Mid-Term Outcomes of a Pre-Cannulated Iliac Branched Device in the Treatment of Abdominal Aortoiliac Aneurysms: A Retrospective Analysis from a Single Center
Abstract
:1. Introduction
2. Patients and Methods
2.1. Cohort Specifications
2.2. Indications, Treatment Strategies, and Follow-up Structure
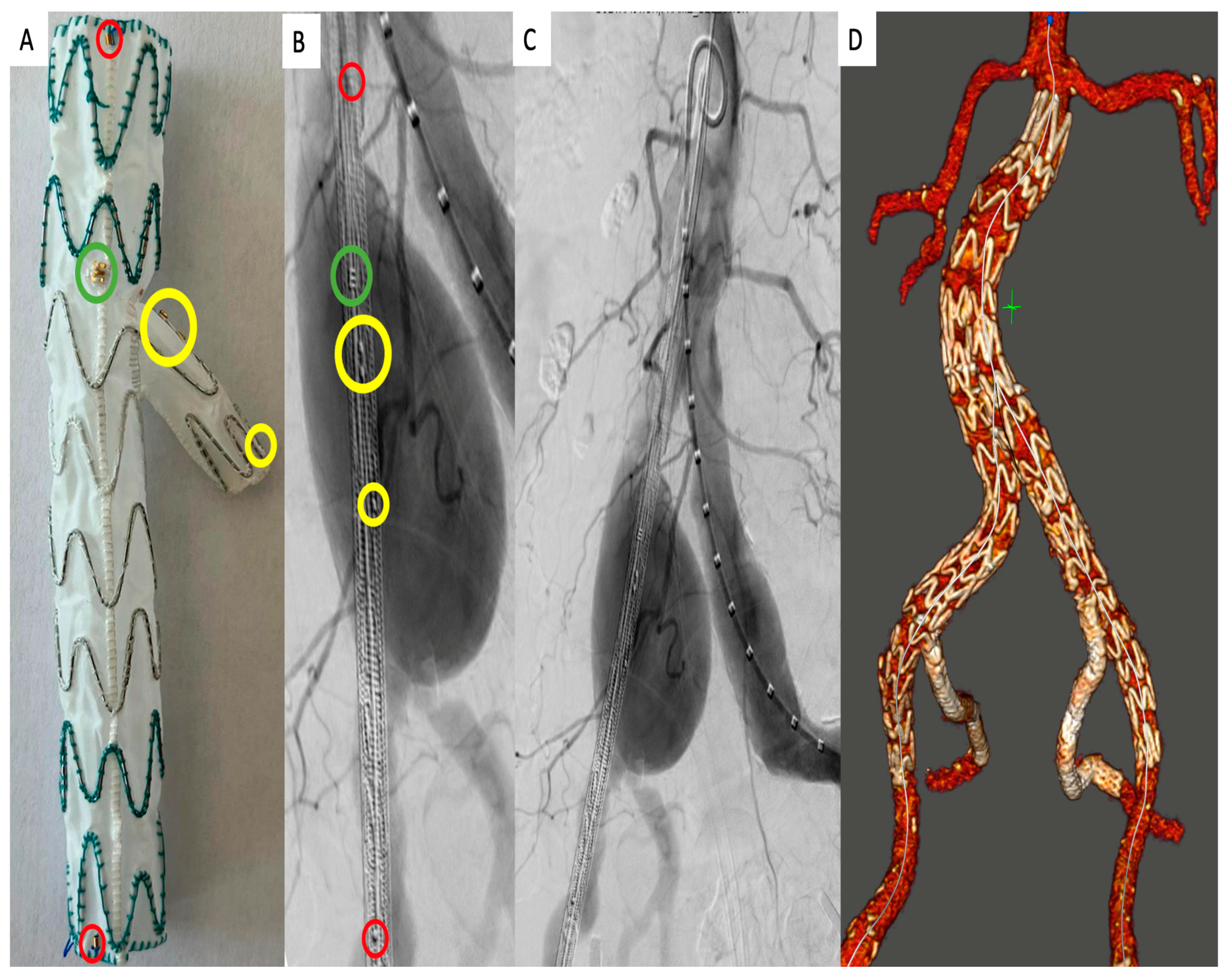
2.3. Operation Technique
2.4. Data Acquisition, CT Analysis, and Outcome Definitions
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort and Procedural Parameters
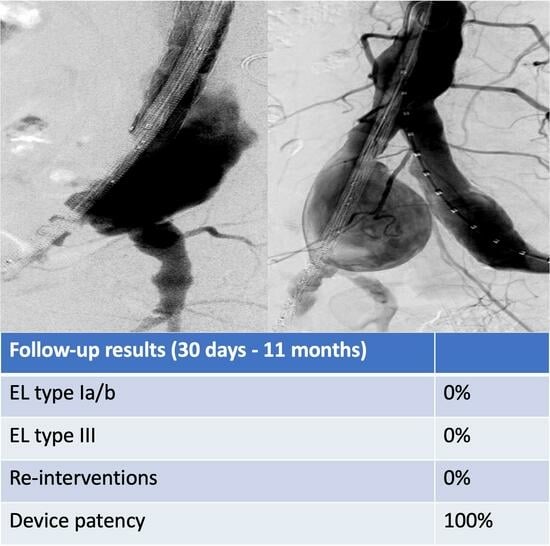
3.2. Early Follow-Up Outcomes
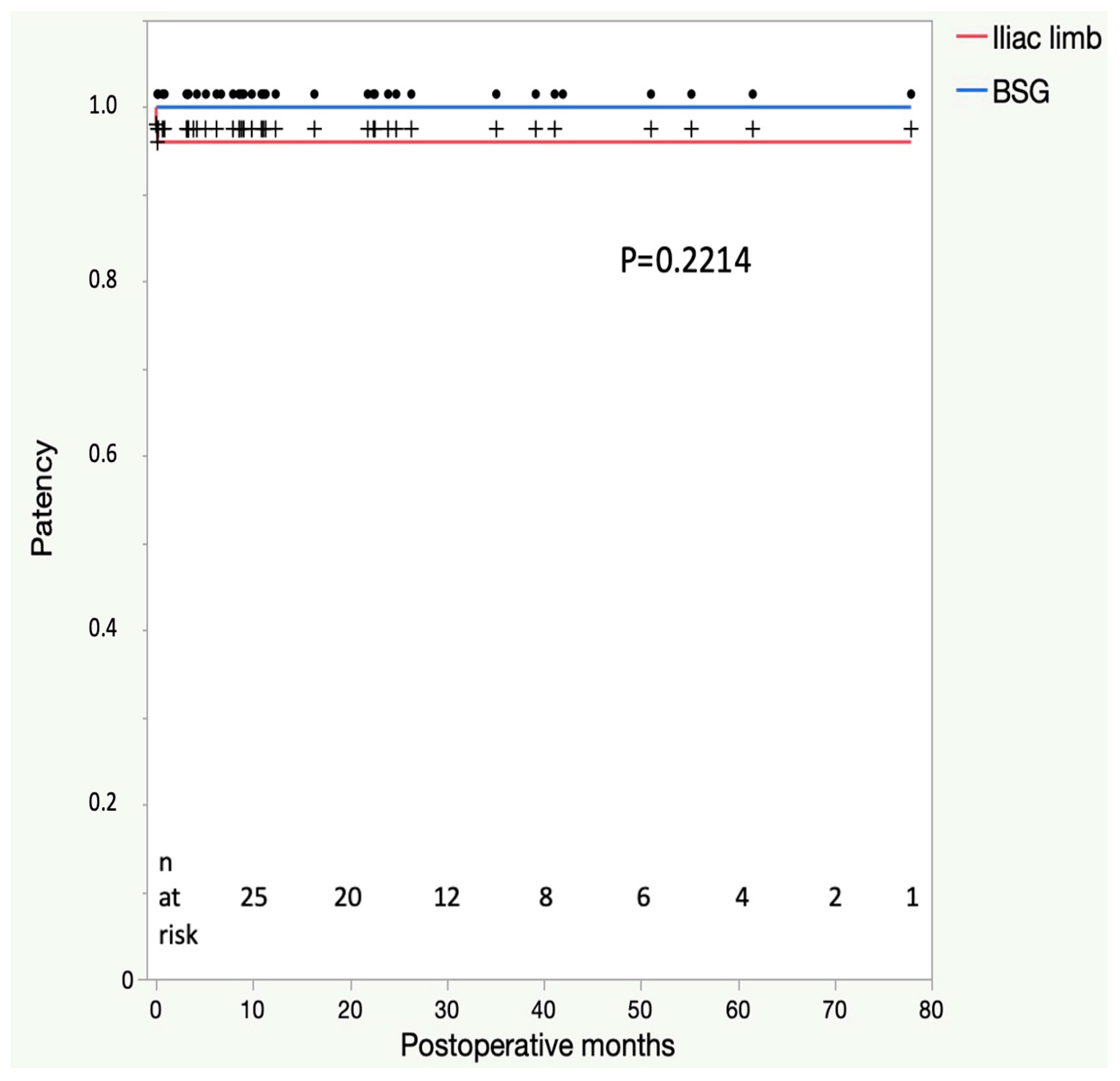
3.3. Mid-Term Follow-Up Outcomes
4. Discussion
4.1. Status of the IBD in the Treatment of Aortoiliac Aneurysms
4.2. Results of the E-iliac IBD Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dix, F.P.; Titi, M.; Al-Khaffaf, H. The isolated internal iliac artery aneurysm—A review. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 119–129. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s choice—European society for vascular surgery (esvs) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Hiromatsu, S.; Hosokawa, Y.; Egawa, N.; Yokokura, H.; Akaiwa, K.; Aoyagi, S. Strategy for isolated iliac artery aneurysms. Asian Cardiovasc. Thorac. Ann. 2007, 15, 280–284. [Google Scholar] [CrossRef]
- Taudorf, M.; Gronvall, J.; Schroeder, T.V.; Lonn, L. Endovascular aneurysm repair treatment of aortoiliac aneurysms: Can iliac branched devices prevent gluteal claudication? J. Vasc. Interv. Radiol. 2016, 27, 174–180. [Google Scholar] [CrossRef]
- Buck, D.B.; Bensley, R.P.; Darling, J.; Curran, T.; McCallum, J.C.; Moll, F.L.; van Herwaarden, J.A.; Schermerhorn, M.L. The effect of endovascular treatment on isolated iliac artery aneurysm treatment and mortality. J. Vasc. Surg. 2015, 62, 331–335. [Google Scholar] [CrossRef]
- Mylonas, S.N.; Rumenapf, G.; Schelzig, H.; Heckenkamp, J.; Youssef, M.; Schafer, J.P.; Ahamd, W.; Brunkwall, J.S.; E-liac Collaborative Group. A multicenter 12-month experience with a new iliac side-branched device for revascularization of hypogastric arteries. J. Vasc. Surg. 2016, 64, 1652–1659.e1. [Google Scholar] [CrossRef]
