Mechanical Dyssynchrony Combined with Septal Scarring Reliably Identifies Responders to Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Echocardiography: Conventional Parameters
2.3. Mechanical Dyssynchrony: Visual Markers
2.3.1. Septal Flash
2.3.2. Apical Rocking
2.3.3. Decision-Making for Septal Flash and Apical Rocking
2.4. Mechanical Dyssynchrony: Quantitative Markers
2.4.1. Systolic Stretch Index
2.4.2. Lateral-to-Septal Work Difference
2.5. Myocardial Scarring
2.6. Cardiac Resynchronization Therapy
2.7. Endpoints
2.8. Statistical Analysis
3. Results
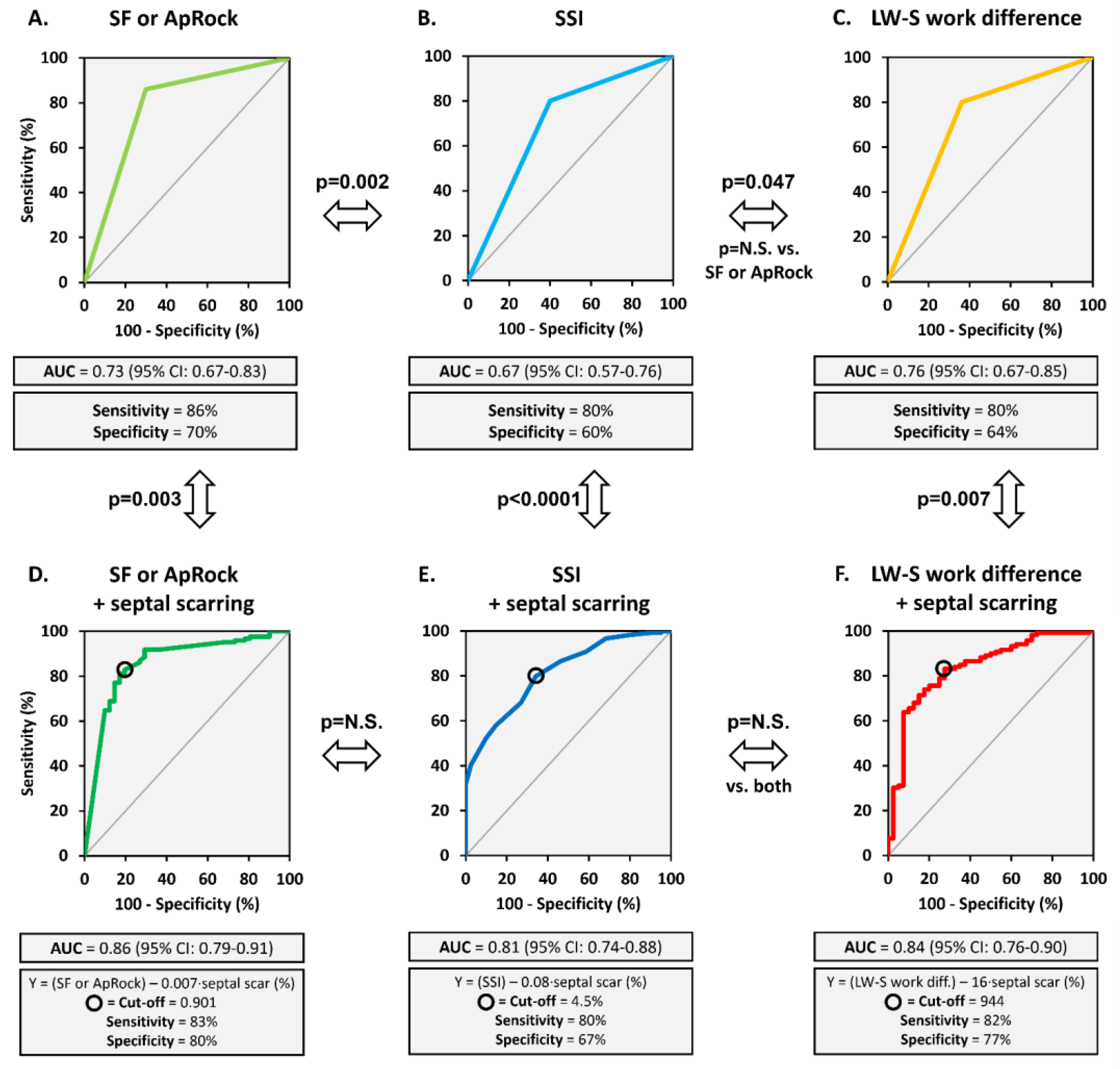
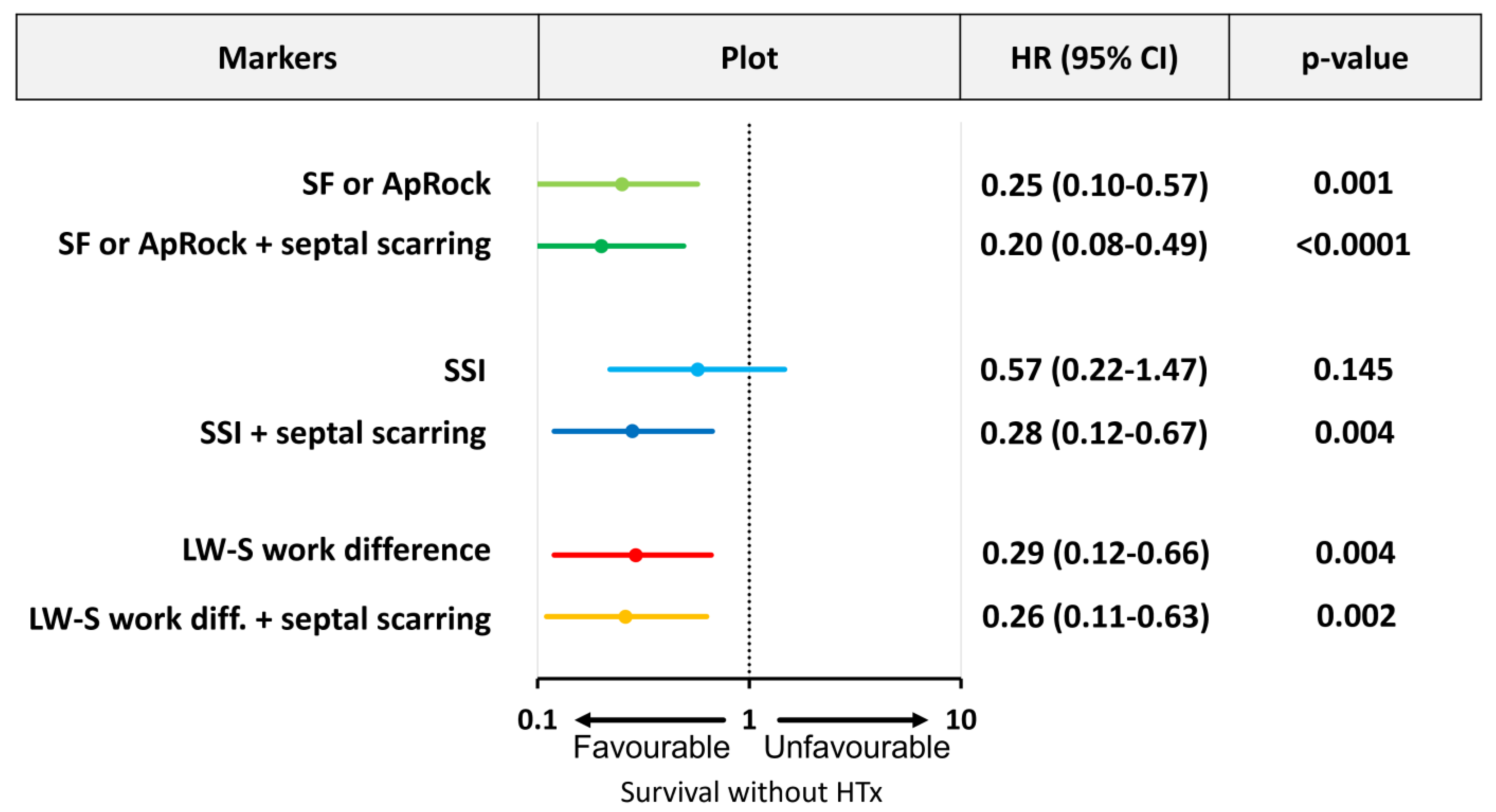
3.1. Predictive Value of Markers of Mechanical Dyssynchrony
3.2. Myocardial Scarring
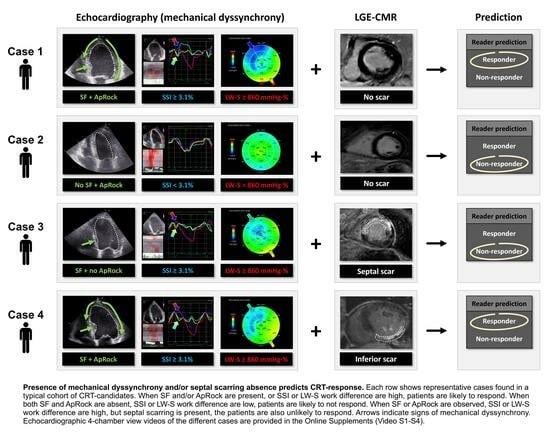
3.3. Combining Mechanical Dyssynchrony and Septal Scarring
3.4. Ischemic vs. Non-Ischemic Patients
3.5. Reproducibility
4. Discussion
4.1. Influence of Myocardial Scarring on Mechanical Dyssynchrony and Response
4.2. Comparison among Different Mechanics-Based Dyssynchrony Parameters
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ApRock | apical rocking |
| CMR | cardiac magnetic resonance imaging |
| CRT | cardiac resynchronization therapy |
| GLS | global longitudinal strain |
| LBBB | left bundle branch block |
| LGE | late gadolinium enhancement |
| LV | left ventricular |
| LVEF | left ventricular ejection fraction |
| LW-S | lateral-to-septal |
| SF | septal flash |
| SSI | systolic stretch index |
References
- Nassif, M.E.; Tang, Y.; Cleland, J.G.; Abraham, W.T.; Linde, C.; Gold, M.R.; Young, J.B.; Daubert, J.C.; Sherfesee, L.; Schaber, D.; et al. Precision Medicine for Cardiac Resynchronization. Circ. Heart Fail. 2017, 10, e00411. [Google Scholar] [CrossRef]
- Beela, A.S.; Ünlü, S.; Duchenne, J.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Penicka, M.; et al. Assessment of Mechanical Dyssynchrony Can Improve the Prognostic Value of Guideline-Based Patient Selection for Cardiac Resynchronization Therapy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Beshai, J.F.; Grimm, R.A.; Nagueh, S.F.; Baker, J.H.; Beau, S.L.; Greenberg, S.M.; Pires, L.A.; Tchou, P.J. Cardiac-Resynchronization Therapy in Heart Failure with Narrow QRS Complexes. N. Engl. J. Med. 2007, 357, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.