Predictive Model for Preeclampsia Combining sFlt-1, PlGF, NT-proBNP, and Uric Acid as Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnostic Criteria
2.3. Laboratory Methods
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
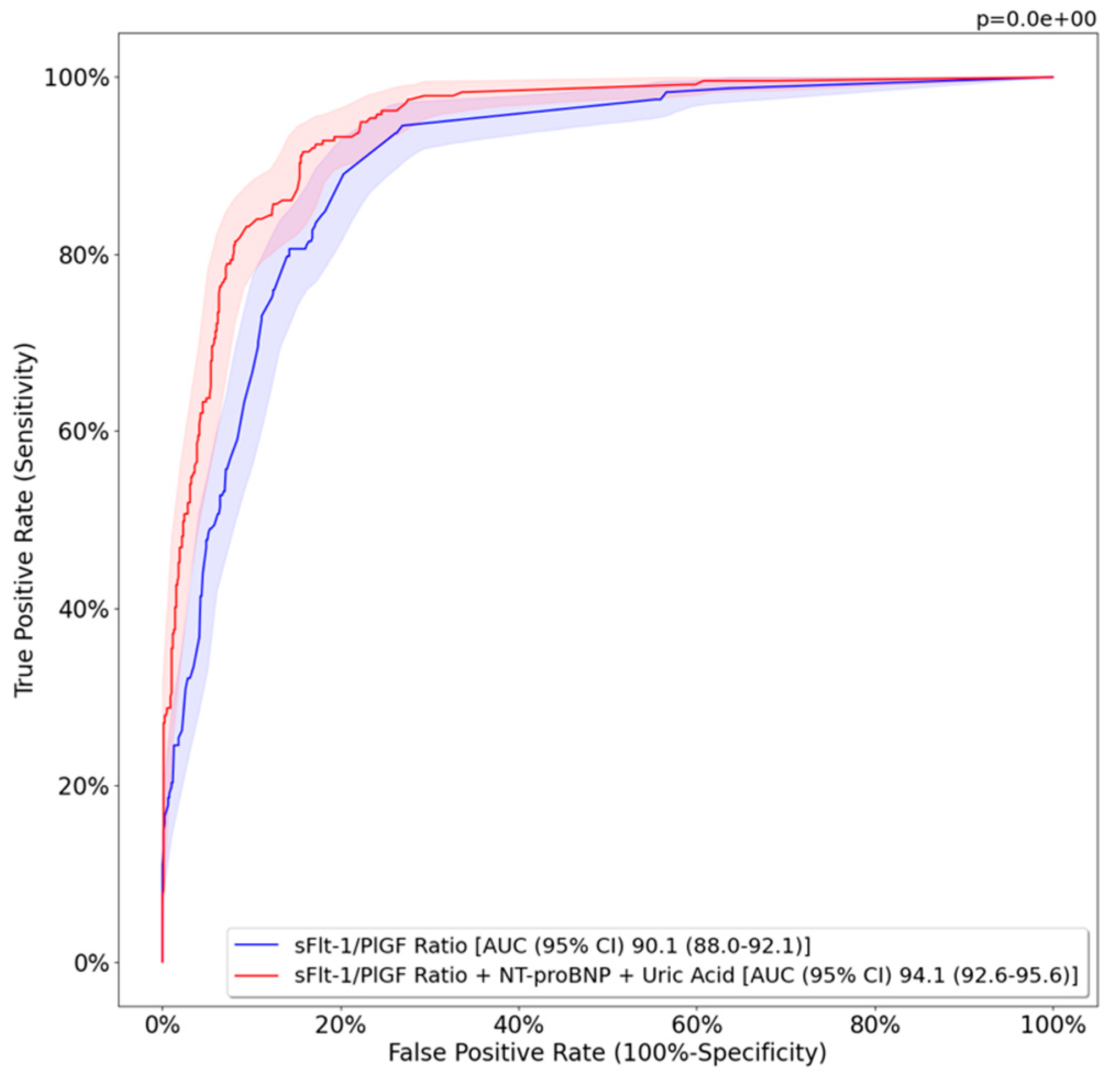
3.2. Predictive Model Results
4. Discussion
4.1. Principal Findings
4.2. Interpretation of Results and Comparison with Existing Literature
4.3. Clinical Implications and Future Research Directions
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ronsmans, C.; Graham, W.J. Maternal mortality: Who, when, where, and why. Lancet 2006, 368, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Staff, A.C.; Braekke, K.; Harsem, N.K.; Lyberg, T.; Holthe, M.R. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 122, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Staff, A.C.; Benton, S.J.; von Dadelszen, P.; Roberts, J.M.; Taylor, R.N.; Powers, R.W.; Charnock-Jones, D.S.; Redman, C.W.G. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 2013, 61, 932–942. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-Like Tyrosine Kinase-1-to-Placental Growth Factor Ratio and Time to Delivery in Women with Suspected Preeclampsia. Obstet. Gynecol. 2016, 128, 261–269. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1: PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Moertl, M.; Zeisler, H.; Calda, P.; Holzgreve, W.; Galindo, A.; Engels, T.; et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am. J. Obstet. Gynecol. 2012, 206, 58.e1-8. [Google Scholar] [CrossRef]
- Herraiz, I.; Llurba, E.; Verlohren, S.; Galindo, A.; Bartha, J.L.; De La Calle, M.; Delgado, J.L.; De Paco, C.; Escudero, A.I.; Moreno, F.; et al. Update on the Diagnosis and Prognosis of Preeclampsia with the Aid of the sFlt-1/PlGF Ratio in Singleton Pregnancies. Fetal Diagn. Ther. 2018, 43, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.J.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: Ruling out pre-eclampsia for up to 4 weeks and value of retesting. Ultrasound Obstet. Gynecol. 2019, 53, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Vatish, M.; Strunz-McKendry, T.; Hund, M.; Allegranza, D.; Wolf, C.; Smare, C. sFlt-1/PlGF ratio test for pre-eclampsia: An economic assessment for the UK. Ultrasound Obstet. Gynecol. 2016, 48, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular physiology of pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Baltabaeva, A.; Liberati, M.; Thilaganathan, B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011, 57, 85–93. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Seong, W.J.; Kim, S.C.; Hong, D.G.; Koo, T.B.; Park, S. Amino-terminal pro-brain natriuretic peptide levels in hypertensive disorders complicating pregnancy. Hypertens. Pregnancy 2011, 30, 287–294. [Google Scholar] [CrossRef]
