Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Statistical Analysis
2.3. Quality Assessment
2.4. Outcomes
2.5. Subgroup and Sensitivity Analyses and Trial Sequential Analysis (TSA)
3. Results
3.1. Primary Outcome
3.2. Secondary Outcomes
3.3. Risk of Bias Assessments and Publication Bias
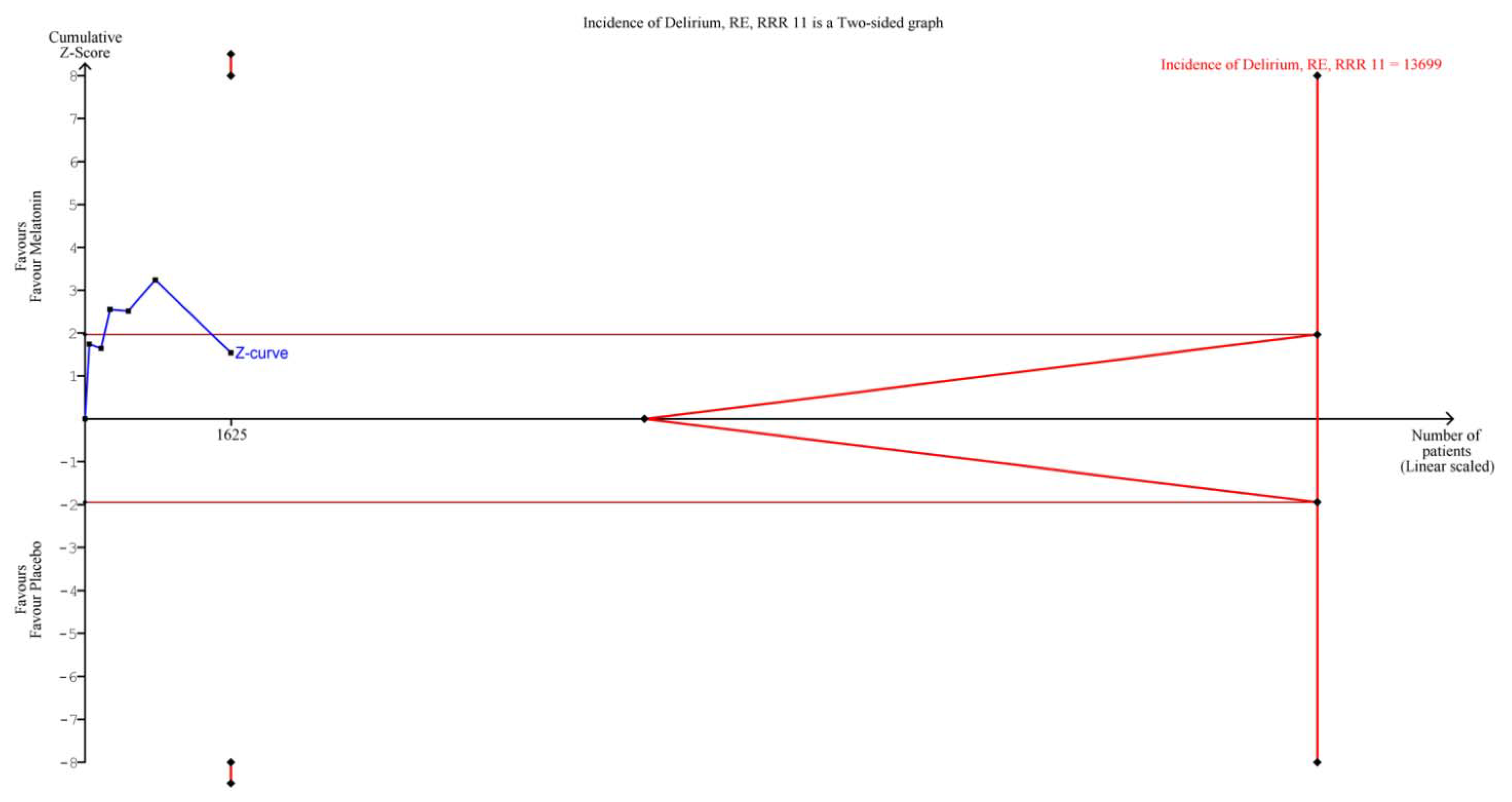
3.4. GRADE of Evidence and TSA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Neufeld, K.J.; Thomas, C. Delirium: Definition, epidemiology, and diagnosis. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2013, 30, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Vasilevskis, E.E.; Han, J.H.; Hughes, C.G.; Ely, E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pract. Research. Clin. Anaesthesiol. 2012, 26, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Bellapart, J.; Appadurai, V.; Lassig-Smith, M.; Stuart, J.; Zappala, C.; Boots, R. Effect of Exogenous Melatonin Administration in Critically Ill Patients on Delirium and Sleep: A Randomized Controlled Trial. Crit. Care Res. Pract. 2020, 2020, 3951828. [Google Scholar] [CrossRef]
- Cavallazzi, R.; Saad, M.; Marik, P.E. Delirium in the ICU: An overview. Ann. Intensive Care 2012, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Page, V.J.; Ely, E.W.; Gates, S.; Zhao, X.B.; Alce, T.; Shintani, A.; Jackson, J.; Perkins, G.D.; McAuley, D.F. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): A randomised, double-blind, placebo-controlled trial. Lancet. Respir. Med. 2013, 1, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Vargas, M.; Buonanno, P.; Iacovazzo, C.; Kotfis, K.; Servillo, G. Haloperidol for preventing delirium in ICU patients: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswar, C.G.; Baldessarini, R.J.; Harsh, V.L.; Alpert, J.E. Adverse endocrine and metabolic effects of psychotropic drugs: Selective clinical review. CNS Drugs 2009, 23, 1003–1021. [Google Scholar] [CrossRef]
- Kram, B.L.; Kram, S.J.; Brooks, K.R. Implications of atypical antipsychotic prescribing in the intensive care unit. J. Crit. Care 2015, 30, 814–818. [Google Scholar] [CrossRef]
- Neufeld, K.J.; Yue, J.; Robinson, T.N.; Inouye, S.K.; Needham, D.M. Antipsychotic Medication for Prevention and Treatment of Delirium in Hospitalized Adults: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 705–714. [Google Scholar] [CrossRef]
