The Effect of Serotonin Transmission on Depressive and Insomnia Symptoms in Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Assessment of Disease Severity and Questionnaire Variables
2.4. Anti-TNF Therapy
2.5. Evaluation of Gene Expression and Protein Concentration
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.H.; eun Kwon, J.; Cho, M. La Immunological Pathogenesis of Inflammatory Bowel Disease. Intestig. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Dudzińska, E.; Gryzinska, M.; Kocki, J. Single Nucleotide Polymorphisms in Selected Genes in Inflammatory Bowel Disease. Biomed. Res. Int. 2018, 2018, 6914346. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Forbes, J.D. Gut Microbiome in Inflammatory Bowel Disease and Other Chronic Immune-Mediated Inflammatory Diseases. Inflamm. Intestig. Dis. 2017, 2, 116–123. [Google Scholar] [CrossRef]
- Bernstein, C.N. Psychological Stress and Depression: Risk Factors for IBD? Dig. Dis. 2016, 34, 58–63. [Google Scholar] [CrossRef]
- Neuendorf, R.; Harding, A.; Stello, N.; Hanes, D.; Wahbeh, H. Depression and Anxiety in Patients with Inflammatory Bowel Disease: A Systematic Review. J. Psychosom. Res. 2016, 87, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut Microbiome Diversity Is Associated with Sleep Physiology in Humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Frey, D.J.; Fleshner, M.; Wright, K.P. The Effects of 40 Hours of Total Sleep Deprivation on Inflammatory Markers in Healthy Young Adults. Brain Behav. Immun. 2007, 21, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Margolis, K.G. Serotonin as a Link between the Gut-Brain-Microbiome Axis in Autism Spectrum Disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut Clock: Implication of Circadian Rhythms in the Gastrointestinal Tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar]
- Mardones, O.; Oyarzún-Salazar, R.; Labbé, B.S.; Miguez, J.M.; Vargas-Chacoff, L.; Muñoz, J.L.P. Intestinal Variation of Serotonin, Melatonin, and Digestive Enzymes Activities along Food Passage Time through GIT in Salmo Salar Fed with Supplemented Diets with Tryptophan and Melatonin. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 266, 111159. [Google Scholar] [CrossRef] [PubMed]
- Young, L.W.; Darios, E.S.; Watts, S.W. An Immunohistochemical Analysis of SERT in the Blood–Brain Barrier of the Male Rat Brain. Histochem. Cell Biol. 2015, 144, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J. Cell Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.R.; Burgess, H.J.; Keshavarzian, A. Sleep Disturbances and Inflammatory Bowel Disease: A Potential Trigger for Disease Flare? Expert. Rev. Clin. Immunol. 2011, 7, 29–36. [Google Scholar] [CrossRef]
