Effectiveness of Regular Aerobic Exercise in Improving Vascular Stiffness in Elderly Korean Women
Abstract
:1. Introduction
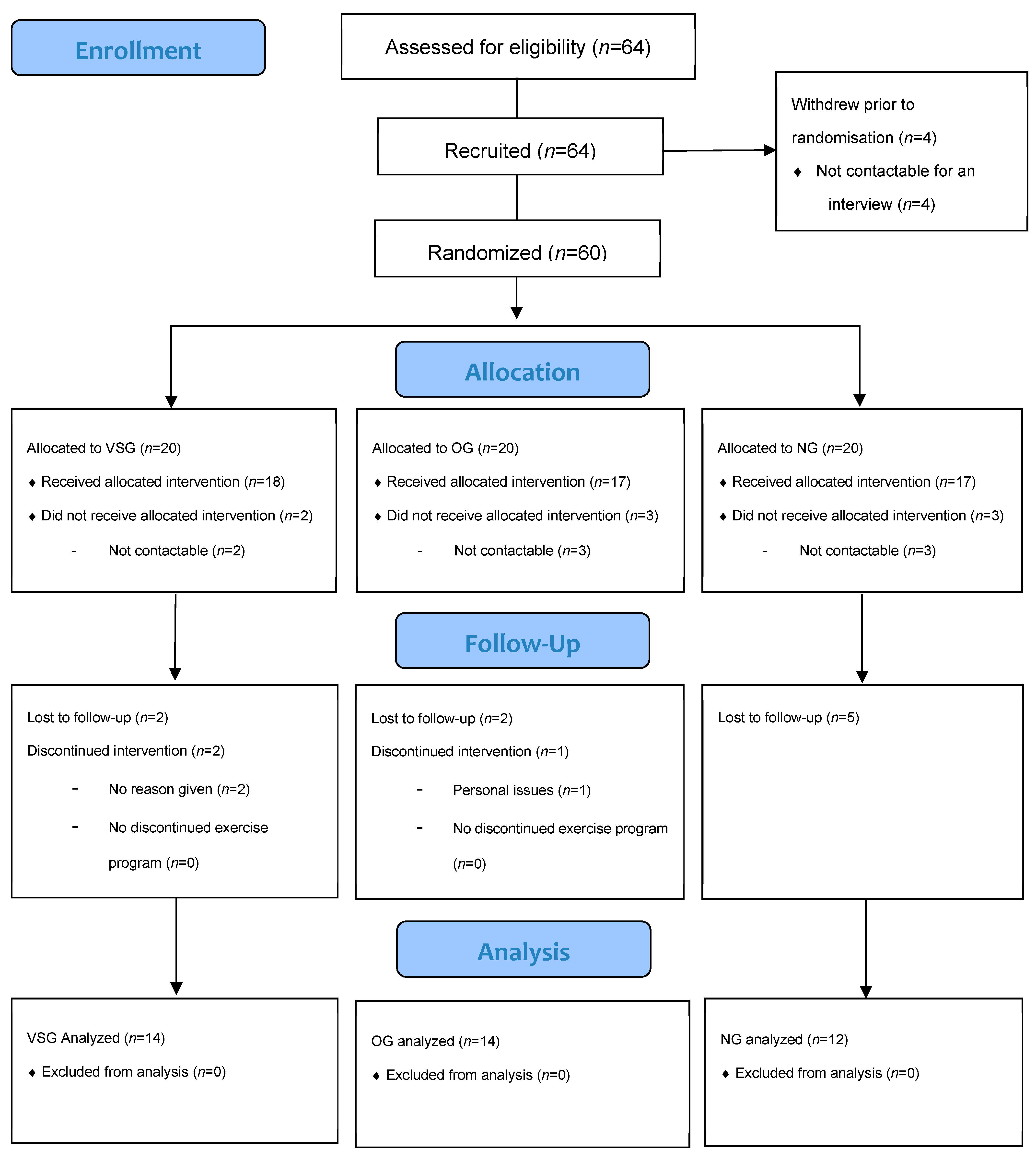
2. Methods
2.1. Participants
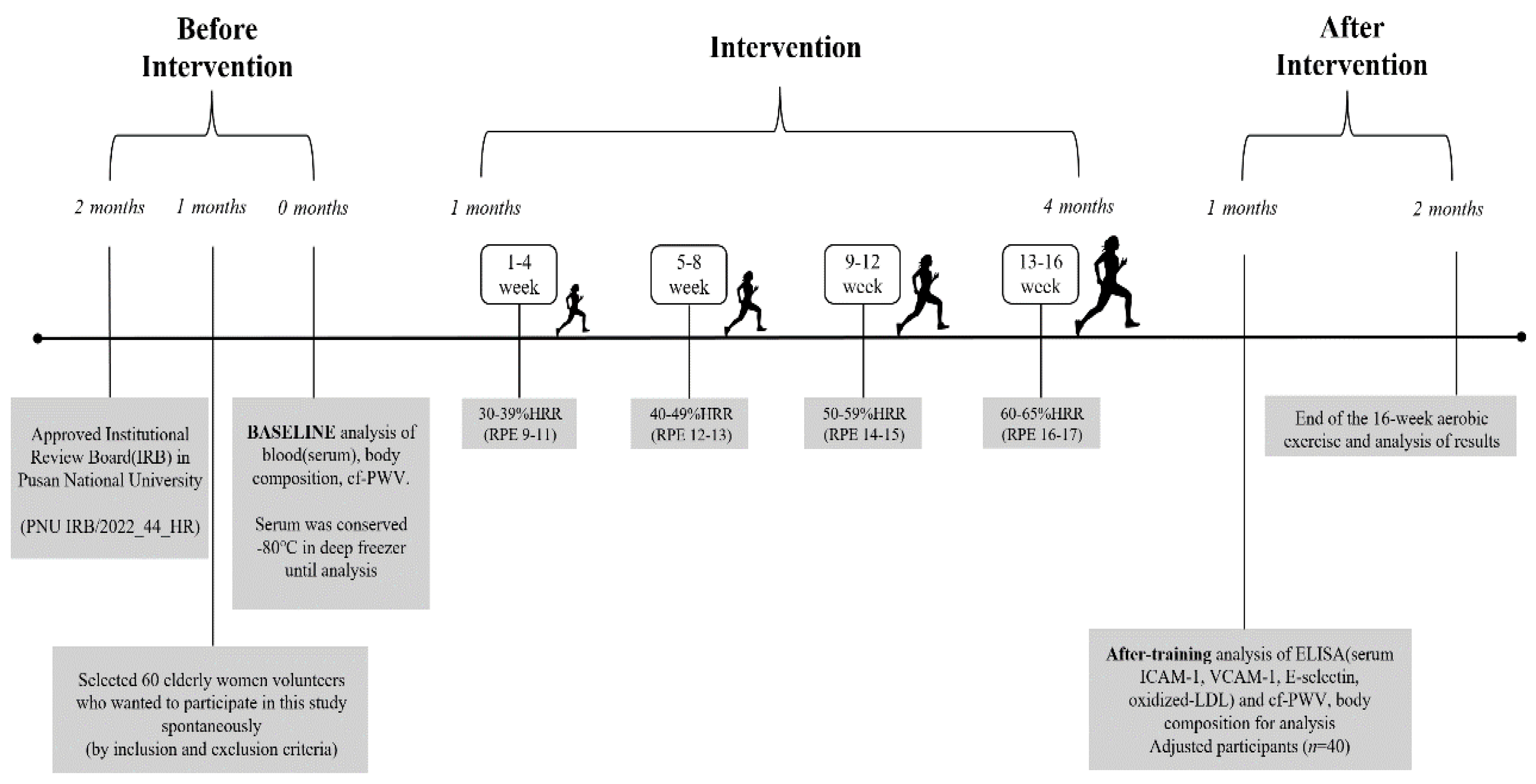
2.2. Aerobic Exercise Program
3. Data Collection
3.1. Body Composition
3.2. Blood Collection
3.3. Vascular Stiffness Analysis
3.4. Blood Analysis
3.5. Data Analysis
4. Results
4.1. Changes in cf-PWV
4.2. Changes in CAM
4.2.1. ICAM-1
4.2.2. VCAM-1
4.2.3. E-Selectin
4.3. Changes in Oxidized LDL
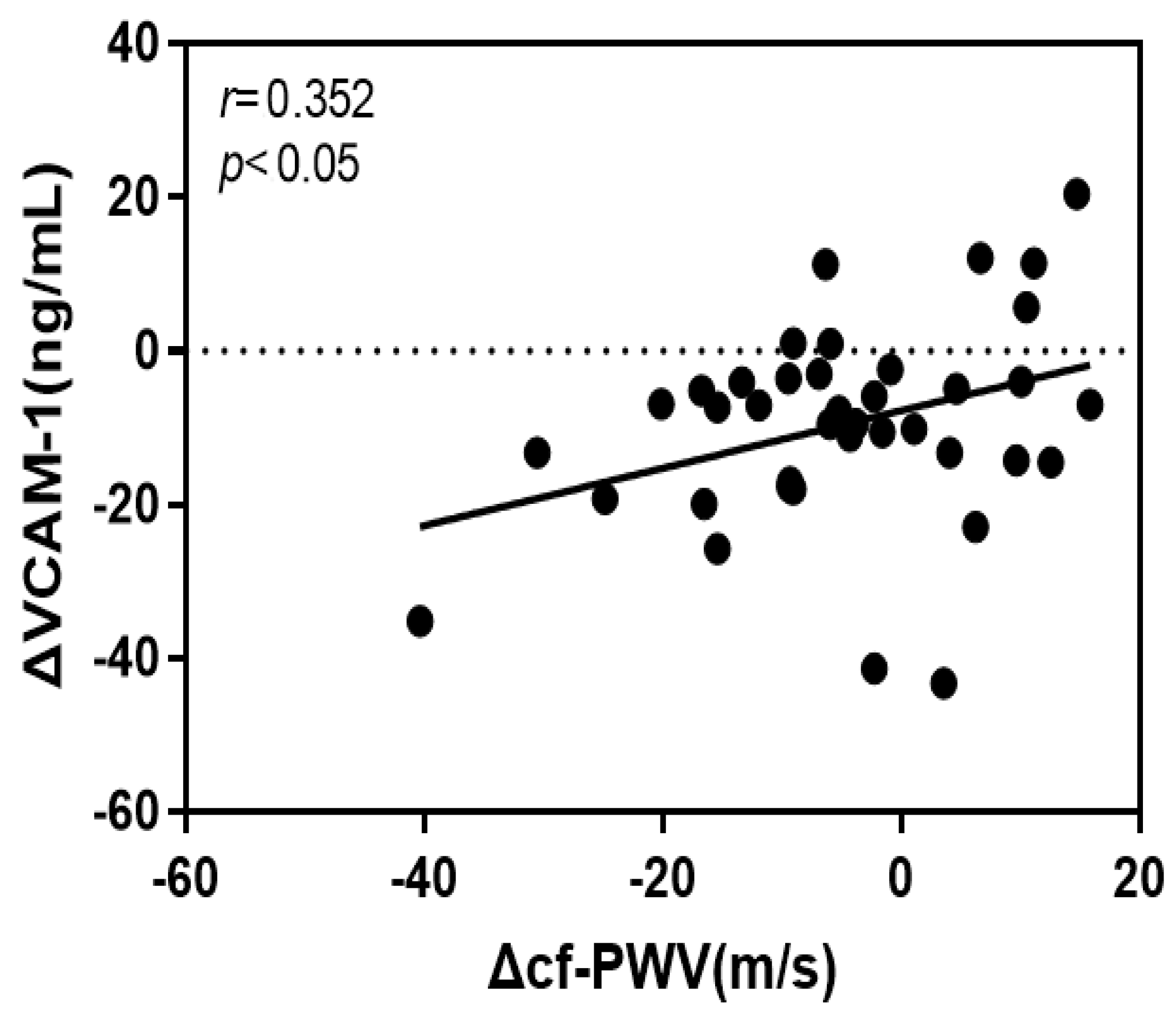
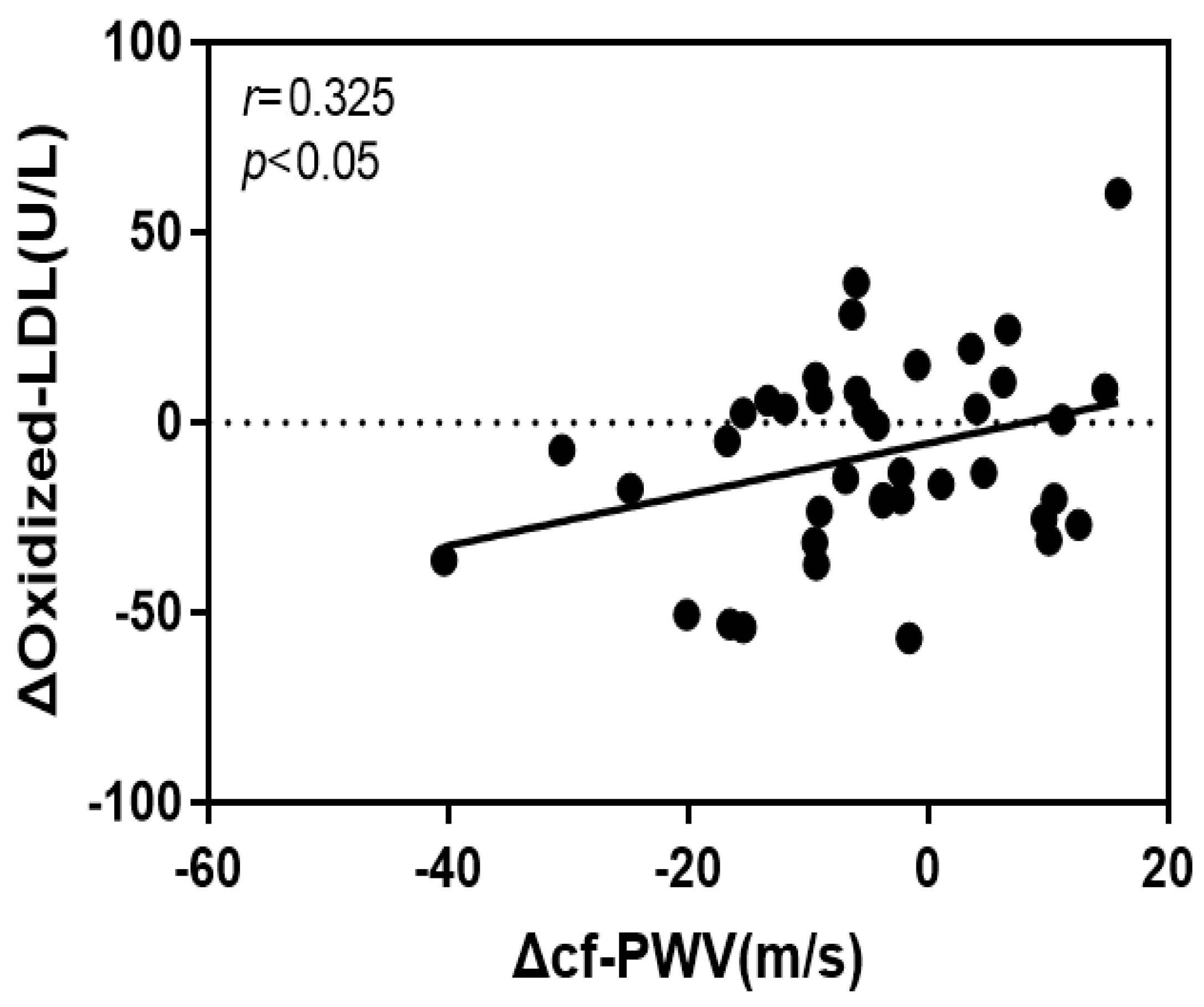
4.4. Correlation of the Rate of Change of cf-PWV with VCAM-1 and Oxidized LDL
4.5. Analysis of the Effects of cf-PWV, VCAM-1, and Oxidized LDL
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habas, K.; Shang, L. Alteration in intercellular adhesion molecule 1(ICAM-1) and vascular cell adhesion molecule 1(VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kuo, F.C.; Tang, W.H.; Lu, C.H.; Su, S.C.; Liu, J.S.; Hsieh, C.H.; Hung, Y.J.; Lin, F.H. Serum E-selectin concentration is associated with risk of metabolic syndrome in female. PLoS ONE 2019, 14, e0222815. [Google Scholar] [CrossRef] [PubMed]
- Qui, S.; Cai, X.; Liu, J.; Yang, B.; Zugel, M.; Steinacker, J.M.; Sun, Z.; Schumann, U. Association between circulating cell adhesion molecules and risk of type 2 diabetes: A meta-analysis. Atherosclerosis 2019, 287, 147–154. [Google Scholar]
