Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavaillé-Coll, M.; Bala, S.; Velidedeoglu, E.; Hernandez, A.; Archdeacon, P.; Gonzalez, G.; Neuland, C.; Meyer, J.; Albrecht, R. Summary of FDA Workshop on Ischemia Reperfusion Injury in Kidney Transplantation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2013, 13, 1134–1148. [Google Scholar] [CrossRef]
- Yarlagadda, S.G.; Coca, S.G.; Formica, R.N.J.; Poggio, E.D.; Parikh, C.R. Association between Delayed Graft Function and Allograft and Patient Survival: A Systematic Review and Meta-Analysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2009, 24, 1039–1047. [Google Scholar] [CrossRef]
- Salguero, J.; Chamorro, L.; Gomez-Gomez, E.; Robles, J.E.; Campos, J.P. Midterm Outcomes of Kidney Transplantation from Expanded Criteria Donors After Circulatory Death: A Single-Center Retrospective Cohort Study. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2023, 21, 481–486. [Google Scholar] [CrossRef]
- Padilla, M.; Coll, E.; Fernández-Pérez, C.; Pont, T.; Ruiz, Á.; Pérez-Redondo, M.; Oliver, E.; Atutxa, L.; Manciño, J.M.; Daga, D.; et al. Improved Short-Term Outcomes of Kidney Transplants in Controlled Donation after the Circulatory Determination of Death with the Use of Normothermic Regional Perfusion. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2021, 21, 3618–3628. [Google Scholar] [CrossRef]
- Rosenthal, J.T.; Danovitch, G.M.; Wilkinson, A.; Ettenger, R.B. The High Cost of Delayed Graft Function in Cadaveric Renal Transplantation. Transplantation 1991, 51, 1115–1118. [Google Scholar] [PubMed]
- Li, M.T.; Ramakrishnan, A.; Yu, M.; Daniel, E.; Sandra, V.; Sanichar, N.; King, K.L.; Stevens, J.S.; Husain, S.A.; Mohan, S. Effects of Delayed Graft Function on Transplant Outcomes: A Meta-Analysis. Transplant. Direct 2023, 9, e1433. [Google Scholar] [CrossRef]