- Vernia, F.; Di Ruscio, M.; Ciccone, A.; Viscido, A.; Frieri, G.; Stefanelli, G.; Latella, G. Sleep Disorders Related to Nutrition and Digestive Diseases: A Neglected Clinical Condition. Int. J. Med. Sci. 2021, 18, 593–603. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Talar-Wojnarowska, R.; Szmyd, B.; Krzywdzińska, M.; Białasiewicz, P. Determinants of Sleep Quality in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 2921. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Bao, C.-H.; Wu, Y.; Liang, S.-H.; Wang, D.; Wu, L.-Y.; Huang, Y.; Liu, H.-R.; Wu, H.-G. Tryptophan-Kynurenine Metabolism: A Link between the Gut and Brain for Depression in Inflammatory Bowel Disease. J. Neuroinflam. 2021, 18, 135. [Google Scholar] [CrossRef]
- Spina, A.; Mazzarella, C.; Dallio, M.; Romeo, M.; Pellegrino, R.; Durante, T.; Romano, M.; Loguercio, C.; Di Mauro, M.; Federico, A.; et al. The Lesson from the First Italian Lockdown: Impacts on Anxiety and Depressive Symptoms and Sleep Quality in Patients with Remission of Inflammatory Bowel Disease. Rev. Recent Clin. Trials. 2022, 17, 109–119. [Google Scholar] [CrossRef]
- Bisgaard, T.H.; Allin, K.H.; Elmahdi, R.; Jess, T. The Bidirectional Risk of Inflammatory Bowel Disease and Anxiety or Depression: A Systematic Review and Meta-Analysis. Gen. Hosp. Psychiatry 2023, 83, 109–116. [Google Scholar] [CrossRef]
- Aardoom, M.A.; Veereman, G.; de Ridder, L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019, 20, 2529. [Google Scholar] [CrossRef]
- Sochal, M.; Krzywdziñska, M.; Gabryelska, A.; Talar-Wojnarowska, R.; Malecka-Panas, E. Efficiency and Safety of One-Year Anti-TNF-α Treatment in Crohn’s Disease: A Polish Single-Centre Experience. Prz. Gastroenterol. 2020, 15, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a Neurotransmitter: The Role of Serotonin in Inflammation and Immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.B.; Vermeire, S. Differential Diagnosis of Inflammatory Bowel Disease: Imitations and Complications. Lancet Gastroenterol. Hepatol. 2018, 3, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, A.N.; Hunter, T.; Dong, Y.; Hoskin, B.; Middleton-Dalby, C.; Hetherington, J.; Stefani-Hunyady, D.; Canavan, J.B. Full, Partial, and Modified Permutations of the Mayo Score: Characterizing Clinical and Patient-Reported Outcomes in Ulcerative Colitis Patients. Crohns Colitis 360 2021, 3, otab007. [Google Scholar] [CrossRef]