- Saud, A.; Ali, N.A.; Gali, F.; Hadi, N. The role of cytokines, adhesion molecules, and toll-like receptors in atherosclerosis progression: The effect of Atorvastatin. J. Med. Life 2022, 15, 751–756. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.; Xiao, Q. Foam cell formation: A new target for fighting atherosclerosis and cardiovascular disease. Vasc. Pharmacol. 2018, 112, 54–71. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, D.; Wang, M.; Zhao, F.; Han, X.; Qi, Y.; Liu, J. Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: A meta-analysis of observational studies. Can. J. Cardiol. 2017, 33, 1624–1632. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.W.; Xu, R.X.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Gao, Y.; Li, J.J. Oxidized-LDL is a useful marker for predicting the very early coronary artery disease and cardiovascular outcomes. Pers. Med. 2018, 15, 521–529. [Google Scholar] [CrossRef]
- Honda, S.; Ikede, K.; Urata, R.; Yamazaki, E.; Emoto, N.; Matoba, S. Cellular senescence promotes endothelial activation through epigenetic alteration and consequently accelerates atherosclerosis. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Koh, Y.; Park, J. Cell adhesion molecules and exercise. J. Inflamm. Res. 2018, 11, 297–306. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.T.; Lim, S.T.; Park, J.K. Effects of a 12-week healthy-life exercise program on oxidized low-density lipoprotein cholesterol and carotid intima-media thickness in obese elderly women. J. Phys. Ther. Sci. 2015, 27, 1435–1439. [Google Scholar] [CrossRef]
- Joint Committee for Guideline Revision. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J. Geriatr. Cardiol. 2019, 16, 182–241. [Google Scholar]
- Korean Society for the Study of Obesity. Guideline for the Management of Obesity; Korean Society for the Study of Obesity: Seoul, Republic of Korea, 2020. [Google Scholar]
- Ha, S.M.; Kim, D.Y.; Kim, J.H. Effects of Circuit Exercise on Functional Fitness, Estradiol, Serotonin, Depression and Cognitive Function in Elderly Women. J. Korean Phys. Educ. Assoc. Girls Women 2017, 31, 199–217. [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Willkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Wang, T.T.; Wang, X.M.; Zhang, X.L. Circulating vascular cell adhesion molecule-1 (VCAM-1) intercellular adhesion molecule (ICAM-1): Relationship with carotid artery elasticity in patients with impaired glucose regulation (IRG). Ann. d’Endocrinol. 2019, 80, 72–76. