Abstract
Objective: It has been reported that monochorionic twin pregnancies conceived through assisted reproductive techniques (ART) display a higher risk of second-trimester miscarriage, cesarean delivery, and neonatal death than those conceived naturally. The aim of this study was to compare the perinatal outcomes of monochorionic diamniotic (MCDA) twin pregnancies conceived naturally and through ART in a tertiary hospital. Methods: This was a retrospective cohort study of all MCDA twin pregnancies that received obstetric care and delivered at La Fe University and Polytechnic Hospital between 2015 and 2021. MCDA pregnancies that were referred to the tertiary hospital for specialized management, follow-up, and delivery were also included. The study was approved by The Health Research Institute Hospital La Fe (IIS La Fe). Results: Among the 184 MCDA pregnancies, 149 (81%) had a natural conception, and 35 (19%) were conceived through ART. Patients with an MCDA pregnancy who conceived through ART had a significantly older maternal age (38.0 [35.5–42.5] vs. 32.0 [29.0–36.0], p < 0.001) and an elevated rate of nulliparity (80.0% vs. 50.3%, p = 0.001). Regarding pregnancy complications, MCDA pregnancies through ART were associated with a significantly higher incidence of gestational diabetes (22.9% vs. 2.7%, p < 0.001), hypertensive disorders during pregnancy (22.9% vs. 9.4%, p = 0.04), and other pregnancy complications such as threatened labor or preterm prelabor rupture of membranes (14.3% vs. 36.2%, p = 0.015), than naturally conceived MCDA pregnancies. No differences were found in the incidence of twin-to-twin transfusion syndrome (20% vs. 33.6%, p = 0.155). MCDA pregnancies through natural conception had a greater rate of vaginal delivery than MCDA through ART (16.8% vs. 2.9%, p = 0.032). When adjusted for confounding factors, MCDA pregnancies through ART were only more likely to develop gestational diabetes than those naturally conceived (aOR 7.86, 95% CI 1.55–39.87). No differences were found regarding neonatal outcomes between groups. Conclusions: Compared with naturally conceived MCDA twin pregnancies, those conceived through ART displayed a significantly higher risk of developing gestational diabetes. No differences regarding other pregnancy complications, mode of delivery, or neonatal outcomes were found between groups.
1. Introduction
Infertility is a major human reproductive health issue [1,2,3,4,5,6,7,8,9,10,11] that affects 48.5 million couples worldwide [12,13]. Infertile patients require assisted reproductive techniques (ART) such as artificial insemination (AI), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI) to achieve pregnancy [14,15]. Additionally, the use of ART is steadily increasing [16] given that same-sex couples [17,18,19,20,21], persons who desire to create single-parent families [18,21,22,23], and individuals who undergo fertility preservation for both medical and nonmedical reasons [24,25,26] also benefit from them to ultimately accomplish parenthood.
The incidence of twin gestations has risen over the past several decades due to advanced maternal age at conception and the increased use of ART [27]. The risk of having a dizygotic twin pregnancy increases with maternal age [28,29,30] due to the greater level of gonadotropins produced with age [29]. Indeed, the twinning rate increases by 300% between 15 and 37 years old [31]. Moreover, the more advanced the maternal age, the lower the number and quality of oocytes, and the higher the need for fertility treatment [32,33]. Twin pregnancies are associated with a higher risk of perinatal complications than singleton gestations [27,34], including preterm birth and the subsequent infant morbidity and mortality [27]. Thus, a single-embryo transfer strategy has been advocated during recent years to minimize dizygotic twin gestations [35,36,37,38,39]. Fortunately, the twin birth rate decreased by 4% during 2014–2018 [27]. Nonetheless, several studies have revealed that monozygotic twinning after single embryo transfer is more common among day 5–6 embryo transfers than among day 2–3 transfers [40,41,42,43,44,45]. Moreover, some authors have described that assisted hatching is associated with an increased risk of monozygotic twinning [42,46].
