Loss of Sour Taste Is the Striking Feature among Four Basic Taste Qualities in Tunisian COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Sample Collection and Determination of Inflammatory Markers
2.4. Tasting Sessions
2.5. Statistical Analysis
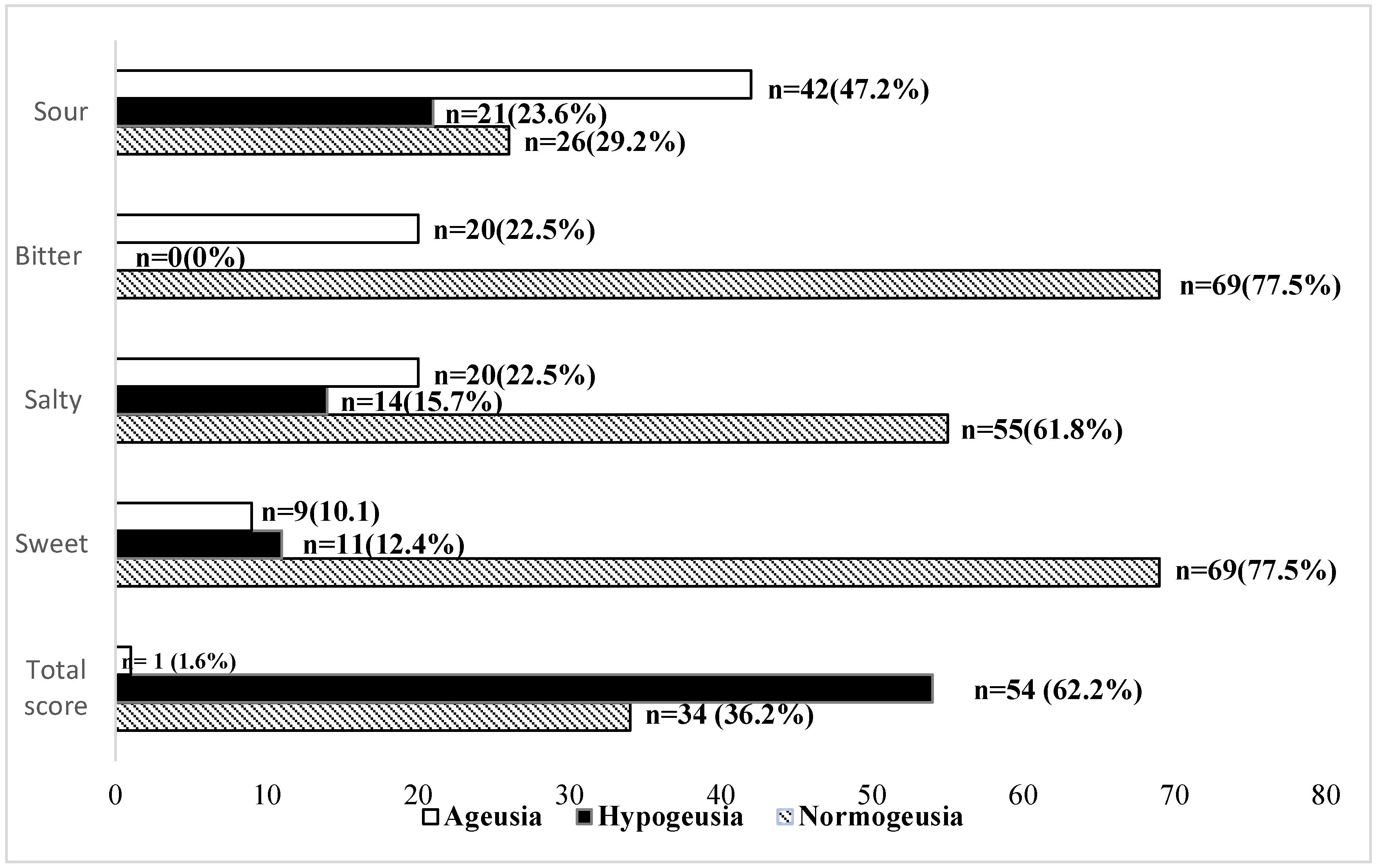
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scotto, G.; Fazio, V.; Lo Muzio, E.; Lo Muzio, L.; Spirito, F. SARS-CoV-2 Infection and Taste Alteration: An Overview. Life 2022, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kusnik, A.; Weiss, C.; Neubauer, M.; Huber, B.; Gerigk, M.; Miethke, T.; Hunter, N.; Rotter, N.; Ludwig, S.; Schell, A.; et al. Presence of gustatory and olfactory dysfunction in the time of the COVID-19 pandemic. BMC Infect. Dis. 2021, 21, 612. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. Oral Symptoms Associated with COVID-19 and Their Pathogenic Mechanisms: A Literature Review. Dent. J. 2021, 9, 32. [Google Scholar] [CrossRef]
- Harizi, C.; Cherif, I.; Najar, N.; Osman, M.; Mallekh, R.; Ayed, O.B.; Ayedi, Y.; Dhaouadi, S.; Hchaichi, A.; Safer, M.; et al. Characteristics and prognostic factors of COVID-19 among infected cases: A nationwide Tunisian analysis. BMC Infect. Dis. 2021, 21, 140. [Google Scholar] [CrossRef]
- Kacem, I.; Gharbi, A.; Harizi, C.; Souissi, E.; Safer, M.; Nasri, A.; Letaief, H.; Akkari, M.; Hechaichi, A.; Mrabet, S.; et al. Characteristics, onset, and evolution of neurological symptoms in patients with COVID-19. Neurol. Sci. 2021, 42, 39–46. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Petrocelli, M.; Lechien, J.R.; Chiesa-Estomba, C.M.; Salzano, G.; Cucurullo, M.; Salzano, F.A.; Saussez, S.; Boscolo-Rizzo, P.; et al. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. J. Laryngol. Otol. 2020, 134, 703–709. [Google Scholar] [CrossRef]
- Singer-Cornelius, T.; Cornelius, J.; Oberle, M.; Metternich, F.U.; Brockmeier, S.J. Objective gustatory and olfactory dysfunction in COVID-19 patients: A prospective cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021, 278, 3325–3332. [Google Scholar] [CrossRef]