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harbor Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Kaplon, R.E.; Hill, S.D.; McNamara, M.N.; Santos-Parker, J.R.; Pierce, G.L.; Seals, D.R.; Donato, A.J. Endothelial cell senescence with aging in healthy humans: Prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular adaptation to exercise in human: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef]
- Hijmans, J.G.; Bammert, T.D.; Stockelman, K.A.; Reiakvam, W.R.; Greiner, J.J.; DeSouza, C.A. High glucose-induced endothelial microparticles increase adhesion molecule expression on endothelial cells. Diabetol. Int. 2019, 10, 143–147. [Google Scholar] [CrossRef]
- Li, G.; Lv, Y.; Su, Q.; You, Q.; Yu, L. The effect of aerobic exercise on pulse wave velocity in middle-aged and elderly people: A systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 2022, 9, e960096. [Google Scholar] [CrossRef]
- LeMaster, E.; Huang, R.T.; Zhang, C.; Bohachkov, Y.; Coles, C.; Shentu, T.P.; Sheng, Y.; Fancher, I.S.; Ng, C.; Christoforidis, T.; et al. Proatherogenic flow increases endothelial stiffness via enhanced CD36-mediated uptake of oxidized low-density lipoproteins. Atheroscler. Thromb. Vasc. Biol. 2018, 38, 64–75. [Google Scholar] [CrossRef]
- Bertani, F.; Di Francesco, D.; Corrado, M.D.; Talmon, M.; Fresu, L.G.; Boccafoschi, F. Paracrine shear stress dependent signaling from endothelial cells affects downstream endothelial function and inflammation. Int. J. Mol. Sci. 2021, 22, 13300. [Google Scholar] [CrossRef]