- Gray, D.; Shahverdyan, R.; Reifferscheid, V.; Gawenda, M.; Brunkwall, J.S. Evar with flared iliac limbs has a high risk of late type 1b endoleak. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 170–176. [Google Scholar] [CrossRef]
- Vedani, S.M.; Petitprez, S.; Weinz, E.; Corpataux, J.M.; Deglise, S.; Deslarzes-Dubuis, C.; Côté, E.; Ricco, J.-B.; Saucy, F. Predictors and consequences of sac shrinkage after endovascular infrarenal aortic aneurysm repair. J. Clin. Med. 2022, 11, 3232. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Blankensteijn, J.D.; Harris, P.L.; White, G.H.; Zarins, C.K.; Bernhard, V.M.; Matsumura, J.S.; May, J.; Veith, F.J.; Fillinger, M.F.; et al. Reporting standards for endovascular aortic aneurysm repair. J. Vasc. Surg. 2002, 35, 1048–1060. [Google Scholar] [CrossRef]
- Houbballah, R.; Majewski, M.; Becquemin, J.P. Significant sac retraction after endovascular aneurysm repair is a robust indicator of durable treatment success. J. Vasc. Surg. 2010, 52, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, J.M.; Tenorio, E.R.; Jain, A.; Mendes, B.C.; Macedo, T.A.; Pather, K.; Gloviczki, P.; Oderich, G. Outcomes of target vessel endoleaks after fenestrated-branched endovascular aortic repair. J. Vasc. Surg. 2020, 72, 445–455. [Google Scholar] [CrossRef]
- Popovic, Z.B.; Thomas, J.D. Assessing observer variability: A user’s guide. Cardiovasc. Diagn. Ther. 2017, 7, 317–324. [Google Scholar] [CrossRef]
- Bosanquet, D.C.; Wilcox, C.; Whitehurst, L.; Cox, A.; Williams, I.M.; Twine, C.P. Systematic review and meta-analysis of the effect of internal iliac artery exclusion for patients undergoing evar. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 534–548. [Google Scholar] [CrossRef]
- Jean-Baptiste, E.; Brizzi, S.; Bartoli, M.A.; Sadaghianloo, N.; Baque, J.; Magnan, P.E.; Hassen-Khodja, R. Pelvic ischemia and quality of life scores after interventional occlusion of the hypogastric artery in patients undergoing endovascular aortic aneurysm repair. J. Vasc. Surg. 2014, 60, 40–49.e1. [Google Scholar] [CrossRef]
- Schneider, D.B.; Matsumura, J.S.; Lee, J.T.; Peterson, B.G.; Chaer, R.A.; Oderich, G.S. Prospective, multicenter study of endovascular repair of aortoiliac and iliac aneurysms using the gore iliac branch endoprosthesis. J. Vasc. Surg. 2017, 66, 775–785. [Google Scholar] [CrossRef]
