Recipient Reaction and Composition of Autologous Sural Nerve Tissue Grafts into the Human Brain
Abstract
:1. Introduction
2. Materials and Methods
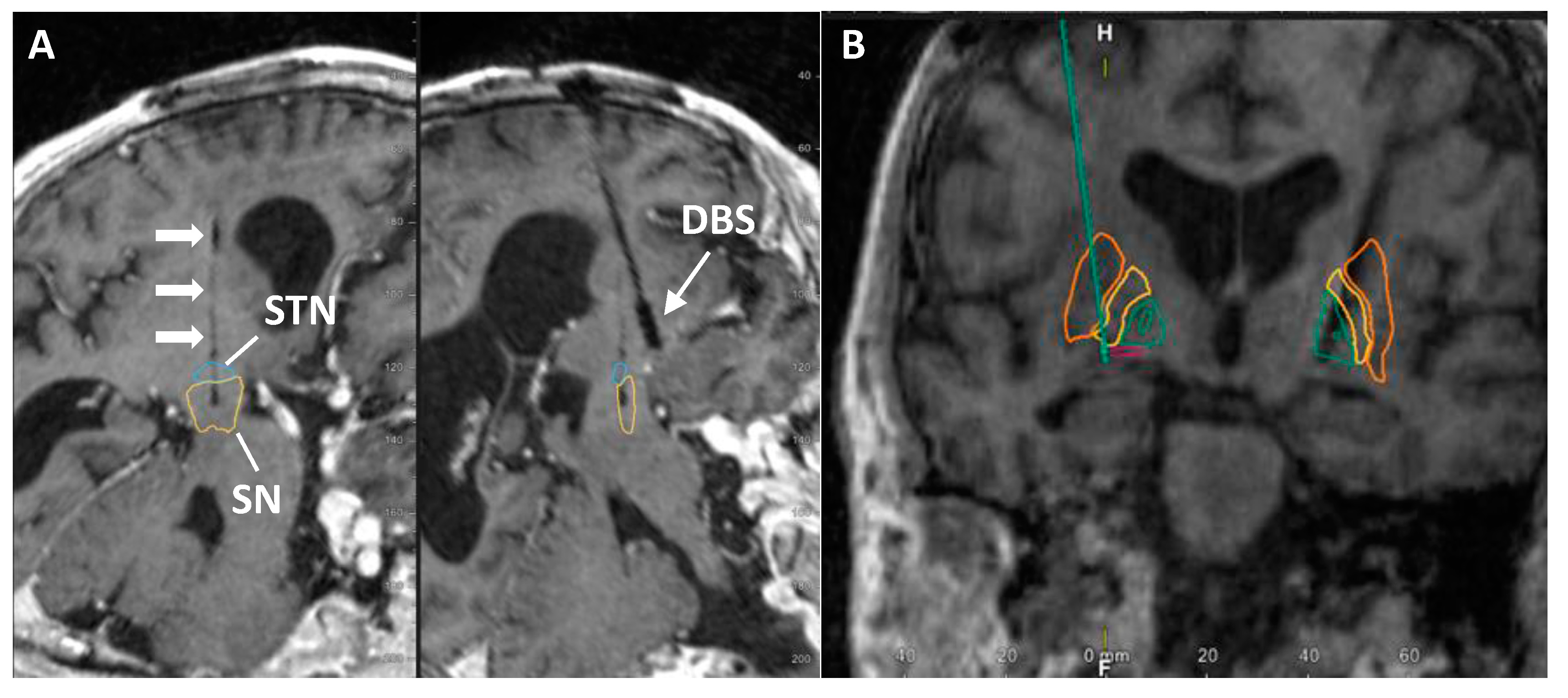
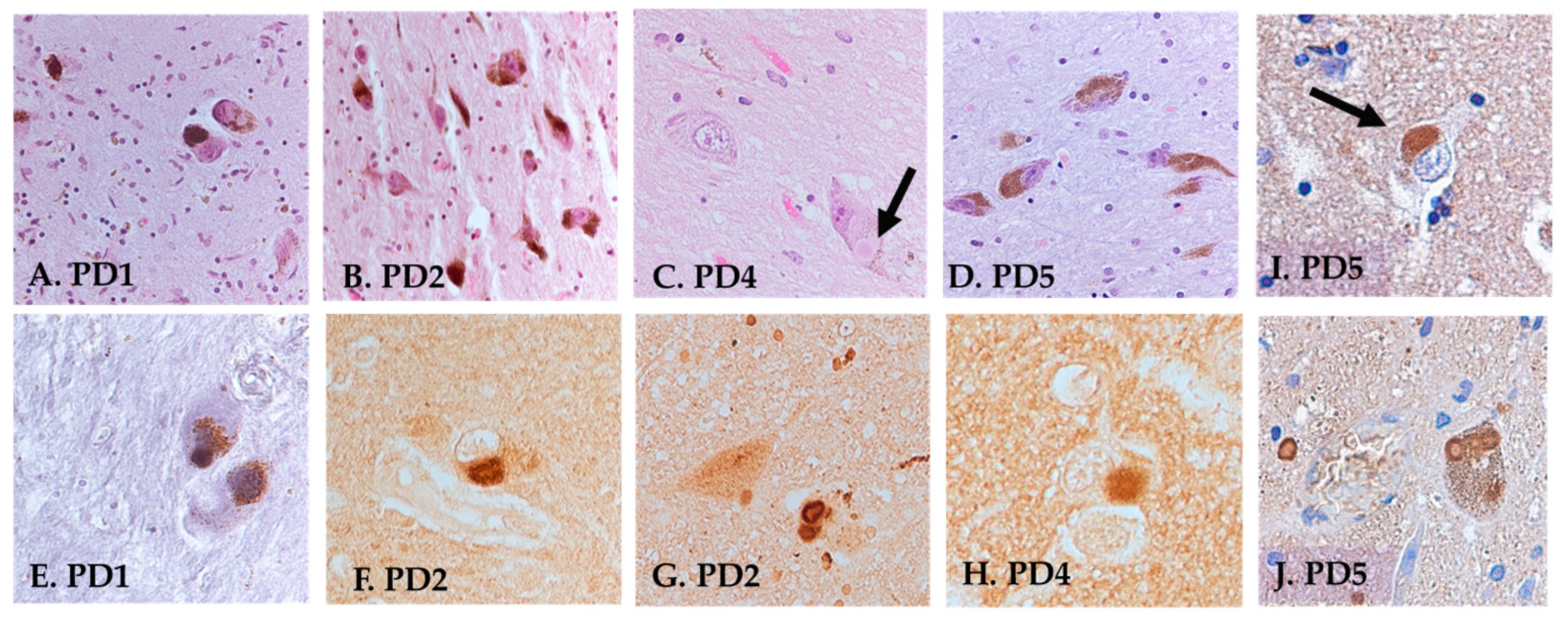
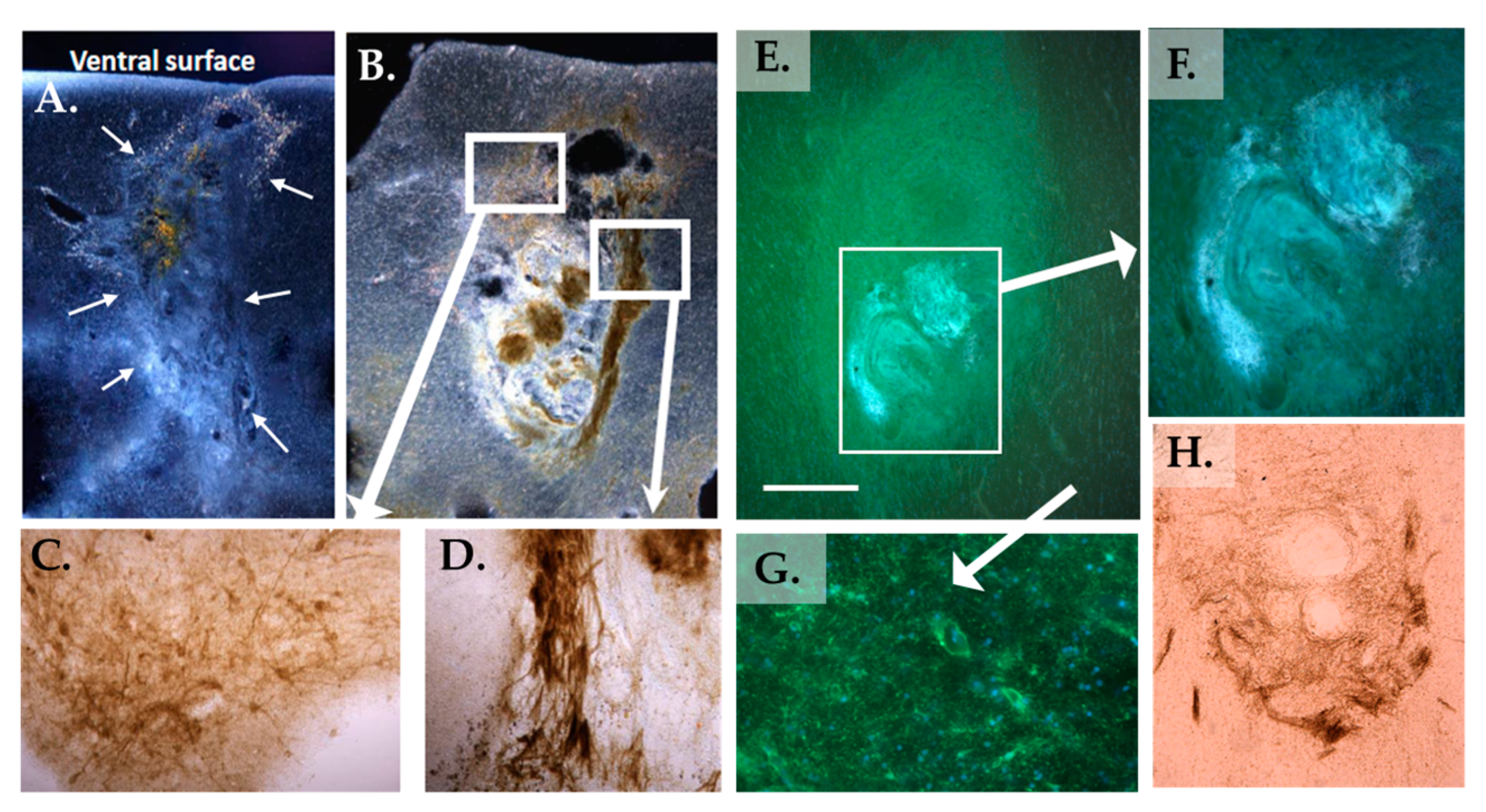
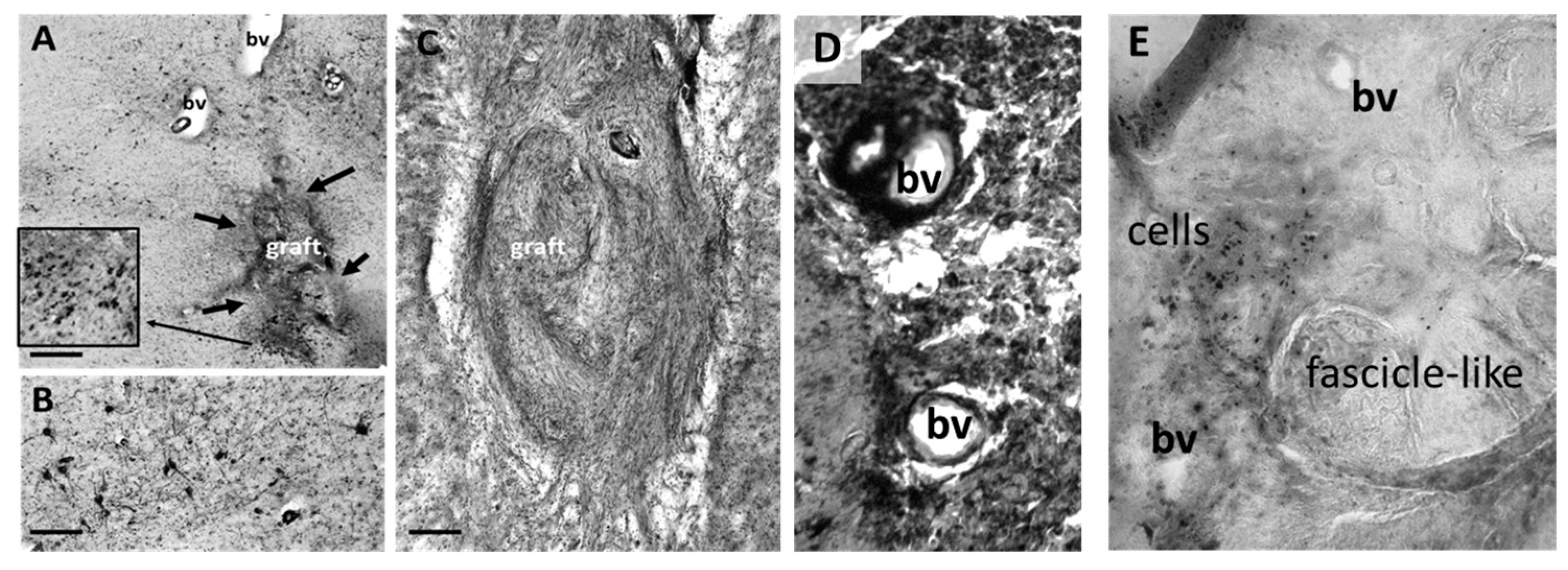
3. Results
3.1. Participant Medical History in the Study
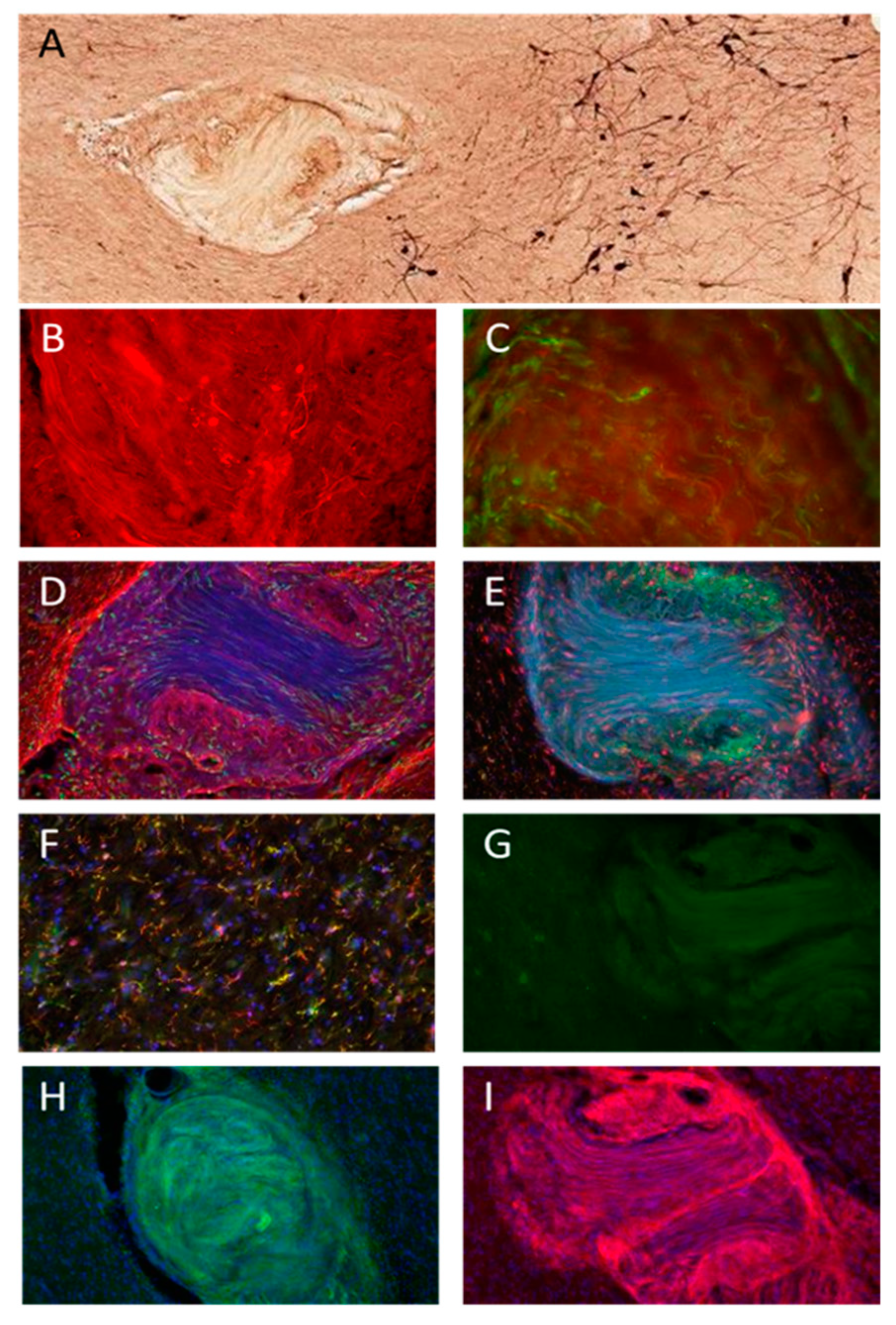
3.2. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aradi, S.D.; Hauser, R.A. Medical Management and Prevention of Motor Complications in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1339–1365. [Google Scholar] [CrossRef]
- Espay, A.J.; Kalia, L.V.; Gan-Or, Z.; Williams-Gray, C.H.; Bedard, P.L.; Rowe, S.M.; Morgante, F.; Fasano, A.; Stecher, B.; Kauffman, M.A.; et al. Disease modification and biomarker development in Parkinson disease: Revision or reconstruction? Neurology 2020, 94, 481–494. [Google Scholar] [CrossRef]
- Gonzalez-Latapi, P.; Bayram, E.; Litvan, I.; Marras, C. Cognitive Impairment in Parkinson’s Disease: Epidemiology, Clinical Profile, Protective and Risk Factors. Behav. Sci. 2021, 11, 74. [Google Scholar] [CrossRef]
- Jia, F.; Fellner, A.; Kumar, K.R. Monogenic Parkinson’s Disease: Genotype, Phenotype, Pathophysiology, and Genetic Testing. Genes 2022, 13, 471. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven Pattern of Dopamine Loss in the Striatum of Patients with Idiopathic Parkinson’s Disease. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Liu, A.K.; Chang, R.C.; Pearce, R.K.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef]
- Horsager, J.; Okkels, N.; Hansen, A.K.; Damholdt, M.F.; Andersen, K.H.; Fedorova, T.D.; Munk, O.L.; Danielsen, E.H.; Pavese, N.; Brooks, D.J.; et al. Mapping Cholinergic Synaptic Loss in Parkinson’s Disease: An [18F]FEOBV PET Case-Control Study. J. Park. Dis. 2022, 12, 2493–2506. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.-Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C. International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson's disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef]