- Echarri, A.; Vera, I.; Ollero, V.; Arajol, C.; Riestra, S.; Robledo, P.; Calvo, M.; Gallego, F.; Ceballos, D.; Castro, B.; et al. The Harvey–Bradshaw Index Adapted to a Mobile Application Compared with In-Clinic Assessment: The MediCrohn Study. Telemed. e-Health 2020, 26, 78–86. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an Instrument Based on ICD-10 Criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Nieto, I.; Robles, E.; Vazquez, C. Self-Reported Cognitive Biases in Depression: A Meta-Analysis. Clin. Psychol. Rev. 2020, 82, 101934. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin Signalling in the Gut-Functions, Dysfunctions and Therapeutic Targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef]
- Shajib, M.S.; Chauhan, U.; Adeeb, S.; Chetty, Y.; Armstrong, D.; Halder, S.L.S.; Marshall, J.K.; Khan, W.I. Characterization of Serotonin Signaling Components in Patients with Inflammatory Bowel Disease. J. Can. Assoc. Gastroenterol. 2019, 2, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Manzella, C.R.; Jayawardena, D.; Pagani, W.; Li, Y.; Alrefai, W.A.; Bauer, J.; Jung, B.; Weber, C.R.; Gill, R.K. Serum Serotonin Differentiates Between Disease Activity States in Crohn’s Patients. Inflamm. Bowel. Dis. 2020, 26, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Sedano, R.; Nguyen, T.M.; Almradi, A.; Rieder, F.; Parker, C.E.; Shackelton, L.M.; D’Haens, G.; Sandborn, W.J.; Feagan, B.G.; Ma, C.; et al. Disease Activity Indices for Pouchitis: A Systematic Review. Inflamm. Bowel. Dis. 2022, 28, 622–638. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, J.R.; Ho, W.; Linden, D.R.; Mawe, G.M.; Sharkey, K.A. Enteroendocrine Cells and 5-HT Availability Are Altered in Mucosa of Guinea Pigs with TNBS Ileitis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2004, 287, G998–G1007. [Google Scholar] [CrossRef]
- Jørandli, J.W.; Thorsvik, S.; Skovdahl, H.K.; Kornfeld, B.; Sæterstad, S.; Gustafsson, B.I.; Sandvik, A.K.; van Beelen Granlund, A. The Serotonin Reuptake Transporter Is Reduced in the Epithelium of Active Crohn’s Disease and Ulcerative Colitis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2020, 319, G761–G768. [Google Scholar] [CrossRef]
- Dong, S.; Chen, M.; Dai, F.; Xuan, Q.; Chen, P.; Feng, D.; Gao, L.; Zhu, C.; Chang, Y.; Chu, F.; et al. 5-Hydroxytryptamine (5-HT)-exacerbated DSS-induced Colitis Is Associated with Elevated NADPH Oxidase Expression in the Colon. J. Cell Biochem. 2019, 120, 9230–9242. [Google Scholar] [CrossRef]
- Qasem, A.; Naser, A.E.; Naser, S.A. Enteropathogenic Infections Modulate Intestinal Serotonin Transporter (SERT) Function by Activating Toll-like Receptor 2 (TLR-2) in Crohn’s Disease. Sci. Rep. 2021, 11, 22624. [Google Scholar] [CrossRef]
- Lowery, C.L.; Elliott, C.; Cooper, A.; Hadden, C.; Sonon, R.N.; Azadi, P.; Williams, D.K.; Marsh, J.D.; Woulfe, D.S.; Kilic, F. Cigarette Smoking-Associated Alterations in Serotonin/Adrenalin Signaling Pathways of Platelets. J. Am. Heart Assoc. 2017, 6, e005465. [Google Scholar] [CrossRef]