- Schneider, D.B.; Matsumura, J.S.; Lee, J.T.; Peterson, B.G.; Chaer, R.A.; Oderich, G.S. Five-year outcomes from a prospective, multicenter study of endovascular repair of iliac artery aneurysms using an iliac branch device. J. Vasc. Surg. 2023, 77, 122–128. [Google Scholar] [CrossRef]
- Masciello, F.; Fargion, A.T.; Pratesi, G.; Dorigo, W.; Pratesi, C. A propensity score-matched comparison of two commercially available iliac branch devices in patients with similar clinical and anatomic preoperative features. J. Vasc. Surg. 2020, 71, 1207–1214. [Google Scholar] [CrossRef]
- Gouveia, E.M.R.; Fenelli, C.; Prendes, C.F.; Oz, T.; Stavroulakis, K.; Rantner, B.; Stana, B.; Tsilimparis, N. A cross-sectional study on the anatomic feasibility of iliac side branch grafts in a real-world setting. J. Vasc. Surg. 2022, 76, 724–732. [Google Scholar] [CrossRef]
- Brunkwall, J.S.; Vaquero-Puerta, C.; Heckenkamp, J.; Barrenechea, J.M.E.; Szopinski, P.; Mertikian, G.; Seifert, S.; Rumenapf, G.; Buz, S.; Assadian, A.; et al. Prospective study of the iliac branch device e-liac in patients with common iliac artery aneurysms: 12 month results. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 831–838. [Google Scholar] [CrossRef]
- Dueppers, P.; Duran, M.; Floros, N.; Schelzig, H.; Wagenhauser, M.U.; Oberhuber, A. The jotec iliac branch device for exclusion of hypogastric artery aneurysms: Abraham study. J. Vasc. Surg. 2019, 70, 748–755. [Google Scholar] [CrossRef]
- Yazar, O.; Willems, S.; Salemans, P.B.; Wong, C.Y.; van Grinsven, B.; Bouwman, L.H. Treatment of aortoiliac aneurysms. Compatibility of the e-liac stent graft (artivion((r)), iliac branch device) with endurant ii or iis (medtronic((r)), evar). Cardiovasc. Interv. Radiol. 2023, 46, 187–193. [Google Scholar] [CrossRef]
| Parameter | Overall |
|---|---|
| n | 38 (100) |
| Age (year; n (%)) | 69 (IQR 62–78) |
| Sex (female; n (%)) | 3 (8) |
| Body mass index | 26 (IQR 24–30) |
| Hypertension, n (%) | 28 (74) |
| Nicotine Abuse, n (%) | 15 (39) |
| Diabetes, n (%) | 5 (13) |