- Ciofalo, A.; Cavaliere, C.; Masieri, S.; Di Chicco, A.; Fatuzzo, I.; Lo Re, F.; Baroncelli, S.; Begvarfaj, E.; Adduci, A.; Mezzaroma, I.; et al. Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative. Smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Niklassen, A.; Draf, J.; Huart, C.; Hintschich, C.; Bocksberger, S.; Trecca, E.; Klimek, L.; Le Bon, S.D.; Altundağ, A.; Hummel, T. COVID-19: Recovery from Chemosensory Dysfunction. A Multicentre study on Smell and Taste. Laryngoscope 2021, 131, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, M.; Stihl, C.; Lüsebrink, E.; Hellmuth, J.C.; Scherer, C.; Freytag, S.; Spiegel, J.L.; Stoycheva, I.; Canis, M.; Weiss, B.G.; et al. The course of subjective and objective chemosensory dysfunction in hospitalized patients with COVID-19: A 6-month follow-up. Eur. Arch. Otorhinolaryngol. 2021, 278, 4855–4861. [Google Scholar] [CrossRef]
- Hintschich, C.; Brosig, A.; Hummel, T.; Andorfer, K.; Wenzel, J.; Bohr, C.; Vielsmeier, V. Gustatory Function in Acute COVID-19—Results from Home-Based Psychophysical Testing. Laryngoscope 2022, 132, 1082–1087. [Google Scholar] [CrossRef]
- Cheon, S.Y.; Koo, B.N. Inflammatory Response in COVID-19 Patients Resulting from the Interaction of the Inflammasome and SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 7914. [Google Scholar] [CrossRef]
- Khan, A.S.; Hichami, A.; Khan, N.A. Obesity and COVID-19: Oro-Naso-Sensory Perception. J. Clin. Med. 2020, 9, 2158. [Google Scholar] [CrossRef]
- Cazzolla, A.P.; Lovero, R.; Lo Muzio, L.; Testa, N.F.; Schirinzi, A.; Palmieri, G.; Pozzessere, P.; Procacci, V.; Di Comite, M.; Ciavarella, D.; et al. Taste and Smell Disorders in COVID-19 Patients: Role of Interleukin-6. ACS Chem. Neurosci. 2020, 11, 2774–2781. [Google Scholar] [CrossRef]
- Khan, N.A. Anakinra for severe forms of COVID-19. Lancet Rheumatol. 2020, 2, e586–e587. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Body Mass Index (BMI). Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index/ (accessed on 21 July 2022).