- Rozich, J.J.; Holmer, A.; Singh, S. Effect of Lifestyle Factors on Outcomes in Patients With Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2020, 115, 832–840. [Google Scholar] [CrossRef]
- Semba, J.; Wakuta, M. Chronic Effect of Nicotine on Serotonin Transporter MRNA in the Raphe Nucleus of Rats: Reversal by Co-Administration of Bupropion. Psychiatry Clin. Neurosci. 2008, 62, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J. Smoking and Diet: Impact on Disease Course? Dig. Dis. 2016, 34, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, L.; Schultz, B.M.; Salazar, G.A.; Pardo-Roa, C.; Sebastián, V.P.; Álvarez-Lobos, M.M.; Bueno, S.M. Impact of Cigarette Smoking on the Gastrointestinal Tract Inflammation: Opposing Effects in Crohn’s Disease and Ulcerative Colitis. Front. Immunol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Behbehani, M. Ovarian Hormones and Migraine Headache: Understanding Mechanisms and Pathogenesis-Part, I. Headache J. Head Face Pain 2006, 46, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Satsangi, J.; Peeters, M.; Parkes, M.; Jewell, D.P.; Vlietinck, R.; Rutgeerts, P. Evidence for Inflammatory Bowel Disease of a Susceptibility Locus on the X Chromosome. Gastroenterology 2001, 120, 834–840. [Google Scholar] [CrossRef]
- Klavdianou, K.; Liossis, S.-N.; Papachristou, D.J.; Theocharis, G.; Sirinian, C.; Kottorou, A.; Filippopoulou, A.; Andonopoulos, A.P.; Daoussis, D. Decreased Serotonin Levels and Serotonin-Mediated Osteoblastic Inhibitory Signaling in Patients With Ankylosing Spondylitis. J. Bone Miner. Res. 2016, 31, 630–639. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Barberio, B.; Zamani, M.; Black, C.J.; Savarino, E.V.; Ford, A.C. Prevalence of Symptoms of Anxiety and Depression in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol Hepatol 2021, 6, 359–370. [Google Scholar] [CrossRef]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Białasiewicz, P.; Małecka-Wojciesko, E.; Sochal, M. Sleep Problems in Chronic Inflammatory Diseases: Prevalence, Treatment, and New Perspectives: A Narrative Review. J. Clin. Med. 2022, 11, 67. [Google Scholar] [CrossRef]
- Fabre, V.; Boutrel, B.; Hanoun, N.; Lanfumey, L.; Fattaccini, C.M.; Demeneix, B.; Adrien, J.; Hamon, M.; Martres, M.-P. Homeostatic Regulation of Serotonergic Function by the Serotonin Transporter As Revealed by Nonviral Gene Transfer. J. Neurosci. 2000, 20, 5065–5075. [Google Scholar] [CrossRef]