- Madrid-Navarro, C.J.; Sanchez-Galvez, R.; Martinez-Nicolas, A.; Marina, R.; Garcia, J.A.; Madrid, J.A.; Rol, M.A. Disruption of Circadian Rhythms and Delirium, Sleep Impairment and Sepsis in Critically ill Patients. Potential Therapeutic Implications for Increased Light-Dark Contrast and Melatonin Therapy in an ICU Environment. Curr. Pharm. Des. 2015, 21, 3453–3468. [Google Scholar] [CrossRef]
- Brzezinski, A. Melatonin in humans. New Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Alling, C.; Lundberg, D.; Malmros, C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol. Scand. 2004, 48, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Khaing, K.; Nair, B.R. Melatonin for delirium prevention in hospitalized patients: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 133, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wibrow, B.; Martinez, F.E.; Myers, E.; Chapman, A.; Litton, E.; Ho, K.M.; Regli, A.; Hawkins, D.; Ford, A.; van Haren, F.M.P.; et al. Prophylactic melatonin for delirium in intensive care (Pro-MEDIC): A randomized controlled trial. Intensive Care Med. 2022, 48, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Clin. Res. Ed.) 2009, 339, b2535. [Google Scholar] [CrossRef]
- Sanfilippo, F.; La Via, L.; Tigano, S.; Morgana, A.; La Rosa, V.; Astuto, M. Trial Sequential Analysis: The evaluation of the robustness of meta-analysis findings and the need for further research. Euromediterranean Biomed. J. 2021, 16, 104–107. [Google Scholar]
- Afshari, A.; Wetterslev, J.; Smith, A.F. Can systematic reviews with sparse data be trusted? Anaesthesia 2017, 72, 12–16. [Google Scholar] [CrossRef]
- Naderi-Behdani, F.; Heydari, F.; Ala, S.; Moradi, S.; Abediankenari, S.; Asgarirad, H.; Khodabakhsh, E. Effect of melatonin on stress-induced hyperglycemia and insulin resistance in critically-ill patients: A randomized double-blind, placebo-controlled clinical trial. Casp. J. Intern. Med. 2022, 13, 51–60. [Google Scholar] [CrossRef]
- Abbasi, S.; Farsaei, S.; Ghasemi, D.; Mansourian, M. Potential Role of Exogenous Melatonin Supplement in Delirium Prevention in Critically Ill Patients: A Double-Blind Randomized Pilot Study. Iran. J. Pharm. Res. IJPR 2018, 17, 1571–1580. [Google Scholar]
- Baumgartner, L.; Lam, K.; Lai, J.; Barnett, M.; Thompson, A.; Gross, K.; Morris, A. Effectiveness of Melatonin for the Prevention of Intensive Care Unit Delirium. Pharmacotherapy 2019, 39, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, H.N.; Ramya, K.; Duggappa, D.R.; Gowda, K.V.; Sudheesh, K.; Nethra, S.S.; Raghavendra Rao, R.S. Effect of melatonin on duration of delirium in organophosphorus compound poisoning patients: A double-blind randomised placebo controlled trial. Indian J. Anaesth. 