- Barreda, P.; Miñambres, E.; Ballesteros, M.Á.; Mazón, J.; Gómez-Román, J.; Gómez Ortega, J.M.; Belmar, L.; Valero, R.; Ruiz, J.C.; Rodrigo, E. Controlled Donation After Circulatory Death Using Normothermic Regional Perfusion Does Not Increase Graft Fibrosis in the First Year Posttransplant Surveillance Biopsy. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2022, 20, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Heilman, R.L.; Smith, M.L.; Smith, B.H.; Qaqish, I.; Khamash, H.; Singer, A.L.; Kaplan, B.; Reddy, K.S. Progression of Interstitial Fibrosis during the First Year after Deceased Donor Kidney Transplantation among Patients with and without Delayed Graft Function. Clin. J. Am. Soc. Nephrol. 2016, 11, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Losappio, V.; Stallone, G.; Infante, B.; Schena, A.; Rossini, M.; Maiorano, A.; Fiorentino, M.; Ditonno, P.; Lucarelli, G.; Battaglia, M.; et al. A Single-Center Cohort Study to Define the Role of Pretransplant Biopsy Score in the Long-Term Outcome of Kidney Transplantation. Transplantation 2014, 97, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; Cravedi, P.; Perna, A.; Dimitrov, B.D.; Turturro, M.; Locatelli, G.; Rigotti, P.; Baldan, N.; Beatini, M.; Valente, U.; et al. Long-Term Outcome of Renal Transplantation from Older Donors. N. Engl. J. Med. 2006, 354, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Klopfer, S.O. Optimal Full Matching and Related Designs via Network Flows. J. Comput. Graph. Stat. 2006, 15, 609–627. [Google Scholar] [CrossRef]
- Rosengard, B.R.; Feng, S.; Alfrey, E.J.; Zaroff, J.G.; Emond, J.C.; Henry, M.L.; Garrity, E.R.; Roberts, J.P.; Wynn, J.J.; Metzger, R.A.; et al. Report of the Crystal City Meeting to Maximize the Use of Organs Recovered from the Cadaver Donor. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2002, 2, 701–711. [Google Scholar]
- Kayler, L.K.; Garzon, P.; Magliocca, J.; Fujita, S.; Kim, R.D.; Hemming, A.W.; Howard, R.; Schold, J.D. Outcomes and Utilization of Kidneys from Deceased Donors with Acute Kidney Injury. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2009, 9, 367–373. [Google Scholar] [CrossRef]