- Dopheide, J.A. Insomnia Overview: Epidemiology, Pathophysiology, Diagnosis and Monitoring, and Nonpharmacologic Therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; van Schooten, F.-J.; Jin, H.; Jonkers, D.; Godschalk, R. The Involvement of Intestinal Tryptophan Metabolism in Inflammatory Bowel Disease Identified by a Meta-Analysis of the Transcriptome and a Systematic Review of the Metabolome. Nutrients 2023, 15, 2886. [Google Scholar] [CrossRef] [PubMed]
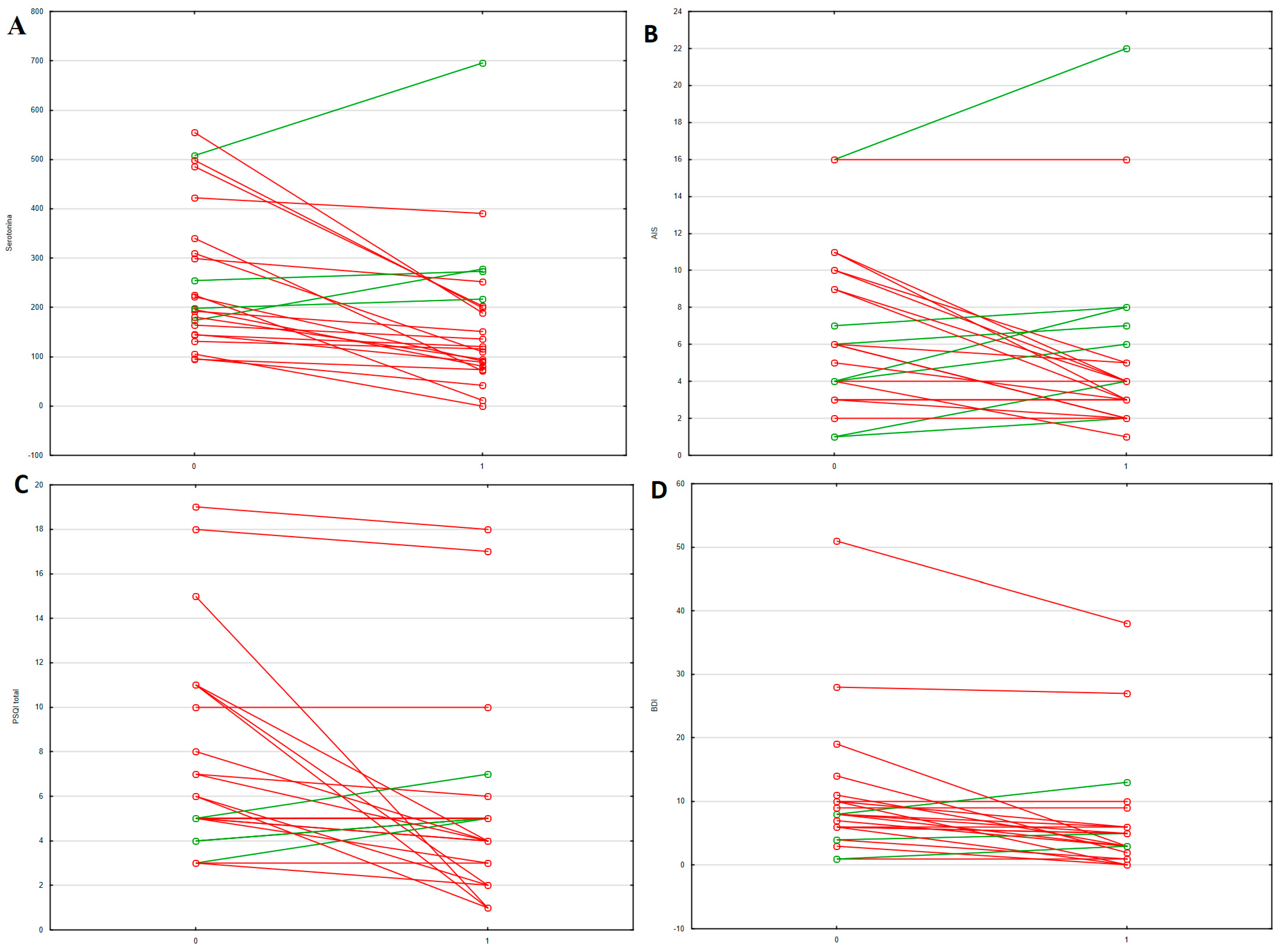
| IBD (n = 77) | AC (n = 45) | NA (n = 32) | HC (n = 41) | p (IBD-HC) | p (AC-HC) | p (NA-HC) | p (AC-NA) | |
|---|---|---|---|---|---|---|---|---|
| Women (n, %) | 43, 55.8 | 24, 53.3 | 19, 59.4 | 20, 48.8 | 0.464 | 0.673 | 0.368 | 0.599 |
| Age | 35 (28–41) | 35 (31–41) | 37 (25–42) | 31 (25–44) | 0.518 | 0.288 | 0.929 | 0.518 |
| BMI | 23.3 (20.8–25.9) | 24.3 (±3.2) | 22.9 (±3.2) | 23.8 (±3.5) | 0.765 | 0.515 | 0.285 | 0.094 |
| Smoker (n, %) | 11, 14.3 | 6, 13.3 | 5, 15.6 | 4, 9.8 | 0.572 | 0.741 | 0.493 | 1.000 |
| Chronic diseases (n, %) | 18, 23.4 | 15, 33.3 | 3, 9.4 | 5, 12.2 | 0.222 | 0.024 | 1.000 | 0.016 |
| Immunomodulators (n, %) | 27, 35.1 | 14, 31.1 | 13, 40.6 | - | - | - | - | 0.389 |
| mRNA SERT | 1.3 (0.6–2.2) | 1.2 (0.7–2.2) | 1.4 (0.5–2.0) | 1.7 (1.0–2.3) | 0.069 | 0.117 | 0.121 | 0.971 |
| Serotonin | 145.1 (100.0–192.4) | 164.1 (105.4–221.0) | 130.0 (87.8–165.7) | 115.3 (76.6–160.5) | 0.015 | 0.001 | 0.480 | 0.014 |
| SERT | 18.6 (±8.8) | 20.5 (±9.4) | 15.9 (±7.1) | 13.2 (±6.0) | <0.001 | <0.001 | 0.079 | 0.021 |
| BDI | 7 (4–10) | 7 (5–10) | 6.5 (3.0–10.5) | 3 (1–8) | 0.005 | 0.003 | 0.101 | 0.268 |
| AIS total | 5 (4–9) | 6 (4–9) | 5 (3–7) | 4 (2–7) | 0.052 | 0.021 | 0.393 | 0.156 |
| ESS | 6 (4–10) | 6 (5–10) | 5 (3–9) | 6 (3–8) | 0.137 | 0.067 | 0.571 | 0.283 |
| Latency | 20 (10–35) | 30 (10–45) | 15 (10–30) | 15 (10–20) | 0.005 | 0.001 | 0.169 | 0.073 |
| Sleep time | 7.0 (6.0–7.7) | 6.9 (6.0–7.5) | 7.0 (6.0–7.9) | 6.8 (5.8–7.7) | 0.651 | 0.907 | 0.465 | 0.537 |
| Time in bed | 8.0 (7.0–8.5) | 8 (7–8.5) | 8.0 (6.8–8.9) | 7 (6–8) | 0.004 | 0.006 | 0.027 | 0.859 |
| Sleep efficiency (%) | 87.5 (81.3–94.1) | 87.5 (80.0–93.8) | 88.2 (83.3–95.1) | 96.7 (90.0–98.6) | <0.001 | <0.001 | 0.002 | 0.399 |
| PSQI | 5 (4–7) | 6 (5–8) | 5 (4–6) | 5 (3–7) | 0.101 | 0.014 | 0.951 | 0.027 |
| LPS | 2 (0–4) | 3 (0–5) | 2 (0–3) | 0 (0–3) | 0.004 | 0.001 | 0.128 | 0.081 |
| VAS | 4 (0–5) | 4 (0–6) | 3 (0–5) | 0 (0–2) | <0.001 | <0.001 | 0.002 | 0.119 |
| CD | UC | HC (n = 41) | p (CD-UC) | p (CD-HC) | p (UC-HC) | p (AC-NA in CD) | p (AC-NA in UC) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 46) | AC (n = 27) | NA (n = 19) | All (n = 31) | AC (n = 18) | NA (n = 13) | |||||||
| mRNA SERT | 1.3 (0.4–2.2) | 1.2 (0.7–2.2) | 1.2 (0.3–2.8) | 1.4 (0.7–2.1) | 1.2 (0.5–2.2) | 1.4 (0.8–1.6) | 1.7 (1.0–2.3) | 0.767 | 0.104 | 0.139 | 0.945 | 0.936 |