Monochorionic twin pregnancies conceived through ART have been reported to display a higher risk of second-trimester miscarriage [47], adverse perinatal outcomes [48], cesarean delivery [49], neonatal morbidity [50], and neonatal death [49,50] than those naturally conceived. However, other authors have not found differences regarding adverse perinatal outcomes [51], gestational age at delivery, rate of preterm birth, type of delivery, or admission to the neonatal intensive care unit (NICU) between monochorionic diamniotic (MCDA) twins conceived through ART and natural conception [52]. Thus, this study aimed to compare the perinatal outcomes of MCDA twin pregnancies conceived naturally and through ART in a tertiary hospital.
2. Materials and Methods
This was a retrospective cohort study among all MCDA twin pregnancies that received obstetric care and delivered at La Fe University and Polytechnic Hospital, Valencia, Spain. MCDA pregnancies that were referred to the tertiary hospital for specialized management, follow up, and delivery were also included. All MCDA twin pregnancies from June 2015 to December 2021 were included. Data of the included patients were collected from the digital clinical history of the hospital. The study was approved by The Health Research Institute Hospital La Fe (IIS La Fe).
Gathered maternal information included age, body mass index, nulliparity, and smoking habit. Considered pregnancy outcomes were miscarriage, considered as pregnancy loss before 24 weeks of gestation; gestational diabetes; hypertensive disorders of pregnancy that included chronic hypertension, gestational hypertension, pre-eclampsia, eclampsia, and HELLP syndrome; twin-to-twin transfusion syndrome; selective fetal growth restriction; fetal growth restriction of both twins; other pregnancy complications that included cholestasis, gestational hypothyroidism, short cervix, cervical insufficiency, threatened preterm labor, previable preterm premature rupture of membranes, and preterm premature rupture of membranes; and mode of delivery. The neonatal outcomes that were retrieved from the first and second newborn at birth involved birth weight, Apgar score, pH of the artery and vein of the umbilical cord, NICU admission, demise, morbidity during the first 30 days of life, and chronic neonatal morbidity.
R version 4.0.3 (The R Foundation for Statistical Computing) was used for the statistical analysis. Quantitative data are shown as mean and interquartile range, while categorical data are presented as absolute and relative frequencies. Comparisons between the characteristics of the groups were performed using Student’s t-test or Kruskal–Wallis test for continuous variables, and Fisher’s exact testing for categorical variables. Multivariable logistic regression analyses were used, and the adjusted odds ratio (aOR) and the 95% confidence interval (CI) are reported. The odds ratios were adjusted by maternal age, nulliparity, body mass index, and smoking habit.
3. Results
A total of 184 MCDA twin pregnancies were included. Among them, 149 (81%) were natural conception, and 35 (19%) were conceived through ART (Table 1).

Table 1.
Characteristics of pregnant women with a monochorionic diamniotic twin pregnancy according to the mode of conception.
Patients with an MCDA twin pregnancy who conceived by ART displayed a significantly higher maternal age than those with an MCDA gestation naturally conceived (38.0 (35.5–42.5) vs. 32.0 (29.0–36.0), p < 0.001). Additionally, women with an MCDA gestation through ART had an elevated rate of nulliparity compared with those with a naturally conceived MCDA twin pregnancy (80.0% vs. 50.3%, p = 0.001, Table 1).
Regarding pregnancy complications, MCDA twin pregnancies conceived through ART were associated with a significantly higher incidence of gestational diabetes (22.9% vs. 2.7%, p < 0.001), hypertensive disorders during pregnancy (22.9% vs. 9.4%, p = 0.04), and other pregnancy complications such as threatened preterm labor or preterm prelabor rupture of membranes (14.3% vs. 36.2%, p = 0.015) than in naturally conceived MCDA pregnancies (Table 2). Interestingly, no differences were found in the incidence of twin-to-twin transfusion syndrome between MCDA twin pregnancies conceived through ART and those naturally conceived (20% vs. 33.6%, p = 0.155).