- Instance Nationale de L’évaluation et de L’accréditation en Santé (INEAS). Guide de Pratique Clinique COVID-19. Available online: https://www.ineas.tn/sites/default/files/gpc_covid_19_version_11_mai_2021.pdf/ (accessed on 21 July 2022).
- Mueller, C.; Kallert, S.; Renner, B.; Stiassny, K.; Temmel, A.F.P.; Hummel, T.; Kobal, G. Quantitative assessment of gustatory function in a clinical context using impregnated “taste strips”. Rhinology 2003, 41, 2–6. [Google Scholar]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Fois, A.G.; Piombino, P.; De Riu, G. Potential pathogenesis of ageusia and anosmia in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Ghods, K.; Alaee, A. Olfactory and Taste Disorders in Patients Suffering from COVID-19, a Review of Literature. J. Dent. 2022, 23, 1–6. [Google Scholar]
- Barón-Sánchez, J.; Santiago, C.; Goizueta-San Martín, G.; Arca, R.; Fernández, R. Smell and taste disorders in Spanish patients with mild COVID-19. Neurologia 2020, 35, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Hannum, M.E.; Koch, R.J.; Ramirez, V.A.; Marks, S.S.; Toskala, A.K.; Herriman, R.D.; Lin, C.; Joseph, P.V.; Reed, D.R. Taste loss as a distinct symptom of COVID-19: A systematic review and meta-analysis. Chem. Senses. 2022, 47, bjac001. [Google Scholar] [CrossRef]
- Agyeman, A.A.; Chin, K.L.; Landersdorfer, C.B.; Liew, D.; Ofori-Asenso, R. Smell and Taste Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2020, 95, 1621–1631. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.R.; Nelson, T.M. Genetics of taste receptors. Curr. Pharm. Des. 2014, 20, 2669–2683. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Suliburska, J.; Duda, G.; Pupek-Musialik, D. The influence of hypotensive drugs on the taste sensitivity in patients with primary hypertension. Acta Pol. Pharm. 2012, 69, 121–127. [Google Scholar]
- El Kady, D.M.; Gomaa, E.A.; Abdella, W.S.; Ashraf Hussien, R.; Abd ElAziz, R.H.; Khater, A.G.A. Oral manifestations of COVID-19 patients: An online survey of the Egyptian population. Clin. Exp. Dent. Res. 2021, 7, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Jothimani, D.; Kailasam, E.; Danielraj, S.; Nallathambi, B.; Ramachandran, H.; Sekar, P.; Manoharan, S.; Ramani, V.; Narasimhan, G.; Kaliamoorthy, I.; et al. COVID-19: Poor outcomes in patients with zinc deficiency. Int. J. Infect. Dis. 2020, 100, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Jain, P. Ageusia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549775/ (accessed on 18 November 2022).