-P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.I.; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Breithardt, O.A.; Stellbrink, C.; Herbots, L.; Claus, P.; Sinha, A.M.; Bijnens, B.; Hanrath, P.; Sutherland, G.R. Cardiac Resynchronization Therapy Can Reverse Abnormal Myocardial Strain Distribution in Patients with Heart Failure and Left Bundle Branch Block. J. Am. Coll. Cardiol. 2003, 42, 486–494. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Schneider, T.-M.; Korder, S.; Szulik, M.; Gürel, E.; Daniel, W.G.; Rademakers, F.; Flachskampf, F.A. Apical Transverse Motion as Surrogate Parameter to Determine Regional Left Ventricular Function Inhomogeneities: A New, Integrative Approach to Left Ventricular Asynchrony Assessment. Eur. Heart J. 2009, 30, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Parsai, C.; Bijnens, B.; Sutherland, G.R.; Baltabaeva, A.; Claus, P.; Marciniak, M.; Paul, V.; Scheffer, M.; Donal, E.; Derumeaux, G.; et al. Toward Understanding Response to Cardiac Resynchronization Therapy: Left Ventricular Dyssynchrony Is Only One of Multiple Mechanisms. Eur. Heart J. 2009, 30, 940–949. [Google Scholar] [CrossRef]
- Gorcsan, J.; Anderson, C.P.; Tayal, B.; Sugahara, M.; Walmsley, J.; Starling, R.C.; Lumens, J. Systolic Stretch Characterizes the Electromechanical Substrate Responsive to Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2019, 12, 1741–1752. [Google Scholar] [CrossRef]
- Salden, O.A.E.; Zweerink, A.; Wouters, P.; Allaart, C.P.; Geelhoed, B.; de Lange, F.J.; Maas, A.H.; Rienstra, M.; Vernooy, K.; Vos, M.A.; et al. The value of septal rebound stretch analysis for the prediction of volumetric response to cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 37–45. [Google Scholar] [CrossRef]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging Predictors of Response to Cardiac Resynchronization Therapy: Left Ventricular Work Asymmetry by Echocardiography and Septal Viability by Cardiac Magnetic Resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Doltra, A.; Bijnens, B.; Tolosana, J.M.; Borràs, R.; Khatib, M.; Penela, D.; De Caralt, T.M.; Castel, M.