- Boeno, F.P.; Ramis, T.R.; Munhoz, S.V.; Farinha, J.B.; Moritz, C.E.; Leal-Menezes, R.; Ribeiro, J.L.; Christou, D.D.; Reischak-Oliveira, A. Effect of aerobic and resistance exercise training on inflammation, endothelial function and ambulatory blood pressure in middle-aged hypertensive patients. J. Hypertens. 2020, 38, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
| Variables Group | Age (Years) | Height (cm) | Weight (kg) | BMI (kg/m2) | %BF (%) | cf-PWV (m/s) |
|---|---|---|---|---|---|---|
| VSG (n = 14) | 78.64 ±4.55 | 152.39 ±4.71 | 51.48 ±6.70 | 22.13 ±2.12 | 29.26 ±5.56 | 14.77 ±1.94 |
| OG (n = 14) | 76.50 ±4.82 | 150.14 ±4.22 | 61.97 ±7.43 | 27.40 ±2.35 | 39.04 ±4.78 | 10.89 ±0.98 |
| NG (n = 12) | 78.75 ±5.79 | 147.83 ±6.70 | 48.55 ±7.06 | 22.14 ±2.03 | 30.08 ±6.34 | 9.94 ±1.31 |
| F | 13.331 *** | 26.535 *** | 13.107 *** | 40.581 *** | ||
| Scheffe | NS | NS | VSG, NG, <OG | VSG, NG <OG | VSG, NG <OG | OG, NG <VSG |
| Variable | Group | Pre | Post | diff(%) | T | F | |
|---|---|---|---|---|---|---|---|
| cf-PWV (m/s) | VSG (n = 14) | 14.77 ±1.94 | 12.61 ±1.73 | −14.62 | 3.712 ** | Group | 31.127 *** |
| OG (n = 14) | 10.89 ±0.98 | 10.88 ±1.30 | −0.09 | 0.058 | Time | 9.422 ** | |
| NG (n = 12) | 9.94 ±1.31 | 9.93 ±1.40 | −0.10 | 0.052 | G × T | 9.319 *** | |
| F | 40.581 *** | 10.983 *** | 7.730 ** | ||||
| Scheffe | OG, NG <VSG | OG, NG <VSG | NG < OG <VSG | ||||