- Cohen, D.J.; St Martin, L.; Christensen, L.L.; Bloom, R.D.; Sung, R.S. Kidney and Pancreas Transplantation in the United States, 1995–2004. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2006, 6, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Kayler, L.K.; Srinivas, T.R.; Schold, J.D. Influence of CIT-Induced DGF on Kidney Transplant Outcomes. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2011, 11, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.G.; Hernández, J.P.C.; López, F.J.M.; Garcia, J.R.; Montemayor, V.G.; Curado, F.A.; Vallejo, M.L.; López, J.C.R.; Cabello, M.D.N.; Aljama, P.; et al. Long-Term Allograft Survival after Kidney Transplantation. Transplant. Proc. 2013, 45, 3599–3602. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Pretagostini, R.; Poli, L.; Melandro, F.; Ferretti, S.; Della Pietra, F.; Rossi, M.; Berloco, P.B. Delayed Graft Function Decreases Early and Intermediate Graft Outcomes after Expanded Criteria Donor Kidney Transplants. Transplant. Proc. 2009, 41, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Lin, M.-Z.; Zhou, H.-L.; Li, H.; Sun, Q.-P.; Huang, Z.-Y.; Hong, L.-Q.; Wang, G.; Cai, R.-M.; Sun, Q.-Q. Delayed Graft Function Is Correlated with Graft Loss in Recipients of Expanded-Criteria Rather than Standard-Criteria Donor Kidneys: A Retrospective, Multicenter, Observation Cohort Study. Chin. Med. J. 2020, 133, 561–570. [Google Scholar] [CrossRef]
- Mezzolla, V.; Pontrelli, P.; Fiorentino, M.; Stasi, A.; Pesce, F.; Franzin, R.; Rascio, F.; Grandaliano, G.; Stallone, G.; Infante, B.; et al. Emerging Biomarkers of Delayed Graft Function in Kidney Transplantation. Transplant. Rev. 2021, 35, 100629. [Google Scholar] [CrossRef]
- Pontrelli, P.; Simone, S.; Rascio, F.; Pesce, F.; Conserva, F.; Infante, B.; Castellano, G.; Sallustio, F.; Fiorentino, M.; Zaza, G.; et al. Pre-Transplant Expression of CCR-2 in Kidney Transplant Recipients Is Associated With the Development of Delayed Graft Function. Front. Immunol. 2022, 13, 804762. [Google Scholar] [CrossRef] [PubMed]

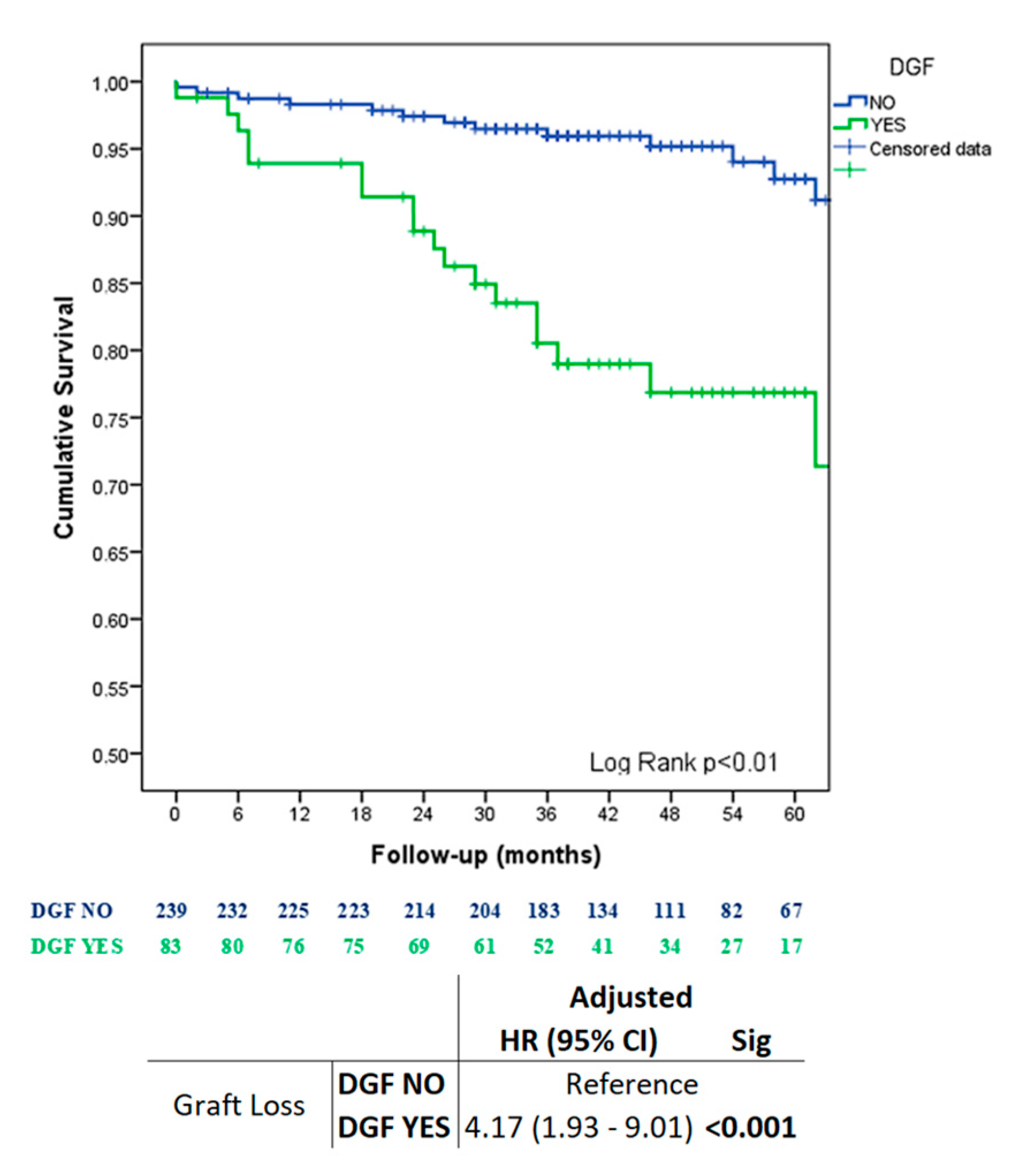
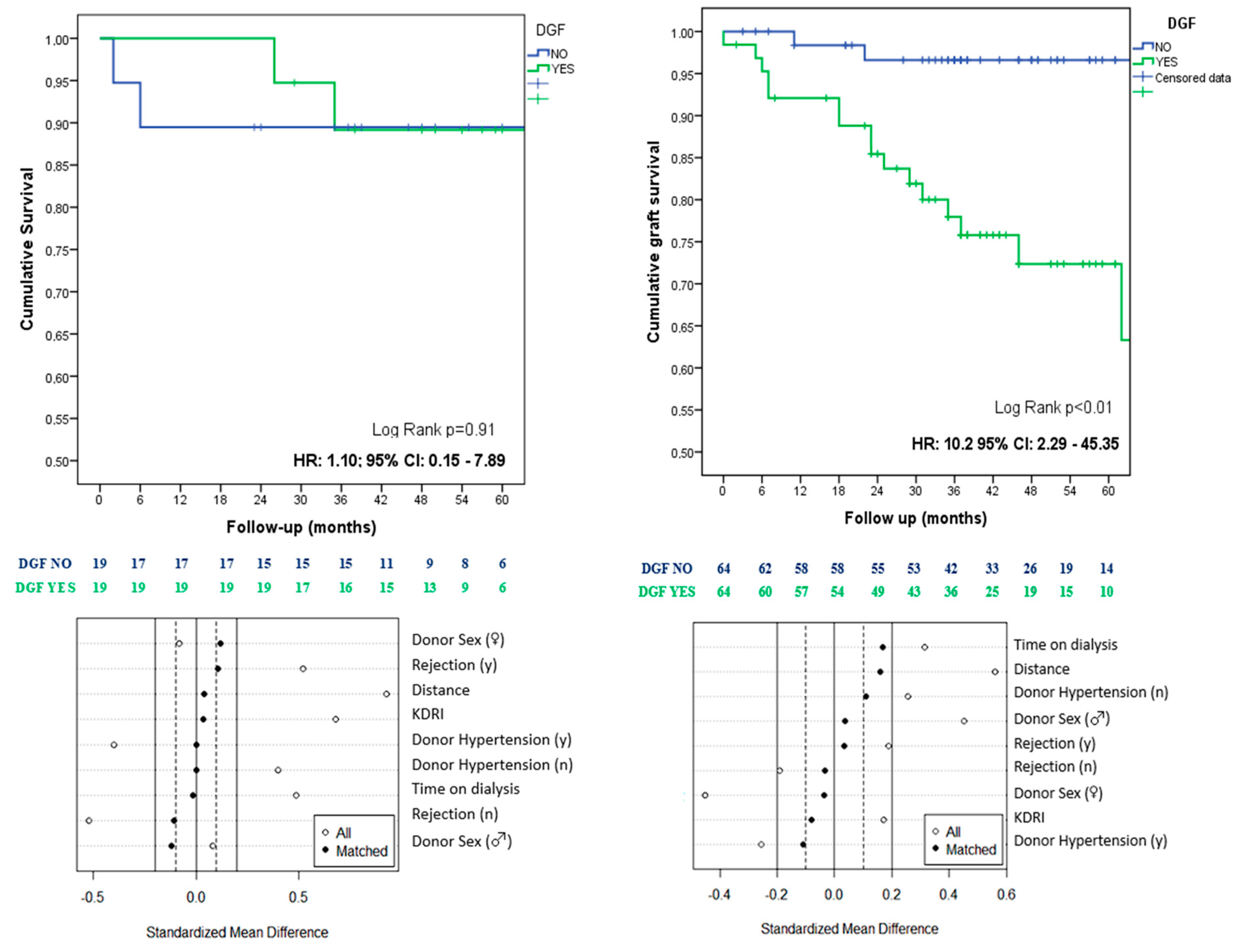
| Characteristics | All | NO DGF | DGF | Sig. |