- Tsuchiya, H. Characterization and Pathogenic Speculation of Xerostomia Associated with COVID-19: A Narrative Review. Dent. J. 2021, 9, 130. [Google Scholar] [CrossRef]
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E.; et al. In-Silico Evidence for a Two Receptor Based Strategy of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 690655. [Google Scholar] [CrossRef]
- dos Santos, J.A.; Normando, A.G.; Da Silva, R.C.; Acevedo, A.; Canto, G.D.L.; Sugaya, N.; Santos-Silva, A.; Guerra, E. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J. Dent. Res. 2021, 100, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.W. Prevalence and Duration of Acute Loss of Smell or Taste in COVID-19 Patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.M.; Shankayi, Z.; Bahrami, F.; Mohammadzadeh, T.; Amini, H.; Naderi, M. Quantitative analysis of taste disorder in COVID-19 patients, the hypersensitivity to salty quality. New Microbes New Infect. 2021, 43, 100919. [Google Scholar] [CrossRef]
- Fortunato, F.; Martinelli, D.; Iannelli, G.; Milazzo, M.; Farina, U.; Di Matteo, G.; De Nittis, R.; Ascatigno, L.; Cassano, M.; Lopalco, P.L.; et al. Self-reported olfactory and gustatory dysfunctions in COVID-19 patients: A 1-year follow-up study in Foggia district, Italy. BMC Infect. Dis. 2022, 22, 77. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Serge Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Risso, D.; Drayna, D.; Morini, G. Alteration, Reduction and Taste Loss: Main Causes and Potential Implications on Dietary Habits. Nutrients 2020, 12, 3284. [Google Scholar] [CrossRef]
- Brion, M.; de Timary, P.; Vander Stappen, C.; Guettat, L.; Lecomte, B.; Rombaux, P.; Maurage, P. Chemosensory Dysfunction in Alcohol-Related Disorders: A Joint Exploration of Olfaction and Taste. Chem. Senses. 2015, 40, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.; Yilmaz, N.H.; Altin, G.; Atakcan, Z.; Mert, A. Olfactory and Gustatory Dysfunctions in COVID-19 Patients: From a Different Perspective. J. Craniofac. Surg. 2021, 32, 2119–2122. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Quintó, L.; de Haro, J.; Bernal-Sprekelsen, M.; Valero, A.; Picado, C.; Marin, C. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: A population-based survey (OLFACAT study). BMJ Open 2012, 2, e001256. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Deng, Y.K.; Wang, H.; Wang, Z.-C.; Liao, B.; Ma, J.; He, C.; Pan, L.; Liu, Y.; Alobid, I.; et al. Self-reported Taste and Smell Disorders in Patients with COVID-19: Distinct Features in China. Curr. Med. Sci. 2021, 41, 14–23. [Google Scholar] [CrossRef] [PubMed]
| n | % | |
|---|---|---|
| Gender | ||
| Male | 36 | 40.4 |
| Female | 53 | 59.6 |
| Age | ||
| 20–35 years | 11 | 12.4 |
| 35–50 years | 29 | 32.6 |
| 50–65 years | 36 | 40.4 |
| <65 years | 13 | 14.6 |
| BMI * class | ||
| Normal weight | 19 | 21.3 |
| Overweight | 28 | 31.5 |
| Obesity | 42 | 47.2 |
| Antecedents | ||
| Type 2 Diabetes | 22 | 18 |
| Allergic rhinitis | 2 | 2.4 |
| Asthma | 3 | 3.7 |
| Sleep apnea syndrome | 4 | 4.9 |
| COVID-19 symptoms | ||
| Fever | 47 | 52.8 |
| Headache | 34 | 38.2 |
| Cough | 38 | 42.7 |
| Asthenia | 48 | 53.9 |
| Anosmia | 34 | 38.2 |
| Ageusia/Hypogeusia (n = 55), (%) | Normogeusia (n = 34), (%) | p Value | |
|---|---|---|---|
| Gender Male (n = 36) Female (n = 53) | 28 (50.9) 27 (49.09) | 8 (23.52) 26 (76.47) | ns |