- Álvarez-Fernández, I.; Prieto, B.; Rodríguez, V.; Ruano, Y.; Escudero, A.I.; Álvarez, F.V. N-terminal pro B-type natriuretic peptide and angiogenic biomarkers in the prognosis of adverse outcomes in women with suspected preeclampsia. Clin. Chim. Acta 2016, 463, 150–157. [Google Scholar] [CrossRef]
- Lam, C.; Lim, K.H.; Kang, D.H.; Karumanchi, S.A. Uric acid and preeclampsia. Semin. Nephrol. 2005, 25, 56–60. [Google Scholar] [CrossRef]
- Germany Paula, L.; Ercília Pinheiro Da Costa, B.; Eduardo Poli-De-Figueiredo, C.; Carlos Ferreira Antonello, I. Does uric acid provide information about maternal condition and fetal outcome in pregnant women with hypertension? Hypertens. Pregnancy 2008, 27, 413–420. [Google Scholar] [CrossRef]
- Townsend, R.; Khalil, A.; Premakumar, Y.; Allotey, J.; Snell, K.I.E.; Chan, C.; Chappell, L.C.; Hooper, R.; Green, M.; Mol, B.W.; et al. Prediction of pre-eclampsia: Review of reviews. Ultrasound Obstet. Gynecol. 2019, 54, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Ganuza, P.; Lequerica-Fernandez, P.; Carretero, F.; Escudero, A.I.; Martinez-Morillo, E.; Sabria, E.; Herraiz, I.; Galindo, A.; Lopez, A.; Martinez-Triguero, M.L.; et al. A more accurate prediction to rule in and rule out pre-eclampsia using the sFlt-1/PlGF ratio and NT-proBNP as biomarkers. Clin. Chem. Lab. Med. 2020, 58, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Meler, E.; Iraola, A.; Eixarch, E.; Coll, O.; Figueras, J.; Francis, A.; Gratacos, E.; Gardosi, J. Customized birthweight standards for a Spanish population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 20–24. [Google Scholar] [CrossRef]
- Arduini, D.; Rizzo, G. Normal values of Pulsatility Index from fetal vessels: A cross-sectional study on 1556 healthy fetuses. J. Perinat. Med. 1990, 18, 165–172. [Google Scholar] [CrossRef]
- American College of Obstetricians; Task Force on Hypertension in Pregnancy Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Borghi, C.; Esposti, D.D.; Immordino, V.; Cassani, A.; Boschi, S.; Bovicelli, L.; Ambrosioni, E. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am. J. Obstet. Gynecol. 2000, 183, 140–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Yu, F. The diagnostic and predictive values of N-terminal pro-B-type natriuretic peptides in pregnancy complications and neonatal outcomes. Am. J. Transl. Res. 2021, 13, 10372–10379. [Google Scholar]