Á.; Berruezo, A.; Brugada, J.; et al. Mechanical Abnormalities Detected with Conventional Echocardiography Are Associated with Response and Midterm Survival in CRT. JACC. Cardiovasc. Imaging 2014, 7, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Maass, A.H.; Vernooy, K.; Wijers, S.C.; Van ’T Sant, J.; Cramer, M.J.; Meine, M.; Allaart, C.P.; De Lange, F.J.; Prinzen, F.W.; Gerritse, B.; et al. Refining Success of Cardiac Resynchronization Therapy Using a Simple Score Predicting the Amount of Reverse Ventricular Remodelling: Results from the Markers and Response to CRT (MARC) Study. Europace 2018, 20, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Ypenburg, C.; Roes, S.D.; Bleeker, G.B.; Kaandorp, T.A.M.; de Roos, A.; Schalij, M.J.; van der Wall, E.E.; Bax, J.J. Effect of Total Scar Burden on Contrast-Enhanced Magnetic Resonance Imaging on Response to Cardiac Resynchronization Therapy. Am. J. Cardiol. 2007, 99, 657–660. [Google Scholar] [CrossRef]
- Duchenne, J.; Larsen, C.; Cvijic, M.; Galli, E.; Aalen, J.; Klop, B.; Puvrez, A.; Mirea, O.; Bézy, S.; Minten, L.; et al. Visual Presence of Mechanical Dyssynchrony Combined with Septal Scarring Identifies Responders to Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2022, 15, 2151–2153. [Google Scholar] [CrossRef]
- Brignole, M.; Auricchio, A.; Baron-Esquivias, G.; Bordachar, P.; Boriani, G.; Breithardt, O.-A.; Cleland, J.; Deharo, J.-C.; Delgado, V.; Elliott, P.M.; et al. 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy: The Task Force on Cardiac Pacing and Resynchronization Therapy of the European Society of Cardiology (ESC). Europace 2013, 15, 1070–1118. [Google Scholar] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A Novel Clinical Method for Quantification of Regional Left Ventricular Pressure-Strain Loop Area: A Non-Invasive Index of Myocardial