Serum YKL-40 Levels, Leukocyte Profiles, and Acute Exacerbations of Advanced COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Measurements
2.3. Statistical Analysis
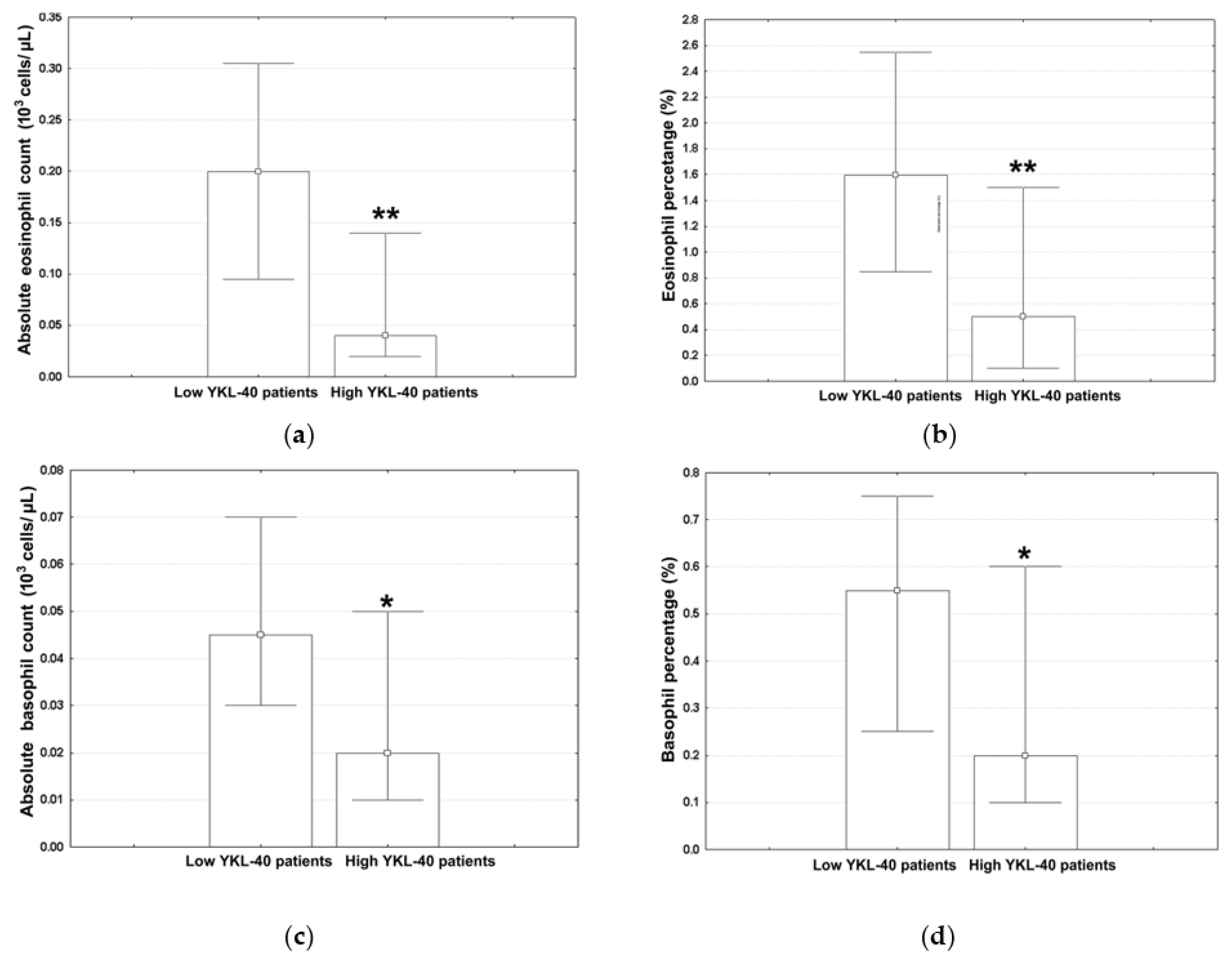
3. Results
3.1. Serum Parameters by COPD Stage (Severity)
3.2. Variables Associated with High YKL-40 Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olortegui-Rodriguez, J.J.; Soriano-Moreno, D.R.; Benites-Bullón, A.; Pelayo-Luis, P.P.; Huaringa-Marcelo, J. Prevalence and incidence of chronic obstructive pulmonary disease in Latin America and the Caribbean: A systematic review and meta-analysis. BMC Pulm. Med. 2022, 22, 273. [Google Scholar] [CrossRef]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.; Heris, J.A.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef]
- Cannavo, M.F.; Coppolino, I.; Monaco, F.; Caramori, G. Overview of Current Management of COPD. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 631–641. [Google Scholar] [CrossRef]
- Crisafulli, E.; Barbeta, E.; Ielpo, A.; Torres, A. Management of severe acute exacerbations of COPD: An updated narrative review. Multidiscip. Respir. Med. 2018, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Bove, D.G.; Lomborg, K.; Jensen, A.K.; Overgaard, D.; Lindhardt, B.Ø.; Midtgaard, J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: A randomised controlled trial. Respir. Med. 2016, 121, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Halpin, D.M.G. Clinical Features and Diagnosis of COPD. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 621–630. [Google Scholar] [CrossRef]
- Lai, T.; Wu, D.; Chen, M.; Cao, C.; Jing, Z.; Huang, L.; Lv, Y.; Zhao, X.; Lv, Q.; Eang, Y.; et al. YKL-40 expression in chronic obstructive pulmonary disease: Relation to acute exacerbations and airway remodeling. Respir. Res. 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, D.; Liu, S.; Ma, Y.; Li, Z.; Tian, P.; Fan, H. The YKL-40 protein is a potential biomarker for COPD: A meta-analysis and systematic review. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 409. [Google Scholar] [CrossRef]
- Laisure, M.; Covill, N.; Ostroff, M.L.; Ostroff, J.L. Summarizing the 2021 updated GOLD guidelines for COPD. US Pharm. 2021, 46, 30–35. [Google Scholar]
- Ramakrishnan, S.; Bafadhel, M. Biomarkers in COPD. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 559–572. [Google Scholar] [CrossRef]
- Gon, Y.; Maruoka, S.; Ito, R.; Mizumura, K.; Kozu, Y.; Hiranuma, H.; Hattori, T.; Takahashi, M.; Hikichi, M.; Hashimoto, S. Utility of serum YKL-40 levels for identification of patients with asthma and COPD. Allergol. Int. 2017, 66, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hirai, K.; Gon, Y.; Maruoka, S.; Mizumura, K.; Hikichi, M.; Hashimoto, S. Combined assessment of serum periostin and YKL-40 may identify asthma-COPD overlap. J. Allergy Clin. Immunol. Pract. 2019, 7, 134–145. [Google Scholar] [CrossRef]