|---|---|---|---|---|
| (n = 322) | (n = 239) | (n = 83) | ||
| Age (yrs), mean (SD) | 55.22 (13.06) | 55.49 (12.79) | 54.44 (13.87) | 0.74 |
| Sex (male), n (%) | 209 (64.9) | 150 (62.8) | 59 (71.1) | 0.17 |
| BMI (Kg/m2), mean (SD) | 27.52 (5.26) | 27.28 (5.23) | 28.21 (5.31) | 0.17 |
| Diabetes mellitus (y), n (%) | 73 (22.7) | 49 (20.5) | 24 (28.9) | 0.11 |
| Arterial hypertension (y), n (%) | 266 (82.6) | 205 (85.8) | 61 (73.5) | 0.01 |
| Peripheral artery disease (y), n (%) | 18 (5.6) | 11 (4.6) | 7 (8.4) | 0.26 |
| Ischemic cardiac disease (y), n (%) | 18 (5.6) | 13 (5.4) | 5 (6.0) | 0.78 |
| Stroke (y), n (%) | 9 (2.8) | 4 (1.7) | 5 (6.0) | 0.05 |
| Primary renal disease | 0.13 | |||
| Diabetes mellitus | 43 (13.4) | 25 (10.5) | 18 (21.7) | |
| Hypertension | 24 (7.5) | 18 (7.5) | 6 (7.2) | |
| Glomerulonephritis | 60 (18.6) | 45 (18.8) | 15 (18.1) | |
| Tubulointerstitial nephritis | 21 (6.5) | 16 (6.7) | 5 (6) | |
| Cystic kidney disease | 53 (16.5) | 44 (18.4) | 9 (10.8) | |
| Urologic | 26 (8.1) | 17 (7.1) | 9 (10.8) | |
| Unknown/missing/others | 95 (29.5) | 74 (31) | 21 (25.3) | |
| Pretransplant renal replacement, n (%) | 0.09 | |||
| None | 11 (3.4) | 11 (4.6) | - | |
| Dialysis peritoneal | 51 (15.8) | 40 (16.7) | 11 (13.3) | |
| Hemodialysis | 260 (80.7) | 188 (78.7) | 72 (86.7) | |
| Time on dialysis (m), mean (SD) | 28.74 (40.96) | 23.97 (33.28) | 42.49 (55.64) | <0.01 |
| Hyperimmunized (y), n (%) | 44 (13.7) | 34 (14.2) | 10 (12.0) | 0.61 |
| History of kidney transplantation (y), n (%) | 55 (17.1) | 39 (16.3) | 16 (19.3) | 0.53 |
| Characteristics | All | NO DGF | DGF | Sig. |
|---|---|---|---|---|
| (n = 322) | (n = 239) | (n = 83) | ||
| Age (yrs), mean (SD) | 56.34 (14.82) | 55.49 (15.70) | 58.78 (11.67) | 0.19 |
| <40 | 38 (11.8) | 33 (13.8) | 5 (6) | |
| 40–49 | 49 (15.2) | 36 (15.1) | 13 (15.7) | |
| 50–59 | 86 (26.7) | 61 (25.5) | 25 (30.1) | |
| 60–69 | 87 (27) | 62 (25.9) | 25 (30.1) | |
| ≥70 | 62 (19.3) | 47 (19.7) | 15 (18.1) | |
| Sex (male), n (%) | 202 (62.7) | 141 (59.0) | 61 (73.5) | 0.01 |