- Artusi, C.A.; Dwivedi, A.K.; Romagnolo, A.; Pal, G.; Kauffman, M.; Mata, I.; Patel, D.; Vizcarra, J.A.; Duker, A.; Marsili, L.; et al. Association of Subthalamic Deep Brain Stimulation With Motor, Functional, and Pharmacologic Outcomes in Patients With Monogenic Parkinson Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2019, 2, e187800. [Google Scholar] [CrossRef]
- Freed, W.J.; Willingham, G.; Heim, R. Effects of adrenal medulla and sciatic nerve co-grafts in rats with unilateral substantia nigra lesions. J. Neural. Transpl. Plast. 1992, 3, 159–167. [Google Scholar] [CrossRef]
- Bjorklund, A.; Lindvall, O. Replacing Dopamine Neurons in Parkinson’s Disease: How did it happen? J. Park. Dis. 2017, 7, S21–S31. [Google Scholar] [CrossRef] [PubMed]
- Marchionini, D.M.; Collier, T.J.; Camargo, M.; McGuire, S.; Pitzer, M.; Sortwell, C.E. Interference with anoikis-induced cell death of dopamine neurons: Implications for augmenting embryonic graft survival in a rat model of Parkinson’s disease. J. Comp. Neurol. 2003, 464, 172–179. [Google Scholar] [CrossRef]
- Shinoda, M.; Hudson, J.L.; Stromberg, I.; Hoffer, B.J.; Moorhead, J.W.; Olson, L. Allogeneic grafts of fetal dopamine neurons: Immunological reactions following active and adoptive immunizations. Brain Res. 1995, 680, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; He, H.; Gao, Q.; Zhou, Y.; Wu, Z.; Zhang, X.; Sun, L.; Hu, G.; Guan, Q.; You, Z.; et al. Human midbrain dopaminergic neuronal differentiation markers predict cell therapy outcomes in a Parkinson’s disease model. J. Clin. Investig. 2022, 132, e156768. [Google Scholar] [CrossRef] [PubMed]
- D’Anglemont de Tassigny, X.; Pascual, A.; Lopez-Barneo, J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front. Neuroanat. 2015, 9, 10. [Google Scholar] [CrossRef]
- Torres, N.; Molet, J.; Moro, C.; Mitrofanis, J.; Benabid, A.L. Neuroprotective Surgical Strategies in Parkinson’s Disease: Role of Preclinical Data. Int. J. Mol. Sci. 2017, 18, 2190. [Google Scholar] [CrossRef]