| Dyslipoproteinemia, n (%) | 7 (18) |
| COPD, n (%) | 6 (16) |
| Previous CABG, n (%) | 3 (8) |
| Atrial fibrillation, n (%) | 8 (21) |
| Previous stroke, n (%) | 5 (13) |
| Peripheral artery disease, n (%) | 5 (13) |
| Glomerular filtration rate, n (%) | |
| CKD Stage I (GFR: ≥90 mL/min) | 14 (37) |
| CKD Stage II (GFR: 60–89 mL/min) | 18 (47) |
| CKD Stage III (GFR: 30–59 mL/min) | 5 (13) |
| CKD Stage IV (GFR: 15–29 mL/min) | 0 (0) |
| CKD Stage V (GFR: <15 mL/min) | 1 (3) |
| Previous abdominal surgery, n (%) | 17 (45) |
| Parameter | Overall |
|---|---|
| Implanted IBD devices, n (%) | 50 (100) |
| Indications (n = 50; IBD units) | |
| Previous EVAR with DLZ dilation/endoleak, n (%) | 10 (20) |
| AAA treatment with DLZ absence, n (%) | 10 (20) |
| CIA aneurysm, n (%) | 18 (36) |
| IIA aneurysm, n (%) | 4 (8) |
| Combined CIA/IIA aneurysm, n (%) | 8 (16) |
| Iliac bifurcation diameter (mm) | 25 (IQR 20–32) |
| BSG access (n = 50; IBD units) | |
| Transfemoral, n (%) | 22 (44) |
| Brachial, n (%) | 17 (34) |
| Transaxillary, n (%) | 11 (22) |
| Procedure description (n = 38; patients) | |
| EVAR + unilateral IBD, n (%) | 6 (16) |
| EVAR + bilateral IBD, n (%) | 9 (24) |
| Unilateral isolated IBD, n (%) | 21 (55) |
| Bilateral isolated IBD, n (%) | 2 (5) |
| Bridging stent grafts per vessel | 1.6 |
| Bridging stent graft type (n = 50; IBD units) | |
| E-ventus, n (%) | 40 (80) |
| VBX, n (%) | 7 (14) |
| Combined, n (%) | 3 (6) |
| Operation time (min) | 197 (IQR 136–262) |
| Primary technical success (n = 38; patients), n (%) | 37 (97) |
| Assisted technical success (n = 38; patients), n (%) | 38 (100) |
| Intraoperative endoleaks (n = 38; patients), n (%) | |
| Type I | 0 (0) |
| Type III | 0 (0) |
| Unplanned adjunctive procedures (n = 38; patients), n (%) | 1 (3) |
| Parameter | Overall |
|---|---|
| n (%) | 38 (100) |
| ICU stay | 0 (0) |
| Stroke, n (%) | 0 (0) |
| Mortality, n (%) | 1 (3) |
| Myocardial infarction, n (%) | 0 (0) |
| Paraplegia, n (%) | 1 (3) |
| Colonic ischemia, n (%) | 0 (0) |
| Bleeding complications needing revision, n (%) | 2 (5) |
| Wound complications needing revision, n (%) | 0 (0) |
| Endoleaks before discharge (patients) | |
| Type I, n (%) | 0 (0) |