| BMI (Kg/m2), mean (SD) | 27.68 (4.68) | 27.42 (4.65) | 28.42 (4.70) | 0.10 |
| Diabetes mellitus (y), n (%) | 44 (13.7) | 27 (11.3) | 17 (20.5) | 0.03 |
| Arterial hypertension (y), n (%) | 131 (40.8) | 90 (37.7) | 41 (49.4) | 0.06 |
| Cause of death, n (%) | 0.39 | |||
| Stroke | 205 (63.7) | 149 (62.3) | 56 (67.5) | |
| Anoxia | 50 (15.5) | 36 (15.1) | 14 (16.9) | |
| Head trauma | 62 (19.3) | 51 (21.3) | 11 (13.3) | |
| Others | 5 (1.6) | 3 (1.3) | 2 (2.4) | |
| Serum creatinine (mg/dL), median (IQR) | 0.85 (0.67) | 0.81 (0.68) | 0.95 (0.63) | <0.01 |
| Tipe of donation, n (%) | 0.03 | |||
| Brain death—standard donor | 113 (35.1) | 88 (36.8) | 25 (30.1) | |
| Brain death—expanded criteria donor | 140 (43.5) | 103 (43.1) | 37 (44.6) | |
| Donation after cardiac death | 69 (24.4) | 48 (20.1) | 21 (25.3) | |
| KDRI, mean (SD) | 1.26 (0.42) | 1.23 (0.42) | 1.36 (0.40) | 0.01 |
| Characteristics | All | NO DGF | DGF | Sig. |
|---|---|---|---|---|
| (n = 322) | (n = 239) | (n = 83) | ||
| Extraction and preservation method, n (%) | 0.01 | |||
| Brain death donor—cold storage | 253 (78.6) | 191 (79.9) | 62 (74.7) | |
| Donation after cardiac death | ||||
| Rapid recovery—cold storage | 51 (15.8) | 31 (13) | 20 (24.1) | |
| Normothermic regional perfusion—cold storage | 18 (5.6) | 17 (7.1) | 1 (1.2) | |
| Preimplantation graft biopsy (score) * | 0.58 | |||
| ≤3 | 32 (9.9) | 20 (8.4) | 12 (14.5) | |
| ≥4 | 87 (27) | 59 (24.7) | 28 (33.7) | |
| Cold ischemia time (h), mean (SD) | 11.67 (4.18) | 11.61 (4.02) | 11.85 (4.63) | 0.76 |
| HLA missmatches, median (IQR) | 4 (4–5) | 4 (4–5) | 5 (4–5) | 0.15 |
| Number of acute rejections, median (IQR) | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0.02 |
| Hospital stay (d), median (IQR) | 12 (10–15) | 11 (9–13) | 17 (14–20) | <0.01 |
| Follow up (m), mean (SD) | 46.07 (19.98) | 47.21 (19.81) | 42.77 (20.22) | 0.09 |
| KDRI ≤ 1 | KDRI > 1 | |||||
|---|---|---|---|---|---|---|
| Characteristics | NO DGF | DGF | Sig. | NO DGF | DGF | Sig. |