| Variable | Group | Pre | Post | diff(%) | t | F | |
|---|---|---|---|---|---|---|---|
| ICAM-1 (ng/mL) | VSG (n = 14) | 10.57 ±2.89 | 9.48 ±2.45 | −10.31 | 3.493 ** | Group | 0.804 |
| OG (n = 14) | 9.92 ±3.69 | 9.62 ±2.75 | −3.02 | 0.674 | Time | 13.462 *** | |
| NG (n = 12) | 11.84 ±4.55 | 10.75 ±3.33 | −9.20 | 2.740 * | G × T | 1.404 | |
| F | 0.873 | 0.760 | 2.898 | ||||
| Scheffe | NS | NS | NS | ||||
| VCAM-1 (ng/mL) | VSG (n = 14) | 33.15 ±6.65 | 26.94 ±4.84 | −18.73 | 5.241 *** | Group | 1.745 |
| OG (n = 14) | 28.13 ±5.81 | 25.67 ±4.20 | −8.75 | 2.144 | Time | 28.254 *** | |
| NG (n = 12) | 29.29 ±3.67 | 28.11 ±2.41 | -4.03 | 1.659 | G × T | 6.006 ** | |
| F | 3.060 | 1.197 | 5.115 * | ||||
| Scheffe | NS | NS | NG < OG <VSG | ||||
| E-selectin (ng/mL) | VSG (n = 14) | 4.74 ±1.37 | 3.71 ±1.50 | −21.73 | 4.236 *** | Group | 1.258 |
| OG (n = 14) | 4.42 ±2.04 | 4.22 ±1.42 | −4.52 | 0.816 | Time | 11.157 ** | |
| NG (n = 12) | 3.57 ±1.30 | 3.39 ±1.28 | −5.04 | 0.771 | G × T | 4.052 * | |
| F | 1.766 | 1.143 | 5.389 ** | ||||
| Scheffe | NS | NS | OG < NG <VSG | ||||
| Variable | Group | Pre | Post | diff(%) | t | F | |
|---|---|---|---|---|---|---|---|
| Oxidized LDL (U/L) | VSG (n = 14) | 10.40 ±3.22 | 7.38 ±2.99 | −29.04 | 4.145 ** | Group | 0.235 |
| OG (n = 14) | 8.69 ±3.33 | 8.67 ±2.87 | −0.23 | 0.027 | Time | 11.895 *** | |