- Nguyen, T.X.; Nguyen, V.T.; Nguyen-Phan, H.N.; Hoang Bui, B. Serum Levels of NT-Pro BNP in Patients with Preeclampsia. Integr. Blood Press. Control 2022, 15, 43–51. [Google Scholar] [CrossRef]
- Fleming, S.M.; O’Byrne, L.; Grimes, H.; Daly, K.M.; Morrison, J.J.; Morrison, J.J. Amino-terminal pro-brain natriuretic peptide in normal and hypertensive pregnancy. Hypertens. Pregnancy 2001, 20, 169–175. [Google Scholar] [CrossRef]
- Verlohren, S.; Perschel, F.H.; Thilaganathan, B.; Dröge, L.A.; Henrich, W.; Busjahn, A.; Khalil, A. Angiogenic Markers and Cardiovascular Indices in the Prediction of Hypertensive Disorders of Pregnancy. Hypertension 2017, 69, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Pihl, K.; Sørensen, S.; Stener Jørgensen, F. Prediction of Preeclampsia in Nulliparous Women according to First Trimester Maternal Factors and Serum Markers. Fetal Diagn. Ther. 2020, 47, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sabriá, E.; Lequerica-Fernández, P.; Lafuente-Ganuza, P.; Eguia-Ángeles, E.; Escudero, A.I.; Martínez-Morillo, E.; Barceló, C.; Álvarez, F.V. Addition of N-terminal pro-B natriuretic peptide to soluble fms-like tyrosine kinase-1/placental growth factor ratio > 38 improves prediction of pre-eclampsia requiring delivery within 1 week: A longitudinal cohort study. Ultrasound Obstet. Gynecol. 2018, 51, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Maternal Serum Uric Acid as a Predictor of Severity of Hypertensive Disorders of Pregnancy: A Prospective Cohort Study. Curr. Hypertens. Rev. 2018, 15, 154–160. [Google Scholar] [CrossRef]
- Hawkins, T.L.A.; Roberts, J.M.; Mangos, G.J.; Davis, G.K.; Roberts, L.M.; Brown, M.A. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: A retrospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 484–492. [Google Scholar] [CrossRef]
- Bellomo, G.; Venanzi, S.; Saronio, P.; Verdura, C.; Narducci, P.L. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension 2011, 58, 704–708. [Google Scholar] [CrossRef]
- Verlohren, S.; Herraiz, I.; Lapaire, O.; Schlembach, D.; Zeisler, H.; Calda, P.; Sabria, J.; Markfeld-Erol, F.; Galindo, A.; Schoofs, K.; et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014, 63, 346–352. [Google Scholar] [CrossRef]