- Popețiu, R.O.; Donath-Miklos, I.; Borta, S.M.; Moldovan, S.D.; Pilat, L.; Nica, D.V.; Pușchiță, M. Serum YKL-40 Levels in Patients with Asthma or COPD: A Pilot Study. Medicina 2023, 59, 383. [Google Scholar] [CrossRef]
- James, A.J.; Reinius, L.E.; Verhoek, M.; Gomes, A.; Kupczyk, M.; Hammar, U.; Ono, J.; Ohta, S.; Izuhara, K.; Bel, E.; et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 193, 131–142. [Google Scholar] [CrossRef]
- Peng, J.; Yu, Q.; Fan, S.; Chen, X.; Tang, R.; Wang, D.; Qi, D. High blood eosinophil and YKL-40 levels, as well as low CXCL9 levels, are associated with increased readmission in patients with acute exacerbation of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, D.B.; Mygind, L.H.; Titlestad, I.L.; Madsen, H.; Pedersen, S.S.; Johansen, J.S.; Pedersen, C. Plasma YKL-40 and all-cause mortality in patients with chronic obstructive pulmonary disease. BMC Pulm. Med. 2013, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.K.; Kang, H.K.; Song, P.; Park, H.K.; Lee, S.S.; Jung, H. Systemic white blood cell count as a biomarker associated with severity of chronic obstructive lung disease. Tuberc. Respir. Dis. 2017, 80, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Xu, M.; Zhao, Y.; Wu, X.; Pudasaini, B.; Liu, J.M. Can we predict the prognosis of COPD with a routine blood test? Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 615–625. [Google Scholar] [CrossRef]
- Aksoy, E.; Karakurt, Z.; Gungor, S.; Ocakli, B.; Ozmen, İ.; Yildirim, E.; Tuncay, E.; Agca, M.C.; Goksenoglu, N.C.; Adigüzel, N. Neutrophil to lymphocyte ratio is a better indicator of COPD exacerbation severity in neutrophilic endotypes than eosinophilic endotypes. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2721–2730. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. The Emerging Clinical Significance of the Red Cell Distribution Width as a Biomarker in Chronic Obstructive Pulmonary Disease: A Systematic Review. J. Clin. Med. 2022, 11, 5642. [Google Scholar] [CrossRef]
- Cui, X.J.; Xie, B.; Zhu, K.W.; Liao, Q.Q.; Zhou, J.C.; Du, S.; Liu, X.X.; Chen, Z.J.; Yang, Y.; Yi, X. Evaluation of the prognostic value of the platelet, neutrophil, monocyte, basophil, and eosinophil to lymphocyte ratios. Res. Sq. 2023; preprint. [Google Scholar] [CrossRef]
- Arad County Clincial Hospital. Available online: https://en.wikipedia.org/wiki/Arad_County_Clinical_Hospital (accessed on 9 September 2023).
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2023. Available online: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf (accessed on 11 September 2023).
- CHI3L1 Research Reagents. Available online: www.cusabio.com/target/CHI3L1.html (accessed on 10 August 2023).