- Bondarenko, O.; Saarma, M. Neurotrophic Factors in Parkinson’s Disease: Clinical Trials, Open Challenges and Nanoparticle-Mediated Delivery to the Brain. Front. Cell. Neurosci. 2021, 15, 682597. [Google Scholar] [CrossRef]
- Whone, A.L.; Boca, M.; Luz, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N.U.; et al. Extended Treatment with Glial Cell Line-Derived Neurotrophic Factor in Parkinson’s Disease. J. Park. Dis. 2019, 9, 301–313. [Google Scholar] [CrossRef]
- Eyjolfsdottir, H.; Eriksdotter, M.; Linderoth, B.; Lind, G.; Juliusson, B.; Kusk, P.; Almkvist, O.; Andreasen, N.; Blennow, K.; Ferreira, D.; et al. Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: Application of a second-generation encapsulated cell biodelivery device. Alzheimer’s Res. 2016, 8, 30. [Google Scholar] [CrossRef]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative Therapy for Alzheimer’s Disease-With Focus on Biodelivery of NGF. Front. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Granholm, A.C.; Henry, S.; Herbert, M.A.; Eken, S.; Gerhardt, G.A.; van Horne, C. Kidney cografts enhance fiber outgrowth from ventral mesencephalic grafts to the 6-OHDA-lesioned striatum, and improve behavioral recovery. Cell. Transpl. 1998, 7, 197–212. [Google Scholar] [CrossRef]
- Kordower, J.H.; Fiandaca, M.S.; Notter, M.F.; Hansen, J.T.; Gash, D.M. NGF-like trophic support from peripheral nerve for grafted rhesus adrenal chromaffin cells. J. Neurosurg. 1990, 73, 418–428. [Google Scholar] [CrossRef]
- Chau, M.J.; Quintero, J.E.; Monje, P.V.; Voss, S.R.; Welleford, A.S.; Gerhardt, G.A.; van Horne, C.G. Using a Transection Paradigm to Enhance the Repair Mechanisms of an Investigational Human Cell Therapy. Cell. Transpl. 2022, 31, 9636897221123515. [Google Scholar] [CrossRef] [PubMed]
- Salado-Manzano, C.; Perpina, U.; Straccia, M.; Molina-Ruiz, F.J.; Cozzi, E.; Rosser, A.E.; Canals, J.M. Is the Immunological Response a Bottleneck for Cell Therapy in Neurodegenerative Diseases? Front. Cell. Neurosci. 2020, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, G.; Marcocci, M.E.; Sgarbanti, R.; Civitelli, L.; Ripoli, C.; Piacentini, R.; Garaci, E.; Grassi, C.; Palamara, A.T. Infectious agents and neurodegeneration. Mol. Neurobiol. 2012, 46, 614–638. [Google Scholar] [CrossRef]