| (n = 19) | (n = 19) | (n = 64) | (n = 64) | |||
| Recipient-Related | ||||||
| Age (yrs), mean (SD) | 47.36 (11.2) | 40.63 (15.04) | 0.22 | 61.9 (9.59) | 58.54 (10.54) | 0.06 |
| Sex (male), n (%) | 13 (68.4) | 14 (73.3) | 0.72 | 41 (64.1) | 45 (70.3) | 0.45 |
| BMI (Kg/m2), mean (SD) | 25.08 (6.23) | 27.98 (6.2) | 0.14 | 27.68 (5.04) | 28.27 (5.07) | 0.58 |
| Diabetes mellitus (y), n (%) | 2 (10.5) | 1 (5.3) | 0.54 | 16 (25) | 23 (35.9) | 0.17 |
| Arterial hypertension (y), n (%) | 12 (63.2) | 12 (63.2) | 1 | 52 (81.3) | 49 (76.6) | 0.51 |
| Peripheral artery disease (y), n (%) | 1 (5.3) | - | 1 | 6 (9.4) | 7 (10.9) | 0.77 |
| Ischemic cardiac disease (y), n (%) | - | 1 (5.3) | 1 | 6 (9.4) | 4 (6.3) | 0.51 |
| Stroke (y), n (%) | - | - | - | 2 (3.1) | 5 (7.8) | 0.24 |
| Pretransplant renal replacement, n (%) | 0.6 | 0.21 | ||||
| None | - | - | 3 (4.7) | - | ||
| Dialysis peritoneal | 3 (15.8) | 1 (5.3) | 10 (15.6) | 10 (15.6) | ||
| Hemodialysis | 16 (84.2) | 18 (94.7) | 51 (79.7) | 54 (84.4) | ||
| Time on dialysis (m), mean (SD) | 53.15 (66.91) | 52.36 (47.46) | 0.72 | 29.87 (36.57) | 39.56 (57.86) | 0.26 |
| Hyperimmunized (y), n (%) | 6 (31.6) | 4 (21.1) | 0.46 | 7 (10.9) | 6 (9.4) | 0.77 |
| History of kidney transplantation (y), n (%) | 6 (31.6) | 7 (36.8) | 0.73 | 5 (7.8) | 9 (14.1) | 0.25 |
| Donor-Related | ||||||
| Age (yrs), mean (SD) | 44.10 (9.72) | 40.63 (15.04) | 0.62 | 66.39 (8.58) | 62.46 (9.84) | 0.03 |
| Sex (male), n (%) | 15 (78.9) | 14 (73.7) | 1 | 46 (71.9) | 47 (73.4) | 0.84 |
| BMI (Kg/m2), mean (SD) | 26.82 (5.33) | 26.78 (1.99) | 0.72 | 27.88 (4.20) | 28.91 (5.16) | 0.36 |
| Diabetes mellitus (y), n (%) | - | - | - | 15 (23.4) | 17 (26.6) | 0.68 |
| Arterial hypertension (y), n (%) | 1 (5.3) | 2 (10.5) | 1 | 33 (51.6) | 39 (60.9) | 0.28 |
| Cause of death, n (%) | 0.68 | 0.5 | ||||
| Stroke | 7 (36.8) | 7 (36.8) | 49 (76.6) | 49 (76.6) | ||
| Anoxia | 6 (31.6) | 6 (31.6) | 8 (12.5) | 8 (12.5) | ||
| Head trauma | 5 (26.3) | 6 (31.6) | 7 (10.9) | 5 (7.8) | ||
| Others | 1 (5.3) | - | - | 2 (3.1) | ||
| Tipe of donation | 1 | 0.1 | ||||