2016, 60, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, J.V.; Di Bernardo, A.P.A.; Chanes, D.A.V.; Martin, D.F.; Joles, V.B.; Amendola, C.P.; Sanches, L.C.; Ciorlia, G.L.; Lobo, S.M. The Effects of Melatonin Supplementation on Sleep Quality and Assessment of the Serum Melatonin in ICU Patients: A Randomized Controlled Trial. Crit. Care Med. 2020, 48, e1286–e1293. [Google Scholar] [CrossRef]
- Hatta, K.; Kishi, Y.; Wada, K.; Takeuchi, T.; Odawara, T.; Usui, C.; Nakamura, H. Preventive effects of ramelteon on delirium: A randomized placebo-controlled trial. JAMA Psychiatry 2014, 71, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Numaguchi, A.; Takahashi, K.; Miyagawa, Y.; Matsui, K.; Higashi, M.; Makishi, G.; Matsui, S.; Matsuda, N. Effect of Administration of Ramelteon, a Melatonin Receptor Agonist, on the Duration of Stay in the ICU: A Single-Center Randomized Placebo-Controlled Trial. Crit. Care Med. 2018, 46, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Dianatkhah, M.; Najafi, A.; Sharifzadeh, M.; Ahmadi, A.; Sharifnia, H.; Mojtahedzadeh, M.; Najmeddin, F.; Moghaddas, A. Melatonin Supplementation May Improve the Outcome of Patients with Hemorrhagic Stroke in the Intensive Care Unit. J. Res. Pharm. Pract. 2017, 6, 173–177. [Google Scholar] [CrossRef]
- Romero, N.; Dube, K.M.; Lupi, K.E. Evaluation of Delirium in Critically Ill Patients Prescribed Melatonin or Ramelteon. Ann. Pharmacother. 2021, 55, 1347–1354. [Google Scholar] [CrossRef]
- Soltani, F.; Salari, A.; Javaherforooshzadeh, F.; Nassajjian, N.; Kalantari, F. The effect of melatonin on reduction in the need for sedative agents and duration of mechanical ventilation in traumatic intracranial hemorrhage patients: A randomized controlled trial. Eur. J. Trauma Emerg. Surg. 2022, 48, 545–551. [Google Scholar] [CrossRef]
- Shi, Y. Effects of Melatonin on Postoperative Delirium After PCI in Elderly Patients: A Randomized, Single-Center, Double-Blind, Placebo-Controlled Trial. Intensive Care Med. 2021, 24, E893–E897. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Yaddanapudi, L.N.; Saini, V.; Sahni, N.; Grove, S. Efcacy of melatonin in prevention of delirium in critically ill adults: A Randomised Controlled Trial. Intensive Care Med. Exp. 2021, 1, 225. [Google Scholar]