| Serotonina | 144.2 (96.0–180.4) | 164.1 (120.8–221.0) | 107.9 (51.5–145.7) | 163.4 (100.0–208.7) | 170.2 (94.9–298.8) | 163.4 (124.0–187.2) | 115.3 (76.6–160.5) | 0.251 | 0.091 | 0.008 | 0.002 | 0.764 |
| SERT | 18.2 (±9.6) | 19.7 (±10.1) | 16.0 (±8.6) | 19.2 (±7.4) | 21.7 (±8.3) | 15.7 (±4.3) | 13.2 (±6.0) | 0.628 | 0.005 | <0.001 | 0.197 | 0.025 |
| BDI | 7 (4–10) | 7 (5–10) | 7 (3–11) | 7 (3–11) | 7.5 (5.0–11.0) | 5 (3–9) | 3 (1–8) | 0.723 | 0.008 | 0.040 | 0.467 | 0.560 |
| AIS total | 6 (4–9) | 7.3 (±4.2) | 6.3 (±3.5) | 4 (3–7) | 5 (3–10) | 4 (3–5) | 4 (2–7) | 0.055 | 0.011 | 0.631 | 0.386 | 0.210 |
| ESS | 6 (4–10) | 6 (4–10) | 5 (3–8) | 7 (4–10) | 7 (5–10) | 6 (3–11) | 6 (3–8) | 0.606 | 0.289 | 0.116 | 0.494 | 0.469 |
| Latency | 30 (10–60) | 30 (15–60) | 20 (10–30) | 15 (10–25) | 17 (10–40) | 15 (10–20) | 15 (10–20) | 0.054 | 0.001 | 0.200 | 0.133 | 0.402 |
| Sleep time | 7 (6–8) | 7 (6–8) | 6.8 (5.9–7.8) | 6.6 (±1.1) | 6.2 (±1.1) | 7.1 (±1.0) | 6.8 (5.8–7.8) | 0.383 | 0.442 | 0.713 | 0.342 | 0.021 |
| Time in bed | 8.0 (7.0–8.5) | 8.0 (7.5–9.0) | 8.0 (6.5–8.5) | 8.0 (7.0–8.5) | 7.4 (±0.9) | 8.2 (±1.4) | 7 (6–8) | 0.397 | 0.004 | 0.050 | 0.172 | 0.050 |
| Sleep efficiency [%] | 87.5 (83.3–93.8) | 87.5 (84.2–93.8) | 87.5 (83.3–94.1) | 87.5 (80.0–95.8) | 84.0 (75.0–95.8) | 88.9 (85.7–95.8) | 96.7 (90.0–98.6) | 0.996 | <0.001 | <0.001 | 0.831 | 0.446 |
| PSQI | 5 (4–8) | 6 (5–10) | 5 (3–8) | 5 (4–7) | 6 (4–8) | 5 (4–5) | 5 (3–7) | 0.405 | 0.099 | 0.279 | 0.113 | 0.139 |
| LPS | 3 (0–5) | 3 (2–7) | 3 (0–5) | 2 (0–2) | 2 (0–3) | 2 (0–2) | 0 (0–3) | 0.007 | <0.001 | 0.342 | 0.154 | 0.246 |
| VAS | 5 (0–6) | 5 (2–7) | 5 (0–5) | 3 (0–4) | 4 (0–5) | 3 (0–3) | 0 (0–2) | 0.031 | <0.001 | 0.005 | 0.302 | 0.109 |
| Serotonin | SERT | mRNA SERT | ||||
|---|---|---|---|---|---|---|
| IBD | HC | IBD | HC | IBD | HC | |
| Serotonin | - | - | 0.11; 0.357 | 0.43; 0.005 | 0.04; 0.760 | −0.02; 0.895 |
| SERT | 0.11; 0.357 | 0.43; 0.005 | - | - | −0.03; 0.786 | 0; 0.981 |
| mRNA SERT | 0.04; 0.760 | −0.02; 0.895 | −0.03; 0.786 | 0; 0.981 | - | - |
| Age | −017; 0.146 | −0.24; 0.137 | 0.06; 0.603 | −0.19; 0.222 | 0.03; 0.768 | 0.2; 0.202 |
| BMI | −015; 0.182 | −0.01; 0.974 | −0.03; 0.806 | 0.16; 0.332 | 0; 0.983 | 0.14; 0.379 |
| HBI | 0.44; 0.002 | - | 0.17; 0.253 | - | −0.06; 0.711 | - |
| PMS | 0.05; 0.772 | - | 0.15; 0.41 | - | 0.15; 0.421 | - |
| mRNA SERT Expression | SERT (ng/mL) | Serotonin (ng/mL) | |||||
|---|---|---|---|---|---|---|---|
| Sex | Women | 1.1 (0.4–1.5) | 0.011 | 19.3 (±9.3) | 0.406 | 163.4 (108.9–201.8) | 0.068 |
| Men | 1.8 (0.8–2.7) | 17.7 (±8.0) | 130.0 (94.9–173.6) | ||||
| Smoking | Yes | 2.4 (1.1–4.1) | 0.049 | 16.8 (±7.2) | 0.468 | 144.3 (96.0–173.6) | 0.585 |