- Fainstein, N.; Ben-Hur, T. Brain Region-Dependent Rejection of Neural Precursor Cell Transplants. Front. Mol. Neurosci. 2018, 11, 136. [Google Scholar] [CrossRef]
- Ideguchi, M.; Shinoyama, M.; Gomi, M.; Hayashi, H.; Hashimoto, N.; Takahashi, J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J. Neurosci. Res. 2008, 86, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Van Horne, C.G.; Quintero, J.E.; Slevin, J.T.; Anderson-Mooney, A.; Gurwell, J.A.; Welleford, A.S.; Lamm, J.R.; Wagner, R.P.; Gerhardt, G.A. Peripheral nerve grafts implanted into the substantia nigra in patients with Parkinson’s disease during deep brain stimulation surgery: 1-year follow-up study of safety, feasibility, and clinical outcome. J. Neurosurg. 2018, 129, 1550–1561. [Google Scholar] [CrossRef]
- Quintero, J.E.; Slevin, J.T.; Gurwell, J.A.; McLouth, C.J.; El Khouli, R.; Chau, M.J.; Guduru, Z.; Gerhardt, G.A.; van Horne, C.G. Direct delivery of an investigational cell therapy in patients with Parkinson’s disease: An interim analysis of feasibility and safety of an open-label study using DBS-Plus clinical trial design. BMJ Neurol. Open. 2022, 4, e000301. [Google Scholar] [CrossRef]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Höke, A. Schwann cell phenotype is regulated by axon modality and central–peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Jellinger, K.A.; Korczyn, A.D. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.X.; Trojanowski, J.Q.; Lee, V.M. Alpha-Synuclein pathology in Parkinson’s disease and related alpha-synucleinopathies. Neurosci. Lett. 2019, 709, 134316. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Sardella-Silva, G.; Mietto, B.S.; Ribeiro-Resende, V.T. Four Seasons for Schwann Cell Biology, Revisiting Key Periods: Development, Homeostasis, Repair, and Aging. Biomolecules 2021, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Yla-Kotola, T.M.; Kauhanen, M.S.; Asko-Seljavaara, S.L.; Haglund, C.H.; Tukiainen, E.; Leivo, I.V. P75 nerve growth factor receptor is expressed in regenerating human nerve grafts. J. Surg. Res. 2008, 146, 254–261. [Google Scholar] [CrossRef]
- Okada, M.; Kawagoe, Y.; Sato, Y.; Nozumi, M.; Ishikawa, Y.; Tamada, A.; Yamazaki, H.; Sekino, Y.; Kanemura, Y.; Shinmyo, Y.; et al. Phosphorylation of GAP-43 T172 is a molecular marker of growing axons in a wide range of mammals including primates. Mol. Brain 2021, 14, 66. [Google Scholar] [CrossRef]
- Sood, D.; Chwalek, K.; Stuntz, E.; Pouli, D.; Du, C.; Tang-Schomer, M.; Georgakoudi, I.; Black, L.D., 3rd; Kaplan, D.L. Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue. ACS Biomater. Sci. Eng. 2016, 2, 131–140. [Google Scholar] [CrossRef]
- Barker, R.A. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 2019, 25, 1045–1053. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Mirsky, R.; Woodhoo, A.; Parkinson, D.B.; Arthur-Farraj, P.; Bhaskaran, A.; Jessen, K.R. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J. Peripher. Nerv. Syst. 2008, 13, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Young, K.F.; Gardner, R.; Sariana, V.; Whitman, S.A.; Bartlett, M.J.; Falk, T.; Morrison, H.W. Can quantifying morphology and TMEM119 expression distinguish between microglia and infiltrating macrophages after ischemic stroke and reperfusion in male and female mice? J. Neuroinflamm. 2021, 18, 58. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.; Melis, M.; Foidart, J.M.; Van Ewijk, W. Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem. 1986, 34, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Favor, J.; Gloeckner, C.J.; Janik, D.; Klempt, M.; Neuhauser-Klaus, A.; Pretsch, W.; Schmahl, W.; Quintanilla-Fend, L. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: An extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics 2007, 175, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Pointer, K.B.; Clark, P.A.; Schroeder, A.B.; Salamat, M.S.; Eliceiri, K.W.; Kuo, J.S. Association of collagen architecture with glioblastoma patient survival. J. Neurosurg. 