| Type II, n (%) | 16 (43) |
| Type III, n (%) | 0 (0) |
| Parameter | Overall |
|---|---|
| n (%) | 37 (100) |
| Follow-up length (months) | 11 (IQR 5–26) |
| Stroke, n (%) | 0 (0) |
| Mortality, n (%) | 2 (5) |
| Myocardial infarction, n (%) | 0 (0) |
| Paraplegia, n (%) | 0 (0) |
| Colonic ischemia, n (%) | 0 (0) |
| Aneurysm rupture, n (%) | 0 (0) |
| Buttock claudication, n (%) | 0 (0) |
| Conversion to open repair, n (%) | 0 (0) |
| Re-interventions | 0 (0) |
| Endoleaks at last follow-up (patients) | |
| Type I, n (%) | 0 (0) |
| Type II, n (%) | 6 (16) |
| Type III, n (%) | 0 (0) |
| Type II endoleak resolution, n (%) | −10 (−63) |
| Aneurysm growth | |
| Sac enlargement, n (%) | 0 (0) |
| Sac stability, n (%) | 31 (84) |
| Sac shrinkage, n (%) | 6 (16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonorden, C.; Shoura, M.; Andic, M.; Hahn, J.K.; Mustafi, M.; Schlensak, C.; Lescan, M. Mid-Term Outcomes of a Pre-Cannulated Iliac Branched Device in the Treatment of Abdominal Aortoiliac Aneurysms: A Retrospective Analysis from a Single Center. J. Clin. Med. 2023, 12, 6395. https://doi.org/10.3390/jcm12196395
Bonorden C, Shoura M, Andic M, Hahn JK, Mustafi M, Schlensak C, Lescan M. Mid-Term Outcomes of a Pre-Cannulated Iliac Branched Device in the Treatment of Abdominal Aortoiliac Aneurysms: A Retrospective Analysis from a Single Center. Journal of Clinical Medicine. 2023; 12(19):6395. https://doi.org/10.3390/jcm12196395
Chicago/Turabian StyleBonorden, Constantin, Mohamed Shoura, Mateja Andic, Julia Kelley Hahn, Migdat Mustafi, Christian Schlensak, and Mario Lescan. 2023. "Mid-Term Outcomes of a Pre-Cannulated Iliac Branched Device in the Treatment of Abdominal Aortoiliac Aneurysms: A Retrospective Analysis from a Single Center" Journal of Clinical Medicine 12, no. 19: 6395. https://doi.org/10.3390/jcm12196395
APA StyleBonorden, C., Shoura, M., Andic, M., Hahn, J. K., Mustafi, M., Schlensak, C., & Lescan, M. (2023). Mid-Term Outcomes of a Pre-Cannulated Iliac Branched Device in the Treatment of Abdominal Aortoiliac Aneurysms: A Retrospective Analysis from a Single Center. Journal of Clinical Medicine, 12(19), 6395. https://doi.org/10.3390/jcm12196395