Work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Aalen, J.M.; Remme, E.W.; Larsen, C.K.; Andersen, O.S.; Krogh, M.; Duchenne, J.; Hopp, E.; Ross, S.; Beela, A.S.; Kongsgaard, E.; et al. Mechanism of Abnormal Septal Motion in Left Bundle Branch Block: Role of Left Ventricular Wall Interactions and Myocardial Scar. JACC Cardiovasc. Imaging 2019, 12, 2402–2413. [Google Scholar] [CrossRef]
- Engblom, H.; Tufvesson, J.; Jablonowski, R.; Carlsson, M.; Aletras, A.H.; Hoffmann, P.; Jacquier, A.; Kober, F.; Metzler, B.; Erlinge, D.; et al. A New Automatic Algorithm for Quantification of Myocardial Infarction Imaged by Late Gadolinium Enhancement Cardiovascular Magnetic Resonance: Experimental Validation and Comparison to Expert Delineations in Multi-Center, Multi-Vendor Patient Data. J. Cardiovasc. Magn. Reson. 2016, 18, 27. [Google Scholar] [CrossRef]
- Steelant, B.; Stankovic, I.; Roijakkers, I.; Aarones, M.; Bogaert, J.; Desmet, W.; Aakhus, S.; Voigt, J.-U. The Impact of Infarct Location and Extent on LV Motion Patterns. JACC Cardiovasc. Imaging 2016, 9, 655–664. [Google Scholar] [CrossRef]
- Lumens, J.; Tayal, B.; Walmsley, J.; Delgado-Montero, A.; Huntjens, P.R.; Schwartzman, D.; Althouse, A.D.; Delhaas, T.; Prinzen, F.W.; Gorcsan, J. Differentiating Electromechanical from Non-Electrical Substrates of Mechanical Discoordination to Identify Responders to Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2015, 8, e003744. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.K.; Galli, E.; Duchenne, J.; Aalen, J.M.; Stokke, C.; Fjeld, J.G.; Degtiarova, G.; Claus, P.; Gheysens, O.; Saberniak, J.; et al. Scar Imaging in the Dyssynchronous Left Ventricle: Accuracy of Myocardial Metabolism by Positron Emission Tomography and Function by Echocardiographic Strain. Int. J. Cardiol. 2022; in press. [Google Scholar] [CrossRef]
- Puvrez, A.; Duchenne, J.; Gorcsan, J.; Marwick, T.H.; Smiseth, O.A.; Voigt, J.U. Why Mechanical Dyssynchrony Remains Relevant to Cardiac Resynchronization Therapy. Letter Regarding the Article ‘Optimized Implementation of Cardiac Resynchronization Therapy: A Call for Action for Referral and Optimization of Care: A Joint Position State. Eur. J. Heart Fail. 2021, 23, 843–844. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 170) | Responders (n = 121) | Non-Responders (n = 49) | |