2017, 126, 1812–1821. [Google Scholar] [CrossRef]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann Cell-Like Cells: Origin and Usability for Repair and Regeneration of the Peripheral and Central Nervous System. Cells 2020, 9, 1990. [Google Scholar] [CrossRef]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef]
- Barton, M.J.; John, J.S.; Clarke, M.; Wright, A.; Ekberg, J. The Glia Response after Peripheral Nerve Injury: A Comparison between Schwann Cells and Olfactory Ensheathing Cells and Their Uses for Neural Regenerative Therapies. Int. J. Mol. Sci. 2017, 18, 287. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Zhu, J.; Wu, Y.; Li, D.; Dong, L.; Li, Y.; Wen, X.; Yu, F.; Zhang, H.; et al. Fibroblast growth factor 21 facilitates peripheral nerve regeneration through suppressing oxidative damage and autophagic cell death. J. Cell. Mol. Med. 2019, 23, 497–511. [Google Scholar] [CrossRef]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration-In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kotliarova, A.; Sidorova, Y.A. Glial Cell Line-Derived Neurotrophic Factor Family Ligands, Players at the Interface of Neuroinflammation and Neuroprotection: Focus Onto the Glia. Front. Cell. Neurosci. 2021, 15, 679034. [Google Scholar] [CrossRef]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef]
- Akhtar, A.A.; Gowing, G.; Kobritz, N.; Savinoff, S.E.; Garcia, L.; Saxon, D.; Cho, N.; Kim, G.; Tom, C.M.; Park, H.; et al. Inducible Expression of GDNF in Transplanted iPSC-Derived Neural Progenitor Cells. Stem Cell Rep. 2018, 10, 1696–1704. [Google Scholar] [CrossRef]
- Quattrone, A.; Barbagallo, G.; Cerasa, A.; Stoessl, A.J. Neurobiology of placebo effect in Parkinson’s disease: What we have learned and where we are going. Mov. Disord. 2018, 33, 1213–1227. [Google Scholar] [CrossRef]