- Bellapart, J.; Roberts, J.A.; Appadurai, V.; Wallis, S.C.; Nuñez-Nuñez, M.; Boots, R.J. Pharmacokinetics of a novel dosing regimen of oral melatonin in critically ill patients. Clin. Chem. Lab. Med. 2016, 54, 467–472. [Google Scholar] [CrossRef]
- Ouimet, S.; Kavanagh, B.P.; Gottfried, S.B.; Skrobik, Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007, 33, 66–73. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Lemoine, P.; Zisapel, N. Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert Opin. Pharmacother. 2012, 13, 895–905. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Bellomo, R.; Hart, G.K.; Norman, T.R.; Goldsmith, D.; Bates, S.; Egi, M. A double-blind placebo-controlled randomised pilot study of nocturnal melatonin in tracheostomised patients. Crit. Care Resusc. J. Australas. Acad. Crit. Care Med. 2006, 8, 187–191. [Google Scholar]
- Wu, Y.; Wang, J.; Wu, A.; Yue, Y. Do fluctuations in endogenous melatonin levels predict the occurrence of postoperative cognitive dysfunction (POCD)? Int. J. Neurosci. 2014, 124, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.J.; Keifer, J.C.; Davies, M.F.; King, T.S.; Bixler, E.O. Melatonin secretion after surgery. Lancet (Lond. Engl.) 2000, 356, 1244–1245. [Google Scholar] [CrossRef]
- Shigeta, H.; Yasui, A.; Nimura, Y.; Machida, N.; Kageyama, M.; Miura, M.; Menjo, M.; Ikeda, K. Postoperative delirium and melatonin levels in elderly patients. Am. J. Surg. 2001, 182, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Yoshitaka, S.; Egi, M.; Morimatsu, H.; Kanazawa, T.; Toda, Y.; Morita, K. Perioperative plasma melatonin concentration in postoperative critically ill patients: Its association with delirium. J. Crit. Care 2013, 28, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lapwood, K.R.; Bhagat, L.; Simpson, M.P. Analgesic effects on endogenous melatonin secretion. J. Pineal Res. 1997, 22, 20–25. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Herxheimer, A.; Petrie, K.J. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst. Rev. 2002, 2010, Cd001520. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.V.; Halladin, N.L.; Rosenberg, J.; Gögenur, I.; Møller, A.M. Melatonin for pre- and postoperative anxiety in adults. Cochrane Database Syst. Rev. 2015, 2015, Cd009861. [Google Scholar] [CrossRef]
- Wurtman, R. Ramelteon: A novel treatment for the treatment of insomnia. Expert Rev. Neurother. 2006, 6, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Erman, M.; Seiden, D.; Zammit, G.; Sainati, S.; Zhang, J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Hirai, K.; Nishiyama, K.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S.; Kawamata, Y.; Hinuma, S.; Miyamoto, M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 2005, 48, 301–310. [Google Scholar] [CrossRef]