- Gicquel, S.; Marion-Gallois, R. Randomization with a posteriori constraints: Description and properties. Stat. Med. 2007, 26, 5033–5045. [Google Scholar] [CrossRef]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef]
- Laniado-Laborín, R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21st century. Int. J. Environ. Res. Public Health 2000, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Takaku, Y.; Ohta, C.; Takayanagi, N.; Yanagisawa, T.; Kanauchi, T.; Takahashi, O. Smoking history and emphysema in asthma—COPD overlap. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 3523. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.A.; Tamminga, A.; Lobb, B.; Huff, R.D.; Nguyen, J.P.; Kim, Y.; Dvorkin-Gheva, A.; Doxey, A.C.; Hirota, J.A. The impact of cigarette smoke exposure, COPD, or asthma status on ABC transporter gene expression in human airway epithelial cells. Sci. Rep. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Bello, C. Smoking in Europe: Which Countries are the Most and Least Addicted to Tobacco and Vaping? May 2023. Available online: www.euronews.com/next/2023/04/11/smoking-in-europe-which-countries-are-the-most-and-least-addicted-to-tobacco-and-vaping (accessed on 6 August 2023).
- Halpin, D.M.G.; Vogelmeier, C.F.; Agusti, A. Lung health for all: Chronic obstructive lung disease and World Lung Day 2022. Am. J. Respir. Crit. 2022, 206, 669–671. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef]
- Fattouh, M.; Alkady, O. Inflammatory biomarkers in chronic obstructive pulmonary disease. Egypt J. Chest. Dis. Tuberc. 2014, 63, 799–804. [Google Scholar] [CrossRef]
- Moon, S.W.; Leem, A.Y.; Kim, Y.S.; Lee, J.H.; Kim, T.H.; Oh, Y.M.; Shin, H.; Chang, J.; Jung, J.Y.; KoLD Study Group. Low serum lymphocyte level is associated with poor exercise capacity and quality of life in chronic obstructive pulmonary disease. Sci. Rep. 2021, 10, 11700. [Google Scholar] [CrossRef]
- Hu, Y.; Long, H.; Cao, Y.; Guo, Y. Prognostic value of lymphocyte count for in-hospital mortality in patients with severe AECOPD. BMC Pulm. Med. 2022, 22, 376. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Carter, J.M.; Hassenbein, D.G.; Howard, B. Cytokines and particle-induced inflammatory cell recruitment. Environ. Health Perspect. 1997, 105, 1159–1164. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Clarke, S.; Barnes, P. Inflammatory and Immune Mechanisms in COPD. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 549–558. [Google Scholar] [CrossRef]