- Slevin, J.T.; Gerhardt, G.A.; Smith, C.D.; Gash, D.M.; Kryscio, R.; Young, B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005, 102, 216–222. [Google Scholar] [CrossRef]
- Spencer, D.D.; Robbins, R.J.; Naftolin, F.; Marek, K.L.; Vollmer, T.; Leranth, C.; Roth, R.H.; Price, L.H.; Gjedde, A.; Bunney, B.S.; et al. Unilateral transplantation of human fetal mesencephalic tissue into the caudate nucleus of patients with Parkinson’s disease. N. Engl. J. Med. 1992, 327, 1541–1548. [Google Scholar] [CrossRef]
- Mansbridge, J.N.; Liu, K.; Pinney, R.E.; Patch, R.; Ratcliffe, A.; Naughton, G.K. Growth factors secreted by fibroblasts: Role in healing diabetic foot ulcers. Diabetes Obes. Metab. 1999, 1, 265–279. [Google Scholar] [CrossRef]
- Han, Y.; Yang, J.; Fang, J.; Zhou, Y.; Candi, E.; Wang, J.; Hua, D.; Shao, C.; Shi, Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct. Target. Ther. 2022, 7, 92. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Catalog # | Company |
|---|---|---|
| TH | AB113 | Abcam, Cambridge, UK |
| Gap43 | PA5-34943 | Invitrogen, Waltham, MA, USA |
| IBA1 (Gt) | NB100-1028 | Novus Biologicals, Littleton, CO, USA |
| TMEM119 | HPA051870 | Sigma-Aldrich, St. Louis, MO, USA |
| GFAP (Gt) | NB100-53809 | Novus Biologicals, Littleton, CO, USA |
| Sox10 | AB155279 | Abcam, Cambridge, UK |
| ER-TR7 | MA1-40076 | Invitrogen, Waltham, MA, USA |
| P75 | MAB367 | R&D Systems, Wiesbaden, Germany |
| GDNF | AF-212 | Novus Biologicals, Littleton, CO, USA |
| PD1 | PD2 | PD4 | PD5 | |
|---|---|---|---|---|
| Age at death | 75 | 75 | 70 | 74 |
| Sex | M | M | M | M |
| Implant side | Left | Left | Right | Right |
| Location of implant | SN | NBM | NBM | SN |
| Postmortem interval (h) | 9.5 | 4 | 54 * | 30 * |
| Duration of PD diagnosis at time of surgery (years) | 6 | 8 | 7 | 8 |
| Time with graft (months) | 33 | 29 | 55 | 54 |
| BASELINE | ||||
| Off-state 1 UPDRS Part III | 46 | 34 | 47 | 40 |
| Lateralized scores (contralateral to graft/ipsilateral to graft) 2 | 17/15 | 17/6 | 17/15 | 18/13 |
| TWO-YEAR VISIT | ||||
| Off-state 1 UPDRS Part III | 37 | N/A | 65 | 35 |