- de Jonghe, A.; van Munster, B.C.; Goslings, J.C.; Kloen, P.; van Rees, C.; Wolvius, R.; van Velde, R.; Levi, M.; de Haan, R.J.; de Rooij, S.E. Effect of melatonin on incidence of delirium among patients with hip fracture: A multicentre, double-blind randomized controlled trial. CMAJ Can. Med. Assoc. J. 2014, 186, E547–E556. [Google Scholar] [CrossRef]
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF Bundle in Critical Care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef]
- Ely, E.W. The ABCDEF Bundle: Science and Philosophy of How ICU Liberation Serves Patients and Families. Crit. Care Med. 2017, 45, 321–330. [Google Scholar] [CrossRef]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Martucci, G.; La Via, L.; Cuttone, G.; Dimarco, G.; Pulizzi, C.; Arcadipane, A.; Astuto, M. Hemoperfusion and blood purification strategies in patients with COVID-19: A systematic review. Artif. Organs 2021, 45, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.B.; Bozza, F.A.; Soares, M.; do Brasil, P.E.; Tura, B.R.; Ely, E.W.; Salluh, J.I. Pharmacologic prevention and treatment of delirium in intensive care patients: A systematic review. J. Crit. Care 2015, 30, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.B. Which medications to avoid in people at risk of delirium: A systematic review. Age Ageing 2011, 40, 23–29. [Google Scholar] [CrossRef]
- Siddiqi, N.; Stockdale, R.; Britton, A.M.; Holmes, J. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst. Rev. 2007, 18, Cd005563. [Google Scholar] [CrossRef]
- Patel, J.; Baldwin, J.; Bunting, P.; Laha, S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia 2014, 69, 540–549. [Google Scholar] [CrossRef]
- Barnes-Daly, M.A.; Phillips, G.; Ely, E.W. Improving Hospital Survival and Reducing Brain Dysfunction at Seven California Community Hospitals: Implementing PAD Guidelines Via the ABCDEF Bundle in 6,064 Patients. Crit. Care Med. 2017, 45, 171–178. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
| Studies | Outcomes Reported | Participants | Intervention | Sample | Age (Mean) | Severity Score APACHE II | Dosage |
|---|---|---|---|---|---|---|---|
| Primary analysis | |||||||
| Wibrow, 2022 Int Care Med [15] | Delirium, MV, LOS, mortality | ICU | Melatonin | 419 | 61.9 | 17.3 | 4 mg/day (2 weeks or LOS) |