|---|---|---|---|
| Clinical characteristics | |||
| Age (years) | 67 ± 10 | 67 ± 10 | 65 ± 11 (*) |
| Male (%) | 71 | 65 | 83 (*) |
| BMI (kg/m2) | 27 ± 5 | 26 ± 5 | 27 ± 5 |
| NYHA-class | 2.4 ± 0.5 | 2.3 ± 0.5 | 2.6 ± 0.5 (*) |
| Ischemic aetiology (%) | 28 | 22 | 44 (*) |
| SBP (mmHg) | 124 ± 21 | 126 ± 20 | 120 ± 21 (*) |
| DBP (mmHg) | 69 ± 11 | 71 ± 12 | 69 ± 10 |
| Medication | |||
| Beta-blocker (%) | 92 | 90 | 92 |
| ACEi/ARB (%) | 94 | 97 | 90 (*) |
| Aldosterone antagonists (%) | 43 | 41 | 46 |
| Diuretics (%) | 71 | 69 | 77 |
| ECG criteria | |||
| QRS morphology: LBBB (%) | 87 | 89 | 81 (*) |
| QRS duration (ms) | 165 ± 19 | 166 ± 18 | 162 ± 19 |
| AFib (%) | 6 | 5 | 8 |
| Paced (%) | 11 | 7 | 19 (*) |
| Device upgrades (%) | 18 | 11 | 35 (*) |
| ESC guideline class | |||
| Class I (%) | 87 | 89 | 80 |
| Class II (%) | 13 | 11 | 20 |
| Implanted CRT | |||
| CRT-P (%) | 14 | 15 | 11 |
| CRT-D (%) | 86 | 85 | 89 |
| All Patients (n = 170) | Responders (n = 121) | Non-Responders (n = 49) | |
|---|---|---|---|
| Pre-CRT | |||
| LVEDV (mL) | 199 ± 75 | 192 ± 70 | 211 ± 85 (*) |
| LVESV (mL) | 140 ± 62 | 136 ± 58 | 151 ± 71 (*) |
| LVEF (%) | 31 ± 8 | 31 ± 7 | 30 ± 8 |
| GLS (%) | 9 ± 3 | 10 ± 4 | 8 ± 3 (*) |
| 12 months follow-up | |||
| LVEDV (mL) | 158 ± 70 | 139 ± 55 (†) | 206 ± 81 (*) |
| LVESV (mL) | 97 ± 59 | 79 ± 42 (†) | 144 ± 69 (*) |
| LVEF (%) | 42 ± 11 | 46 ± 10 (†) | 33 ± 10 (*) |
| GLS (%) | 11 ± 3 | 14 ± 4 (†) | 8 ± 4 (*) |
| Regression Variable | B | 95%CI | VIF | p-Value |
|---|---|---|---|---|
| Model 1: SF or ApRock (1) | ||||
| Constant | 1.170 | |||
| SF or ApRock | −26.589 | −34.790 to −18.388 | 1.161 | <0.0001 |
| Ischaemic aetiology | 5.335 | 2.256 to 13.327 | 1.144 | 0.036 |
| QRS-morphology | −4.034 | −14.147 to 6.078 | 1.048 | 0.432 |
| QRS-duration | −0.059 | −0.236 to 0.118 | 1.019 | 0.511 |
| LVEF | −0.015 | −0.459 to 0.429 | 1.038 | 0.947 |
| Model 2: SSI (2) | ||||
| Constant | 0.532 | |||