- Álvarez-Fernández, I.; Prieto, B.; Rodríguez, V.; Ruano, Y.; Escudero, A.I.; Álvarez, F.V. New biomarkers in diagnosis of early onset preeclampsia and imminent delivery prognosis. Clin. Chem. Lab. Med. 2014, 52, 1159–1168. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Resnik, J.L.; Hong, C.; Resnik, R.; Kazanegra, R.; Beede, J.; Bhalla, V.; Maisel, A. Evaluation of B-type natriuretic peptide (BNP) levels in normal and preeclamptic women. Am. J. Obstet. Gynecol. 2005, 193, 450–454. [Google Scholar] [CrossRef]
- Lafuente-Ganuza, P.; Carretero, F.; Lequerica-FernÃindez, P.; Fernandez-Bernardo, A.; Escudero, A.I.; De La Hera-Galarza, J.M.; Garcia-Iglesias, D.; Alvarez-Velasco, R.; Alvarez, F.V. NT-proBNP levels in preeclampsia, intrauterine growth restriction as well as in the prediction on an imminent delivery. Clin. Chem. Lab. Med. 2021, 59, 1077–1085. [Google Scholar] [CrossRef]
- Zheng, Z.; Lin, X.; Cheng, X. Serum Levels of N-Terminal Pro-Brain Natriuretic Peptide in Gestational Hypertension, Mild Preeclampsia, and Severe Preeclampsia: A Study from a Center in Zhejiang Province, China. Med. Sci. Monit. 2021, 28, e934285-1. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, I.; Dröge, L.A.; Gómez-Montes, E.; Henrich, W.; Galindo, A.; Verlohren, S. Characterization of the soluble fms-like tyrosine kinase-1 to placental growth factor ratio in pregnancies complicated by fetal growth restriction. Obstet. Gynecol. 2014, 124, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Foo, L.; Masini, G.; Bennett, P.R.; McEniery, C.M.; Wilkinson, I.B.; Lees, C.C. Early and late preeclampsia are characterized by high cardiac output, but in the presence of fetal growth restriction, cardiac output is low: Insights from a prospective study. Am. J. Obstet. Gynecol. 2018, 218, 517.e1–517.e12. [Google Scholar] [CrossRef] [PubMed]

| No Preeclampsia (n = 390) | Early-Preterm Preeclampsia (n = 90) | Late-Preterm Preeclampsia (n = 67) | p-Value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (years) | 34.02 ± 6 | 35.5 ± 5.9 | 34.8 ± 7.6 | 0.422 |
| Ethnicity Caucasian Black Asian | 88.1 (258) 7.2 (21) 4.8 (14) | 76.5 (26) 11.8 (4) 11.8 (4) | 94.2 (49) 1.9 (1) 3.8 (2) | 0.143 |
| Smoking | 7.6 (22) | 8.8 (3) | 0 (0) | 0.112 |
| Nulliparity | 39.8 (117) | 55.9 (19) | 44.2 (23) | 0.184 |
| Maternal morbidity | ||||
| Diabetes mellitus type 1 | 1.4 (4) | 2.9 (1) | 3.8 (2) | 0.415 |
| Diabetes mellitus type 2 | 1.7 (5) | 0 (0) | 5.8 (5) | 0.114 |
| Hypertension | 17 (50) | 17.6 (6) | 13.5 (7) | 0.806 |
| Cardiovascular disease | 0.7 (2) | 0 (0) | 0 (0) | 0.745 |
| Renal disease | 2.4 (7) | 2.9 (1) | 1.9 (1) | 0.955 |
| Biomarker data | ||||