| Placebo | 422 | 61.9 | 17.5 | ||||
| Shi, 2021 Heart Surg Forum [29] | Delirium, mortality | ICU after PCI | Melatonin | 148 | 71.5 | ** | 3 mg/day for 1 week |
| Placebo | 149 | 71.6 | ** | ||||
| Gandolfi, 2020 Crit. Care Med. [23] | Delirium, LOS, mortality | ICU | Melatonin | 102 | 60.0 | 1.5 * | 10 mg/day for 7 days |
| Placebo | 101 | 57.0 | 1.42 * | ||||
| Abbasi,2018 Iran J Pharm Res [20] | Delirium, LOS, mortality | mixed ICU surgical/medical | Melatonin | 67 | 52.5 | 8.1 | 3 mg/day for 5 days |
| Placebo | 70 | 49.9 | 7.3 | ||||
| Nishikimi, 2018 Crit Care Med [25] | Delirium, LOS, mortality | medical ICU | Ramelteon | 45 | 67.0 | 23.98 | 8 mg/day |
| Placebo | 43 | 66.5 | 23.95 | ||||
| Vijayakumar, 2016 Indian J Anaesth. [22] | Delirium, MV, LOS | ICU poisoning with organophosphorus | Melatonin | 26 | 36.9 | 10.2 | 3 mg/day |
| Placebo | 30 | 38 | 8.56 | ||||
| Bellapart, 2020 Crit Care Res Pract [31] | LOS | ICU | Melatonin | 21 | 54.75 | 21.5 | 6 mg/day |
| Placebo | 12 | 57.25 | 22.5 | ||||
| Soltani, 2020 Eur J Trauma Emerg Surg [28] | MV, LOS, mortality | ICU for intracranial hemorrhage | Melatonin | 26 | 34.62 | 7.45 | 3 mg/day |
| Placebo | 26 | 36.85 | 8.14 | ||||
| Dianatkhah, 2017 J Res Pharm Pract [26] | MV, LOS, mortality | ICU for hemorrhagic stroke | Melatonin | 20 | 57.7 | 17.6 | 30 mg/day |
| Placebo | 20 | 52.9 | 16.9 | ||||
| Sensitivity analysis | |||||||
| Bandyopadhya, 2021 ESICM Lives 2021 [30] | Delirium | ICU | Melatonin | 54 | / | / | 3 mg/day |
| Placebo | 54 | / | / | ||||
| Romero, 2021 Pharmacotherapy [27] | Delirium, MV, LOS | ICU | Melatonin/ramelteon | 131 | 65.51 | 4 * | 3 mg/day melatonin |
| Placebo | 27 | 60.7 | 4.125 * | 8 mg/day ramelteon | |||
| Baumgartner, 2019 Pharmacotherapy [21] | Delirium, MV, LOS, mortality | Mixed or cardiac ICU medical/surgical | Melatonin | 117 | 60.5 | 17.5 | 3.5 mg/day (1–10 mg range) |
| Placebo | 115 | 59.5 | 15.75 | ||||
| Hatta, 2014 JAMA Psychiatry [24] | Delirium | Medical ICU and acute wards | Ramelteon | 33 | 78.3 | 14.6 | 8 mg/day |
| Placebo | 34 | 78.2 | 13.5 | ||||
| Outcome | Studies | Patients (n) | RR or MD (95% CI) | p-Value | Heterogeneity | |
|---|---|---|---|---|---|---|
| I2 | p-Value | |||||
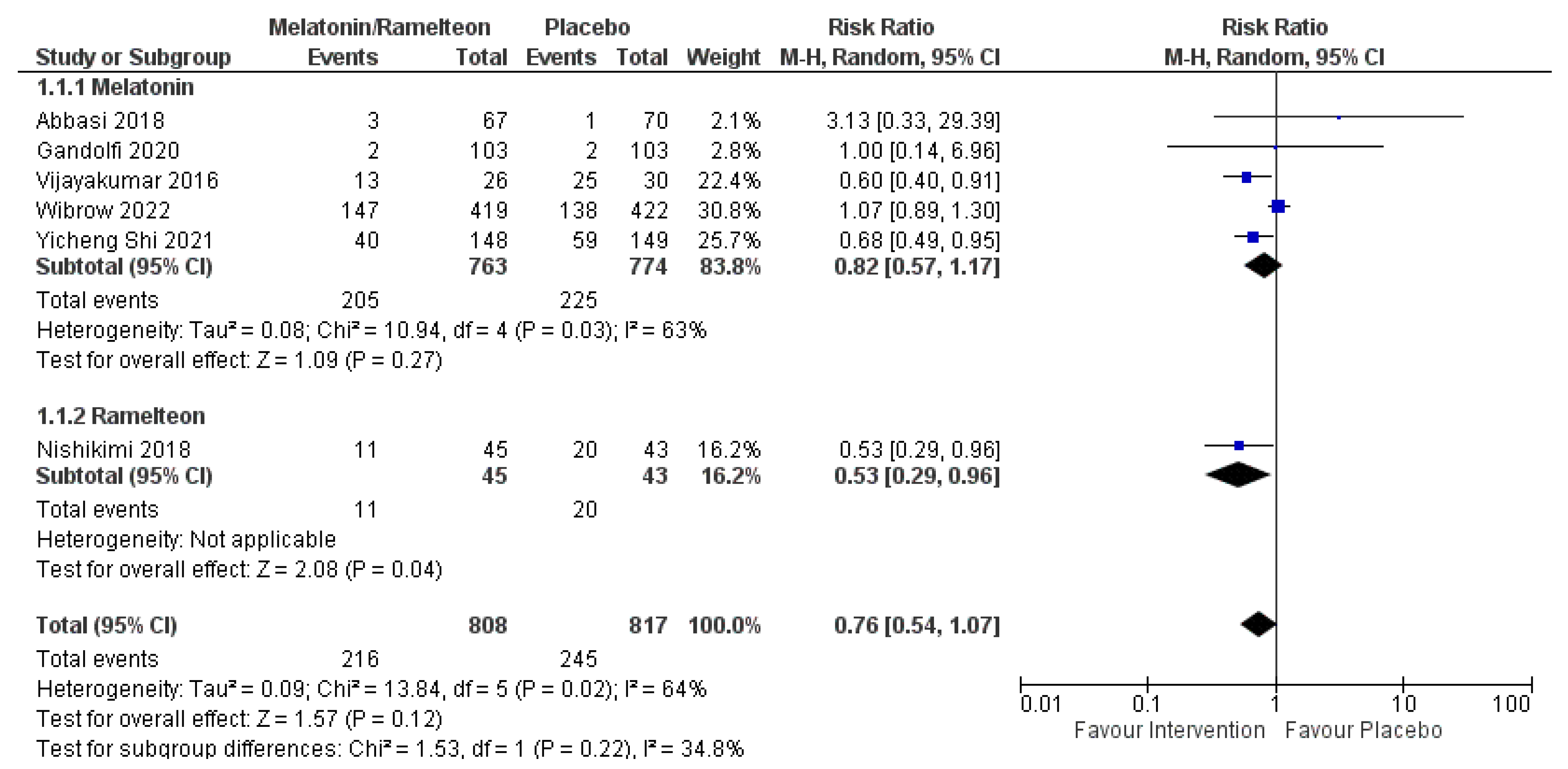
| Delirium incidence | 6 | 1625 | RR 0.76 (0.54, 1.07) | 0.12 | 64% | 0.02 |
| Days of MV | 4 | 989 | MD −2.80 (−6.06, 0.47) | 0.09 | 94% | <0.00001 |
| Mortality | 7 | 1661 | RR 0.84 (0.62, 1.13) | 0.26 | 0% | 0.60 |