| SSI | −2.768 | −3.687 to −1.849 | 1.098 | <0.0001 |
| Ischaemic aetiology | 12.292 | 4.774 to 19.810 | 1.037 | 0.002 |
| QRS-morphology | −2.151 | −12.442 to 8.140 | 1.075 | 0.680 |
| QRS-duration | −0.067 | −0.247 to 0.113 | 1.024 | 0.462 |
| LVEF | −0.087 | −0.539 to 0.364 | 1.061 | 0.704 |
| Model 3: LW-S work difference (3) | ||||
| Constant | −13.891 | |||
| LW-S work difference | −0.013 | −0.017 to −0.008 | 1.168 | <0.0001 |
| Ischaemic aetiology | 13.267 | 5.622 to 20.913 | 1.033 | 0.001 |
| QRS-morphology | −1.912 | −12.476 to 8.653 | 1.093 | 0.721 |
| QRS-duration | −0.102 | −0.284 to 0.080 | 1.013 | 0.269 |
| LVEF | 0.457 | −0.012 to 0.926 | 1.095 | 0.056 |
| Regression Variable | B | VIF | 95%CI | p-Value |
|---|---|---|---|---|
| Constant | −35.107 | |||
| Anterior scar (%) | 0.001 | 2.921 | −0.423 to 0.425 | 0.999 |
| Septal scar (%) | 0.466 | 3.250 | 0.010 to 0.921 | 0.015 |
| Inferior scar (%) | 0.028 | 3.100 | −0.370 to 0.426 | 0.889 |
| Lateral scar (%) | 0.198 | 2.947 | −0.199 to 0.595 | 0.327 |
| Regression Variable | B | 95%CI | VIF | p-Value |
|---|---|---|---|---|
| Model 1: SF or ApRock + septal scarring (1) | ||||
| Constant | −6.639 | |||
| SF or ApRock | −25.689 | −33.643 to −17.735 | 1.123 | <0.0001 |
| Septal scar (%) | 0.315 | 0.072 to 0.558 | 1.160 | 0.011 |
| QRS-morphology | −3.530 | −13.504 to 6.443 | 1.048 | 0.486 |
| QRS-duration | −0.036 | −0.212 to 0.140 | 1.034 | 0.686 |
| LVEF | 0.065 | −0.380 to 0.509 | 1.067 | 0.775 |
| Model 2: SSI + septal scarring (2) | ||||
| Constant | −5.405 | |||
| SSI | −2.694 | −3.605 to −1.772 | 1.110 | <0.0001 |
| Septal scar | 0.413 | 0.172 to 0.653 | 1.100 | 0.001 |
| QRS-morphology | −1.694 | −11.958 to 8.570 | 1.076 | 0.745 |
| QRS-duration | −0.045 | −0.226 to 0.135 | 1.040 | 0.622 |
| LVEF | −0.017 | −0.473 to 0.440 | 1.092 | 0.942 |
| Model 3: LW-S work difference + septal scarring (3) | ||||
| Constant | −20.610 | |||
| LW-S work difference | −0.013 | −0.017 to −0.008 | 1.171 | <0.0001 |
| Septal scar | 0.460 | 0.218 to 0.703 | 1.088 | <0.0001 |
| QRS-morphology | −1.288 | −11.789 to 9.212 | 1.095 | 0.809 |
| QRS-duration | −0.075 | −0.258 to 0.107 | 1.031 | 0.417 |