| No | 1.2 (0.5–2.0) | 18.9 (±9.0) | 148.6 (100.0–195.8) | ||||
| History of surgery | Yes | 1.1 (0.6–2.0) | 0.581 | 16.5 (±10.4) | 0.190 | 161.8 (107.9–219.1) | 0.401 |
| No | 1.3 (0.5–2.2) | 19.4 (±8.0) | 144.1 (96.0–188.8) | ||||
| Other chronic diseases | Yes | 1.1 (0.5–2.2) | 0.516 | 18.7 (±9.5) | 0.959 | 157.7 (105.4–205.0) | 0.559 |
| No | 1.3 (0.6–2.2) | 18.6 (±8.6) | 144.3 (96.1–188.8) | ||||
| BDI | >10 points | 1.4 (0.5–1.9) | 0.754 | 18.5 (±9.7) | 0.950 | 145.7 (51.5–254.1) | 0.864 |
| <11 points | 1.2 (0.6–2.2) | 18.6 (±8.5) | 144.7 (105.0–188.8) | ||||
| AIS | >5 points | 1.5 (0.7–2.7) | 0.170 | 21.4 (±9.3) | 0.006 | 145.1 (105.0–178.1) | 0.744 |
| <6 points | 1.1 (0.5–1.7) | 16.0 (±7.5) | 149.7 (98.1–197.0) | ||||
| ESS | >10 | 1.4 (1.1–1.6) | 0.665 | 17.9 (±7.8) | 0.716 | 142.8 (74.0–282.0) | 0.811 |
| <11 | 1.2 (0.5–2.2) | 18.8 (±9.0) | 151.5 (100.3–188.8) | ||||
| PSQI | >5 | 1.2 (0.5–2.2) | 0.923 | 19.1 (±10.6) | 0.654 | 145.1 (68.0–254.1) | 0.826 |
| <6 | 1.3 (0.7–2.2) | 18.2 (±6.9) | 145.0 (105.4–188.8) | ||||
| Glucocorticoids | Yes | 1.2 (0.4–2.9) | 0.751 | 18.3 (±10.6) | 0.279 | 145.1 (68.0–254.1) | 0.076 |
| No | 1.3 (0.7–1.9) | 18.2 (±6.9) | 145.0 (105.4–188.8) | ||||
| Azathioprine | Yes | 1.3 (0.4–2.5) | 0.860 | 15.2 (±7.6) | 0.448 | 145.7 (100.27–187.2) | 0.677 |
| No | 1.3 (0.7–2.0) | 17.9 (±6.1) | 144.7 (96.1–205.0) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sochal, M.; Witkowska, A.; Binienda, A.; Gabryelska, A.; Białasiewicz, P.; Fichna, J.; Talar-Wojnarowska, R.; Małecka-Wojciesko, E. The Effect of Serotonin Transmission on Depressive and Insomnia Symptoms in Inflammatory Bowel Diseases. J. Clin. Med. 2023, 12, 6353. https://doi.org/10.3390/jcm12196353
Sochal M, Witkowska A, Binienda A, Gabryelska A, Białasiewicz P, Fichna J, Talar-Wojnarowska R, Małecka-Wojciesko E. The Effect of Serotonin Transmission on Depressive and Insomnia Symptoms in Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2023; 12(19):6353. https://doi.org/10.3390/jcm12196353
Chicago/Turabian StyleSochal, Marcin, Alicja Witkowska, Agata Binienda, Agata Gabryelska, Piotr Białasiewicz, Jakub Fichna, Renata Talar-Wojnarowska, and Ewa Małecka-Wojciesko. 2023. "The Effect of Serotonin Transmission on Depressive and Insomnia Symptoms in Inflammatory Bowel Diseases" Journal of Clinical Medicine 12, no. 19: 6353. https://doi.org/10.3390/jcm12196353
APA StyleSochal, M., Witkowska, A., Binienda, A., Gabryelska, A., Białasiewicz, P., Fichna, J., Talar-Wojnarowska, R., & Małecka-Wojciesko, E. (2023). The Effect of Serotonin Transmission on Depressive and Insomnia Symptoms in Inflammatory Bowel Diseases. Journal of Clinical Medicine, 12(19), 6353. https://doi.org/10.3390/jcm12196353










