ullman, J.; Keith, B.; Donehey, J.; Mitter, S.S.; et al. Early use of remote dielectric sensing after hospitalization to reduce heart failure readmissions. ESC Heart Fail. 2021, 8, 1047–1054. [Google Scholar] [CrossRef] [PubMed]

| All (n = 458) | Non-Heart Failure (n = 328) | Heart Failure (n = 130) | |
|---|---|---|---|
| Demographics | |||
| Age, years | 76 (69, 82) | 76 (69, 82) | 78 (64, 83) |
| Man | 267 (58%) | 180 (55%) | 87 (67%) |
| Height, cm | 159 (151, 166) | 159 (151, 166) | 158 (152, 169) |
| Body mass index, kg/m2 | 22.8 (20.5, 25.2) | 23.0 (20.9, 25.3) | 21.9 (19.4, 25.0) |
| Laboratory data | |||
| Hemoglobin, g/dL | 12.5 (11.1, 13.8) | 12.5 (11.1, 13.7) | 12.7 (11.3, 14.0) |
| Serum albumin, g/dL | 3.9 (3.6, 4.2) | 3.9 (3.6, 4.2) | 3.8 (3.5, 4.1) |
| Serum creatinine, mg/dL | 1.0 (0.8, 1.5) | 0.9 (0.8, 1.4) | 1.2 (0.9, 1.6) |
| Plasma B-type natriuretic peptide, pg/mL | 95 (33, 253) | 69 (25, 156) | 285 (100, 604) |
| Echocardiographic data | |||
| Left ventricular ejection fraction, % | 62 (51, 69) | 66 (59, 71) | 43 (32, 49) |
| Left ventricular end-diastolic diameter, mm | 48 (43, 53) | 46 (42, 50) | 52 (48, 61) |
| Left ventricular end-systolic diameter, mm | 31 (27, 38) | 29 (26, 33) | 42 (35, 50) |
| Left atrial diameter, mm | 41 (35, 47) | 40 (34, 46) | 43 (37, 49) |
| Past medical history | |||
| Heart failure | 130 (28%) | ||
| Stroke | 84 (18%) | 67 (20%) | 17 (13%) |
| History of coronary intervention | 107 (23%) | 77 (23%) | 30 (23%) |
| Hypertension | 339 (74%) | 247 (75%) | 92 (71%) |
| Dyslipidemia | 251 (55%) | 182 (55%) | 69 (53%) |
| Diabetes mellitus | 163 (36%) | 111 (34%) | 52 (40%) |
| Valvular diseases | 149 (33%) | 90 (27%) | 59 (45%) |
| Chronic kidney diseases | 305 (66%) | 200 (61%) | 105 (81%) |
| Atrial fibrillation | 161 (35%) | 102 (31%) | 59 (45%) |
| ReDS, % | 28 (25, 33) | 28 (24, 32) | 28 (25, 33) |
| Chest X-ray | |||
| Congestion score index | 0.08 (0.00, 0.25) | 0.00 (0.00, 0.17) | 0.08 (0.00, 0.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumida, T.; Imamura, T.; Hori, M.; Nakagaito, M.; Onoda, H.; Tanaka, S.; Ushijima, R.; Kinugawa, K. Correlation between Remote Dielectric Sensing and Chest X-Ray to Assess Pulmonary Congestion. J. Clin. Med. 2023, 12, 598. https://doi.org/10.3390/jcm12020598
Izumida T, Imamura T, Hori M, Nakagaito M, Onoda H, Tanaka S, Ushijima R, Kinugawa K. Correlation between Remote Dielectric Sensing and Chest X-Ray to Assess Pulmonary Congestion. Journal of Clinical Medicine. 2023; 12(2):598. https://doi.org/10.3390/jcm12020598
Chicago/Turabian StyleIzumida, Toshihide, Teruhiko Imamura, Masakazu Hori, Masaki Nakagaito, Hiroshi Onoda, Shuhei Tanaka, Ryuichi Ushijima, and Koichiro Kinugawa. 2023. "Correlation between Remote Dielectric Sensing and Chest X-Ray to Assess Pulmonary Congestion" Journal of Clinical Medicine 12, no. 2: 598. https://doi.org/10.3390/jcm12020598
APA StyleIzumida, T., Imamura, T., Hori, M., Nakagaito, M., Onoda, H., Tanaka, S., Ushijima, R., & Kinugawa, K. (2023). Correlation between Remote Dielectric Sensing and Chest X-Ray to Assess Pulmonary Congestion. Journal of Clinical Medicine, 12(2), 598. https://doi.org/10.3390/jcm12020598