| NG (n = 12) | 8.32 ±2.98 | 7.96 ±2.72 | −4.33 | 0.730 | G × T | 8.740 *** | |
| F | 1.630 | 0.712 | 8.920 *** | ||||
| Scheffe | NS | NS | OG, NG < VSG | ||||
| Dependent Variable | B | SE | β | t | F | R2 |
|---|---|---|---|---|---|---|
| Constant | −7.723 | 2.110 | −3.660 *** | |||
| VCAM-1 | 0.374 | 0.162 | 0.352 | 2.315 * | 5.359 * | 0.124 |
| Oxidized LDL | 0.675 | 0.319 | 0.325 | 2.118 * | 4.485 * | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, S.; Kim, T.; Kang, D.; Kim, D. Effectiveness of Regular Aerobic Exercise in Improving Vascular Stiffness in Elderly Korean Women. J. Clin. Med. 2023, 12, 6119. https://doi.org/10.3390/jcm12196119
Koh S, Kim T, Kang D, Kim D. Effectiveness of Regular Aerobic Exercise in Improving Vascular Stiffness in Elderly Korean Women. Journal of Clinical Medicine. 2023; 12(19):6119. https://doi.org/10.3390/jcm12196119
Chicago/Turabian StyleKoh, Suhan, Taekyu Kim, Duwang Kang, and Doyeon Kim. 2023. "Effectiveness of Regular Aerobic Exercise in Improving Vascular Stiffness in Elderly Korean Women" Journal of Clinical Medicine 12, no. 19: 6119. https://doi.org/10.3390/jcm12196119
APA StyleKoh, S., Kim, T., Kang, D., & Kim, D. (2023). Effectiveness of Regular Aerobic Exercise in Improving Vascular Stiffness in Elderly Korean Women. Journal of Clinical Medicine, 12(19), 6119. https://doi.org/10.3390/jcm12196119