| GA at sampling (weeks) | 33.6 ± 3.3 | 30.8 ± 2.1 * | 34.9 ± 1.2 ¥ | <0.001 |
| sFlt-1 (pg/mL) | 3252 ± 2815.2 | 15043.1 ± 9289 * | 12044.8 ± 9167.7 *,¥ | <0.001 |
| PlGF (pg/mL) | 361.2 ± 394.7 | 66.9 ± 84.4 | 83.4 ± 72.9 | 0.227 |
| sFlt-1/PlGF ratio | 28.9 ± 56.8 | 443.7 ± 329.1 * | 220.8 ± 229.9 *,¥ | <0.001 |
| NT-proBNP (ng/L) | 44.3 ± 46.3 | 883.5 ± 2391.5 * | 261.6 ± 247.2 *,¥ | <0.001 |
| Uric acid (mg/dL) | 3.9 ± 1 | 5.9 ± 1.4 * | 5.8 ± 1.5 *,¥ | <0.001 |
| Pregnancy outcomes | ||||
| GA at delivery (weeks) | 38.3 ± 2.3 | 31.4 ± 2.1 * | 35.3 ± 1 *,¥ | <0.001 |
| Mode of delivery Vaginal Operative vaginal Cesarean section | 53.3 (154) 6.6 (19) 39.9 (115) | 11.8 (4) * 0 (0) 88.2 (30) * | 32.7 (17) * 0 (0) 67.3 (35) * | <0.001 |
| Maternal admission to OICU | 0.7 (2) | 70.6 (24) * | 63.5 (33) * | <0.001 |
| Neonatal outcomes | ||||
| Birth weight (grams) | 2755.4 ± 654.5 | 1315.5 ± 372 * | 2249.1 ± 458.1 *,¥ | <0.001 |
| IUGR | 23.7 (72) | 82.9 (29) * | 43.6 (24) *,¥ | <0.001 |
| 1′ Apgar score | 8 ± 1 | 6 ± 2 * | 8 ± 0 ¥ | <0.001 |
| 5′ Apgar score | 9 ± 0 | 8 ± 1 * | 9 ± 1 ¥ | <0.001 |
| Umbilical artery pH | 7.2 ± 0.6 | 7.3 ± 0.1 | 7.2 ± 0.1 | 0.943 |
| Umbilical vein pH | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 | 0.05 |
| Admission to NICU | 13 (37) | 97 (32) * | 52.9 (27) *,¥ | <0.001 |
| Adverse neonatal outcome | 11.3 (32) | 87.9 (29) * | 28.8 (15) *,¥ | <0.001 |
| sFlt-1/PlGF Ratio | sFlt-1/PlGF Ratio + NT-proBNP + Uric Acid | p-Value | |
|---|---|---|---|
| Sensitivity (%) | 77.5 (71.9–83.0) | 79.6 (74.4–84.5) | 0.210 |
| Specificity (%) | 91.0 (89.0–93.0) | 94.9 (93.4–96.5) | <0.05 |
| PPV (%) | 72.8 (67.4–78.4) | 83.1 (78.5–88.2) | <0.05 |
| NPV (%) | 92.8 (91.0–94.7) | 93.7 (92.0–95.3) | 0.140 |
| Biomarkers | sFlt-1/PlGF Ratio | sFlt-1/PlGF Ratio + NT-proBNP + Uric Acid |
|---|---|---|
| Early-preterm PE | ||
| Sensitivity (%) | 82.2 (76.3–88.5) | 86.7 (81.8–92.6) |
| Specificity (%) | 90.8 (88.4–93.5) | 93.8 (91.7–95.9) * |
| PPV (%) | 72.5 (66.1–79.4) | 80.4 (74.2–86.4) * |
| NPV (%) | 94.5 (92.6–96.6) | 96.0 (94.3–97.8) |
| Late-preterm PE | ||
| Sensitivity (%) | 63.5 (53.1–75.0) | 63.5 (53.1–75.0) |
| Specificity (%) | 90.2 (87.6–92.8) | 96.0 (94.2–97.8) * |
| PPV (%) | 55.0 (45.7–64.9) | 75.0 (65.2–86.2) * |
| NPV (%) | 92.9 (90.7–95.5) | 93.3 (91.1–95.7) |
| Early-Preterm PE | sFlt-1/PlGF Ratio | sFlt-1/PlGF Ratio + NT-proBNP + Uric Acid | Late-Preterm PE | sFlt-1/PlGF Ratio | sFlt-1/PlGF Ratio + NT-proBNP + Uric Acid |
|---|---|---|---|---|---|