| LVEF | 0.528 | 0.057 to 0.998 | 1.117 | 0.082 |
| AUC | 95%CI | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Ischemic heart disease | ||||
| SF or ApRock + septal scar | 0.80 | 0.67 to 0.93 | 74 | 91 |
| SSI + septal scar | 0.76 | 0.63 to 0.90 | 46 | 91 |
| LW-S work difference + septal scar | 0.75 | 0.59 to 0.88 | 47 | 91 |
| Non-ischemic heart disease | ||||
| SF or ApRock + septal scar | 0.87 | 0.77 to 0.96 | 94 | 64 |
| SSI + septal scar | 0.82 | 0.74 to 0.90 | 90 | 63 |
| LW-S work difference + septal scar | 0.79 | 0.68 to 0.90 | 91 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchenne, J.; Larsen, C.K.; Cvijic, M.; Galli, E.; Aalen, J.M.; Klop, B.; Mirea, O.; Puvrez, A.; Bézy, S.; Wouters, L.; et al. Mechanical Dyssynchrony Combined with Septal Scarring Reliably Identifies Responders to Cardiac Resynchronization Therapy. J. Clin. Med. 2023, 12, 6108. https://doi.org/10.3390/jcm12186108
Duchenne J, Larsen CK, Cvijic M, Galli E, Aalen JM, Klop B, Mirea O, Puvrez A, Bézy S, Wouters L, et al. Mechanical Dyssynchrony Combined with Septal Scarring Reliably Identifies Responders to Cardiac Resynchronization Therapy. Journal of Clinical Medicine. 2023; 12(18):6108. https://doi.org/10.3390/jcm12186108
Chicago/Turabian StyleDuchenne, Jürgen, Camilla K. Larsen, Marta Cvijic, Elena Galli, John M. Aalen, Boudewijn Klop, Oana Mirea, Alexis Puvrez, Stéphanie Bézy, Laurine Wouters, and et al. 2023. "Mechanical Dyssynchrony Combined with Septal Scarring Reliably Identifies Responders to Cardiac Resynchronization Therapy" Journal of Clinical Medicine 12, no. 18: 6108. https://doi.org/10.3390/jcm12186108
APA StyleDuchenne, J., Larsen, C. K., Cvijic, M., Galli, E., Aalen, J. M., Klop, B., Mirea, O., Puvrez, A., Bézy, S., Wouters, L., Minten, L., Sirnes, P. A., Khan, F. H., Voros, G., Willems, R., Penicka, M., Kongsgård, E., Hopp, E., Bogaert, J., ... Voigt, J.-U. (2023). Mechanical Dyssynchrony Combined with Septal Scarring Reliably Identifies Responders to Cardiac Resynchronization Therapy. Journal of Clinical Medicine, 12(18), 6108. https://doi.org/10.3390/jcm12186108