| LOS in ICU | 8 | 1453 | MD −0.26 (−0.89, 0.37) | 0.42 | 75% | 0.0002 |
| Melatonin or Ramelteon as Compared to Placebo for Prevention of Delirium in the Intensive Care Unit | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty Assessment | Summary of Findings | ||||||||||
| Participants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
| Placebo | Melatonin or Ramelteon | Risk with Placebo | Risk Difference with Melatonin or Ramelteon | ||||||||
| Delirium incidence | |||||||||||
| 1625 (6 RCTs) | Serious | Not serious | Very serious a | Not serious | None | Very low | 245/817 (30.0%) | 216/808 (26.7%) | RR 0.76 (0.54 to 1.07) | 300 per 1000 | 72 fewer per 1000 (from 138 fewer to 21 more) |
| Days of mechanical ventilation | |||||||||||
| 989 (4 RCTs) | Serious | Not serious | Very serious | Serious b | None | Very low | 498 | 491 | - | The mean duration of mechanical ventilation was 0 days | MD 2.8 lower (6.06 lower to 0.47 higher) |
| Mortality at longest follow-up | |||||||||||
| 1661 (7 RCTs) | Serious | Not serious | Very serious | Serious c | None | Very low | 85/833 (10.2%) | 71/828 (8.6%) | RR 0.84 (0.62 to 1.13) | 102 per 1000 | 16 fewer per 1000 (from 39 fewer to 13 more) |
| Length of stay in the intensive care unit | |||||||||||
| 1453 (8 RCTs) | Very serious | Not serious | Very serious | Serious d | None | Very low | 726 | 727 | - | The mean length of stay was 0 days | MD 0.26 lower (0.89 lower to 0.37 higher) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, G.; Cuocina, M.; La Via, L.; Messina, S.; Attaguile, G.A.; Cantarella, G.; Sanfilippo, F.; Bernardini, R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 435. https://doi.org/10.3390/jcm12020435
Aiello G, Cuocina M, La Via L, Messina S, Attaguile GA, Cantarella G, Sanfilippo F, Bernardini R. Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(2):435. https://doi.org/10.3390/jcm12020435
Chicago/Turabian StyleAiello, Giuseppe, Micol Cuocina, Luigi La Via, Simone Messina, Giuseppe A. Attaguile, Giuseppina Cantarella, Filippo Sanfilippo, and Renato Bernardini. 2023. "Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 2: 435. https://doi.org/10.3390/jcm12020435
APA StyleAiello, G., Cuocina, M., La Via, L., Messina, S., Attaguile, G. A., Cantarella, G., Sanfilippo, F., & Bernardini, R. (2023). Melatonin or Ramelteon for Delirium Prevention in the Intensive Care Unit: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 12(2), 435. https://doi.org/10.3390/jcm12020435