- Ju, J. An increased proportion of apoptosis in CD4+ T lymphocytes isolated from the peripheral blood in patients with stable chronic obstructive pulmonary disease. Tuberc. Respir. Dis. 2018, 81, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ai, X.; Liao, Z.; You, C.; Cheng, Y. The prognostic values of neutrophil to lymphocyte ratio for outcomes in chronic obstructive pulmonary disease. Medicine 2019, 98, e16371. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Rad, M.H.; Asgari, B.; Hosseinzadeh, N.; Eishi, A. Eosinopenia as a marker of outcome in acute exacerbations of chronic obstructive pulmonary disease. Maedica 2015, 10, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Ruiying, W.; Jianying, X. Clinical features and three-year prognosis of AECOPD patients with different levels of blood eosinophils. Heart Lung 2022, 56, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Liu, Y.; Zhang, L.; Zheng, J.; Wang, J.; Hansbro, P.M.; Wang, L.; Wang, G.; Hsu, A.C.Y. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir. Res. 2019, 20, 95. [Google Scholar] [CrossRef]
- Libreros, S.; Iragavarapu-Charyulu, V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J. Leukoc. Biol. 2015, 98, 931–936. [Google Scholar] [CrossRef]
- Jogdand, P.; Siddhuraj, P.; Mori, M.; Sanden, C.; Jönsson, J.; Walls, A.F.; Kearley, J.; Humbles, A.A.; Kolbeck, R.; Bjemer, L.; et al. Eosinophils, basophils and type 2 immune microenvironments in COPD-affected lung tissue. Eur. Respir. J. 2020, 55, 1900110. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef]
- Oishi, K.; Matsunaga, K.; Shirai, T.; Hirai, K.; Gon, Y. Role of type2 inflammatory biomarkers in chronic obstructive pulmonary disease. J. Clin. Med. 2020, 9, 2670. [Google Scholar] [CrossRef]
- Hirano, T.; Matsunaga, K. Measurement of blood eosinophils in asthma and chronic obstructive pulmonary disease. Intern. Med. 2023, 62, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, P.; Bikov, A.; Jensen, J.U. Using Blood Eosinophil Count as a Biomarker to Guide Corticosteroid Treatment for Chronic Obstructive Pulmonary Disease. Diagnostic 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A. Basic Statistics and Epidemiology: A Practical Guide, 4th ed.; CRC Press: London, UK, 2016; pp. 80–156. [Google Scholar] [CrossRef]
- Baker, J.R.; Donnelly, L.E. Leukocyte function in COPD: Clinical relevance and potential for drug therapy. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2227–2242. [Google Scholar] [CrossRef]
- Hansel, N.N.; Washko, G.R.; Foreman, M.G.; Han, M.K.; Hoffman, E.A.; DeMeo, D.L.; Graham, B.R.; Van Beek, E.J.R.; Kazerooni, E.A.; Wise, R.A. Racial differences in CT phenotypes in COPD. COPD J. Chronic. Obstr. Pulm. Dis. 2013, 10, 20–27. [Google Scholar] [CrossRef] [PubMed][Green Version]

| COPD Stage | Age | Sex | Smoking Status | ||
|---|---|---|---|---|---|
| Male | Female | Ever Smoker | Never Smoker | ||
| Severe COPD | 66 (61; 72) | 15 (65.21%) | 8 (34.79%) | 21 (91.30%) | 2 (8.70%) |
| Very severe COPD | 66.5 (57; 70) | 17 (70.84%) | 7 (29.16%) | 22 (91.67%) | 2 (8.33%) |