| Brain death | 13 (68.4) | 13 (68.4) | 52 (81.3) | 49 (76.6) | ||
| Donation after cardiac death | 5 (26.3) | 5 (26.3) | 9 (14.1) | 15 (23.4) | ||
| Normotermic regional perfusion | 1 (5.3) | 1 (5.3) | 3 (4.7) | - | ||
| Serum creatinine (mg/dL), median (IQR) | 0.97 (1.23) | 0.73 (0.23) | 0.62 | 0.77 (0.31) | 1.02 (0.69) | 0.03 |
| KDRI, mean (SD) | 0.86 (0.08) | 0.86 (0.11) | 0.79 | 1.54 (0.38) | 1.51 (0.33) | 0.90 |
| Transplantation-Procedure-Related | ||||||
| Cold ischemia time (h), mean (SD) | 10.73 (3.91) | 10.99 (5.38) | 0.90 | 12.38 (4.05) | 12.11 (4.40) | 0.75 |
| HLA mismatches, median (IQR) | 3 (3–5) | 4 (3–5) | 0.17 | 5 (4–5) | 5 (4–6) | 0.47 |
| Number of acute rejections, median (IQR) | 0 (0–1) | 0 (0–1) | 0.97 | 0 (0–1) | 0 (0–1) | 0.67 |
| Hospital stay (d), median (IQR) | 11 (10–14) | 14.5 (12–18.5) | <0.01 | 11 (9–13) | 17 (14–20) | <0.01 |
| Follow-up (m), mean (SD) | 50.15 (26.50) | 56.31 (16.93) | 0.48 | 45.15 (19.70) | 38.75 (19.46) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salguero, J.; Chamorro, L.; Gómez-Gómez, E.; de Benito, P.; Robles, J.E.; Campos, J.P. Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study. J. Clin. Med. 2023, 12, 6397. https://doi.org/10.3390/jcm12196397
Salguero J, Chamorro L, Gómez-Gómez E, de Benito P, Robles JE, Campos JP. Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study. Journal of Clinical Medicine. 2023; 12(19):6397. https://doi.org/10.3390/jcm12196397
Chicago/Turabian StyleSalguero, Joseba, Laura Chamorro, Enrique Gómez-Gómez, Patricia de Benito, Jose E. Robles, and Juan P. Campos. 2023. "Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study" Journal of Clinical Medicine 12, no. 19: 6397. https://doi.org/10.3390/jcm12196397
APA StyleSalguero, J., Chamorro, L., Gómez-Gómez, E., de Benito, P., Robles, J. E., & Campos, J. P. (2023). Kidney Survival Impact of Delayed Graft Function Depends on Kidney Donor Risk Index: A Single-Center Cohort Study. Journal of Clinical Medicine, 12(19), 6397. https://doi.org/10.3390/jcm12196397