| Lateralized scores (contralateral to graft/ipsilateral to graft) 2 | 14/9 | N/A | 17/21 | 10/6 |
| PD1 | PD2 | PD4 | PD5 | |
|---|---|---|---|---|
| Neuropathological diagnosis | LBD, amygdala predominant | LBD, neocortical | LBD, neocortical | LBD, neocortical |
| Cerebrovascular pathology | +++ | + | + | + |
| Focal lesions | 0 | 0 | 0 | 0 |
| Tauopathy | 0 | + (rare) | 0 | 0 |
| Amyloid deposits | 0 | 0 | 0 | 0 |
| α-synucleinopathy | ++ | ++ | +++ | +++ |
| Cortical α-syn | 0 | + | ++ | + |
| Amygdala/Thal α-syn | + | + (not Thal) | + | +++ |
| Glial inclusions or GCIs | 0 | 0 | 0 | 0 |
| Brainstem LBs | + | ++ | ++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colvett, I.; Gilmore, A.; Guzman, S.; Ledreux, A.; Quintero, J.E.; Ginjupally, D.R.; Gurwell, J.A.; Slevin, J.T.; Guduru, Z.; Gerhardt, G.A.; et al. Recipient Reaction and Composition of Autologous Sural Nerve Tissue Grafts into the Human Brain. J. Clin. Med. 2023, 12, 6121. https://doi.org/10.3390/jcm12196121
Colvett I, Gilmore A, Guzman S, Ledreux A, Quintero JE, Ginjupally DR, Gurwell JA, Slevin JT, Guduru Z, Gerhardt GA, et al. Recipient Reaction and Composition of Autologous Sural Nerve Tissue Grafts into the Human Brain. Journal of Clinical Medicine. 2023; 12(19):6121. https://doi.org/10.3390/jcm12196121
Chicago/Turabian StyleColvett, Isaac, Anah Gilmore, Samuel Guzman, Aurélie Ledreux, Jorge E. Quintero, Dhanunjaya Rao Ginjupally, Julie A. Gurwell, John T. Slevin, Zain Guduru, Greg A. Gerhardt, and et al. 2023. "Recipient Reaction and Composition of Autologous Sural Nerve Tissue Grafts into the Human Brain" Journal of Clinical Medicine 12, no. 19: 6121. https://doi.org/10.3390/jcm12196121
APA StyleColvett, I., Gilmore, A., Guzman, S., Ledreux, A., Quintero, J. E., Ginjupally, D. R., Gurwell, J. A., Slevin, J. T., Guduru, Z., Gerhardt, G. A., van Horne, C. G., & Granholm, A.-C. (2023). Recipient Reaction and Composition of Autologous Sural Nerve Tissue Grafts into the Human Brain. Journal of Clinical Medicine, 12(19), 6121. https://doi.org/10.3390/jcm12196121