| Characteristic | Severe COPD (n = 23) | Very Severe COPD (n = 24) | Reference Range |
|---|---|---|---|
| YKL–40 | 3960.5 (3027.5; 4947.25) | 3925.5 (2924.25; 4904.5) | |
| ALLC (103 cells/μL) | 11.36 (9.34; 14.31) | 9.35 (7.38; 11.77) | 1–4 |
| ANC (103 cells/μL) | 8.49 (6.71; 10.49) | 7.13 (4.93; 8.41) | 2–8 |
| Neutrophil percentage (%) | 71 (62.7; 81) | 70.1 (65.4; 79.5) | 45–80 |
| ALC (103 cells/μL) | 1.75 (1.05; 2.91) | 1.54 (0.94; 2.04) | 4–10 |
| Lymphocyte percentage (%) | 17 (10.6; 20.9) | 17.3 (11.05; 23.55) | 20–55 |
| NLR | 3.99 (2.56; 7.94) | 3.90 (2.90; 7.35) | |
| AEC (103 cells/μL) | 0.12 (0.02; 0.27) | 0.10 (0.03; 0.19) | 0.05–0.7 |
| Eosinophil percentage (%) | 1.4 (0.2; 2.1) | 1.0 (0.35; 1.65) | 0–7 |
| AMC (103 cells/μL) | 1.05 (0.71; 1.30) | 0.85 (0.58; 0.97) | 0.3–1 |
| Monocyte percentage (%) | 7.70 (6.50; 10.70) | 8.25 (6.55; 10.35) | 0–15 |
| ABC (103 cells/μL) | 0.04 (0.01; 0.08) | 0.04 (0.02; 0.06) | 0–0.2 |
| Basophil percentage (%) | 0.4 (0.1; 0.7) | 0.4 (0.2; 0.6) | 0–2 |
| Hemoglobin (g/dL) | 13.5 (12; 14.2) | 14.10 (12.4; 15.55) | 12.6–17.4 |
| Hematocrit (%) | 41.6 (38.5; 43.6) | 43.95 (37.8; 49) | 37–51 |
| Characteristic | Low YKL-40 Patients (n = 24) | High YKL-40 Patients (n = 23) |
|---|---|---|
| ALLC (103 cells/μL) | 10.81 (8.05; 13.04) | 10.57 (8.16; 13.71) |
| ANC (103 cells/μL) | 7.25 (5.25; 8.70) | 7.91 (5.79; 10.81) |
| AMC (103 cells/μL) | 0.87 (0.70; 1.08) | 0.80 (0.63; 1.04) |
| Monocyte percentage (%) | 8.10 (6.70; 9.65) | 7.70 (6.10; 12.70) |
| Hemoglobin (g/dL) | 14.05 (12.75; 15.25) | 13.50 (12.10; 14.20) |
| Hematocrit (%) | 43.40 (38.00; 42.75) | 42.20 (38.30; 44.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popețiu, R.O.; Donath-Miklos, I.; Borta, S.M.; Rus, L.A.; Vîlcea, A.; Nica, D.V.; Pușchiță, M. Serum YKL-40 Levels, Leukocyte Profiles, and Acute Exacerbations of Advanced COPD. J. Clin. Med. 2023, 12, 6106. https://doi.org/10.3390/jcm12186106
Popețiu RO, Donath-Miklos I, Borta SM, Rus LA, Vîlcea A, Nica DV, Pușchiță M. Serum YKL-40 Levels, Leukocyte Profiles, and Acute Exacerbations of Advanced COPD. Journal of Clinical Medicine. 2023; 12(18):6106. https://doi.org/10.3390/jcm12186106
Chicago/Turabian StylePopețiu, Romana Olivia, Imola Donath-Miklos, Simona Maria Borta, Larisa Alexandra Rus, Anamaria Vîlcea, Dragoș Vasile Nica, and Maria Pușchiță. 2023. "Serum YKL-40 Levels, Leukocyte Profiles, and Acute Exacerbations of Advanced COPD" Journal of Clinical Medicine 12, no. 18: 6106. https://doi.org/10.3390/jcm12186106
APA StylePopețiu, R. O., Donath-Miklos, I., Borta, S. M., Rus, L. A., Vîlcea, A., Nica, D. V., & Pușchiță, M. (2023). Serum YKL-40 Levels, Leukocyte Profiles, and Acute Exacerbations of Advanced COPD. Journal of Clinical Medicine, 12(18), 6106. https://doi.org/10.3390/jcm12186106