| Age (years) 20–35 (n = 11) 35–50 (n = 29) 50–65 (n = 36) <65 years (n = 13) | 1 (9%) 19 (65%) 25 (69%) 10 (76%) | 10 (91%) 10 (35%) 11 (31%) 3 (24%) | 0.01 |
| BMI class Normal weight (n = 19) Overweight (n = 28) Obesity (n = 42) | 6 (11) 16 (29) 33 (60) | 13 (38) 12 (35) 9 (27) | 0.003 ns 0.002 |
| COVID-19 form Minor/Moderate (n = 30) Severe (n = 59) | 15 (27.27) 40 (72.72) | 15 (44.11) 19 (55.88) | ns |
| Markers of Inflammation IL-1B (pg/mL) IL-6 (pg/mL) TNF (pg/mL) CRP (mg/L) | Mean ± SD 8.05 ± 8.39 27.16 ± 65.2 1.39 ± 0.49 88.36 ± 73.28 | Mean ± SD 7.22 ± 2.00 28.44 ± 43.18 1.33 ± 0.42 78.23 ± 71.54 | ns ns ns ns |
| Disease Form | |||
|---|---|---|---|
| Taste | Minor/Moderate (n = 30) | Severe (n = 59) | p Value |
| Total Score | 7.96 ± 3.30 | 6.86 ± 3.13 | ns |
| Bitter | 2.36 ± 1.44 | 1.66 ± 1.28 | 0.021 |
| Sour | 1.36 ± 0.85 | 0.57 ± 0.81 | <0.001 |
| Salty | 1.83 ± 1.44 | 2.01 ± 1.34 | ns |
| Sweet | 2.40 ± 1.42 | 2.61 ± 1.27 | ns |
| [IL-1β] pg/mL | [IL-6] pg/mL | [TNF-α] pg/mL | CRP (mg/L) | |
|---|---|---|---|---|
| Sweet Ageusia/hypo (n = 20) Normogeusia (n = 69) p value | 10.75 ± 1.2 7.12 ± 2.32 0.004 | 19.18 ± 20.4 30.08 ± 63.85 ns | 1.4 ± 0.25 1.35 ± 0.49 ns | 60.04 ± 26.65 40.9 ± 7.17 ns |
| Salty Ageusia/hypo (n = 34) Normogeusia (n = 55) p value | 8.54 ± 2.16 7.07 ± 2.35 ns | 12.93 ± 10.81 28.60 ± 36.87 ns | 1.35 ± 0.28 1.36 ± 0.48 ns | 85.58 ± 66.92 22.50 ± 18.75 ns |
| Sour Ageusia/hypo (n = 63) Normogeusia (n = 26) p value | 7.6 ± 1.5 7.59 ± 2.24 ns | 25.04 ± 21.49 28.69 ± 47.43 ns | 1.31 ± 0.28 1.38 ± 0.48 ns | 84.16 ± 72.58 86.15 ± 78.35 ns |
| Bitter Ageusia/hypo (n = 20) Normogeusia (n = 69) p value | 10.83 ± 13.44 6.83 ± 1.99 0.018 | 22.64 ± 22.5 29.10 ± 64.29 ns | 1.43 ± 0.64 1.34 ± 0.39 ns | 96.8 ± 82.29 80.51 ± 67.52 ns |
| Total score Ageusia/hypo (n = 55) Normogeusia (n = 34) p value | 8.05 ± 8.39 7.22 ± 2.00 ns | 27.16 ± 65.2 28.44 ± 43.18 ns | 1.39 ± 0.49 1.33 ± 0.42 ns | 88.36 ± 73.28 78.23 ± 71.54 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmous, I.; Sayed Khan, A.; Sahnoun, I.; Ben Othman, R.; Ben Jemaa, H.; Mahjoub, F.; Gamoudi, A.; Douik El Gharbi, L.; Mestiri, T.; Khan, N.A.; et al. Loss of Sour Taste Is the Striking Feature among Four Basic Taste Qualities in Tunisian COVID-19 Patients. J. Clin. Med. 2023, 12, 597. https://doi.org/10.3390/jcm12020597
Karmous I, Sayed Khan A, Sahnoun I, Ben Othman R, Ben Jemaa H, Mahjoub F, Gamoudi A, Douik El Gharbi L, Mestiri T, Khan NA, et al. Loss of Sour Taste Is the Striking Feature among Four Basic Taste Qualities in Tunisian COVID-19 Patients. Journal of Clinical Medicine. 2023; 12(2):597. https://doi.org/10.3390/jcm12020597
Chicago/Turabian StyleKarmous, Inchirah, Amira Sayed Khan, Imen Sahnoun, Rym Ben Othman, Houda Ben Jemaa, Faten Mahjoub, Amel Gamoudi, Leila Douik El Gharbi, Tahar Mestiri, Naim Akhtar Khan, and et al. 2023. "Loss of Sour Taste Is the Striking Feature among Four Basic Taste Qualities in Tunisian COVID-19 Patients" Journal of Clinical Medicine 12, no. 2: 597. https://doi.org/10.3390/jcm12020597
APA StyleKarmous, I., Sayed Khan, A., Sahnoun, I., Ben Othman, R., Ben Jemaa, H., Mahjoub, F., Gamoudi, A., Douik El Gharbi, L., Mestiri, T., Khan, N. A., & Jamoussi, H. (2023). Loss of Sour Taste Is the Striking Feature among Four Basic Taste Qualities in Tunisian COVID-19 Patients. Journal of Clinical Medicine, 12(2), 597. https://doi.org/10.3390/jcm12020597