| Within 1 week | Within 1 week | ||||
| Sensitivity (%) | 87.0 (81.4–92.7) | 90.9 (86.0–95.9) | Sensitivity (%) | 65.3 (54.8–75.9) | 65.3 (54.8–75.9) |
| PPV (%) | 70.5 (63.8–77.6) | 78.7 (72.3–84.9) * | PPV (%) | 54.2 (44.7–63.6) | 74.4 (64.3–84.0) * |
| Specificity (%) | 90.8 (88.1–93.2) | 93.8 (91.8–95.8) * | Specificity (%) | 90.2 (87.6–92.9) | 96.0 (94.3–97.8) * |
| NPV (%) | 96.5 (94.9–98.2) | 97.6 (96.2–98.9) | NPV (%) | 93.6 (91.5–95.9) | 94.0 (92.0–96.1) |
| Within 3 weeks | Within 2 weeks | ||||
| Sensitivity (%) | 71.4 (50.0–100.0) | 50 (25.0–71.4) | Sensitivity (%) | 40.0 (18.2–60.0) | 40.0 (18.2–60.0) |
| PPV (%) | 26.3 (13.6–36.4) | 26.9 (11.8–40.0) | PPV (%) | 18.2 (5.9–27.8) | 35.3 (15.4–55.6) |
| Specificity (%) | 90.8 (88.4–93.3) | 93.8 (91.7–95.9) * | Specificity (%) | 90.2 (87.6–92.9) * | 96.0 (94.3–97.8) * |
| NPV (%) | 98.6 (97.7–100.0) | 97.6 (96.3–99.0) | NPV (%) | 96.5 (94.8–98.2) | 96.7 (95.1–98.3) |
Disclaimer/Publisher’s Note:The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido-Giménez, C.; Cruz-Lemini, M.; Álvarez, F.V.; Nan, M.N.; Carretero, F.; Fernández-Oliva, A.; Mora, J.; Sánchez-García, O.; García-Osuna, Á.; Alijotas-Reig, J.; et al. Predictive Model for Preeclampsia Combining sFlt-1, PlGF, NT-proBNP, and Uric Acid as Biomarkers. J. Clin. Med. 2023, 12, 431. https://doi.org/10.3390/jcm12020431
Garrido-Giménez C, Cruz-Lemini M, Álvarez FV, Nan MN, Carretero F, Fernández-Oliva A, Mora J, Sánchez-García O, García-Osuna Á, Alijotas-Reig J, et al. Predictive Model for Preeclampsia Combining sFlt-1, PlGF, NT-proBNP, and Uric Acid as Biomarkers. Journal of Clinical Medicine. 2023; 12(2):431. https://doi.org/10.3390/jcm12020431
Chicago/Turabian StyleGarrido-Giménez, Carmen, Mónica Cruz-Lemini, Francisco V. Álvarez, Madalina Nicoleta Nan, Francisco Carretero, Antonio Fernández-Oliva, Josefina Mora, Olga Sánchez-García, Álvaro García-Osuna, Jaume Alijotas-Reig, and et al. 2023. "Predictive Model for Preeclampsia Combining sFlt-1, PlGF, NT-proBNP, and Uric Acid as Biomarkers" Journal of Clinical Medicine 12, no. 2: 431. https://doi.org/10.3390/jcm12020431
APA StyleGarrido-Giménez, C., Cruz-Lemini, M., Álvarez, F. V., Nan, M. N., Carretero, F., Fernández-Oliva, A., Mora, J., Sánchez-García, O., García-Osuna, Á., Alijotas-Reig, J., Llurba, E., & on behalf of the EuroPE Working Group. (2023). Predictive Model for Preeclampsia Combining sFlt-1, PlGF, NT-proBNP, and Uric Acid as Biomarkers. Journal of Clinical Medicine, 12(2), 431. https://doi.org/10.3390/jcm12020431