Table 2.
Pregnancy outcomes of monochorionic diamniotic twin pregnancies through natural conception vs. through assisted reproductive techniques.
The mode of delivery was compared between MCDA twin pregnancies naturally conceived and those through ART (Table 2). Naturally conceived MCDA twin pregnancies showed a greater rate of vaginal delivery than MCDA through ART (16.8% vs. 2.9%, p = 0.032).
Multivariate analysis revealed that MCDA twin pregnancies through ART were more likely to develop gestational diabetes than those naturally conceived (aOR 7.86, 95% CI 1.55–39.87). When adjusting for confounding factors including maternal age, nulliparity, body mass index, and smoking habit, no statistical differences were found regarding other pregnancy complications or mode of delivery between MCDA twin pregnancies naturally conceived and those through ART (Figure 1).
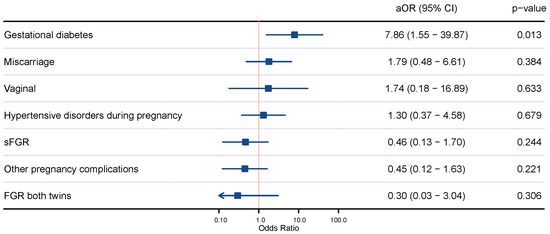
Figure 1.
Comparison of pregnancy outcomes of naturally conceived monochorionic diamniotic twin pregnancies and those through assisted reproductive techniques. Adjusted odds ratio (aOR) by maternal age, nulliparity, body mass index, and smoking habit; 95% confidence interval (CI); vaginal delivery (vaginal); selective fetal growth restriction (sFGR); fetal growth restriction (FGR).
Neonatal outcomes including birthweight, Apgar score, NICU admission, and neonatal morbidity were compared between MCDA twin gestations that were naturally conceived and those through ART. Nonetheless, no differences were found regarding neonatal outcomes (Table 3). When comparing specific neonatal outcomes of the first and the second newborn at delivery between MCDA twin pregnancies naturally conceived and those through ART, no differences were found between the groups (Table 4).

Table 3.
Neonatal outcomes of monochorionic diamniotic twin pregnancies naturally conceived vs. through assisted reproductive techniques.

Table 4.
Neonatal outcomes of the first and the second newborn at delivery of monochorionic diamniotic twin pregnancies through natural conception vs. through assisted reproductive techniques.
4. Discussion
The main findings of this study are that, compared with naturally conceived MCDA twin pregnancies, those conceived through ART have a significantly higher incidence of gestational diabetes. Remarkably, no differences regarding other pregnancy complications, mode of delivery, or neonatal outcomes were found.
The occurrence of twin gestations has risen worldwide over the recent decades due to advanced maternal age at conception and the heightened use of ART [27]. Actually, the twinning rate rose by 76% from 1980 to 2009 (19 to 33 twins per 1000 births), was stable from 2009 to 2012, and rose until 2014 (34 per 1000 births), before declining by 8% from 2014 to 2020 [53]. Noticeably, from 2020 to 2021, the twinning rates were the lowest in two decades (31 per 1000 births) [53]. Twin pregnancies are associated with a higher risk of perinatal mortality and morbidity than singleton gestations [27,34]. Thus, a single-embryo transfer strategy has been advocated during recent years to lessen dizygotic twin gestations [35,36,37,38,39]. Nevertheless, it has been reported that ART increases the incidence of monozygotic twins from 1 in 250 in natural conceptions to approximately 1 in 50 [41,47]. Particularly, extended culture or embryo transfer among days 5–6 [40,41,42,43,44,45] and assisted hatching [42,46] have been described to confer a higher risk of monozygotic twinning after single-embryo transfer. Due to vascular anastomoses [54], monochorionic twin pregnancies are associated with higher perinatal morbidity and mortality than dichorionic twin gestations [50,55]. Transfusion imbalances through the vascular anastomoses cause pregnancy complications specific to MCDA twin pregnancies [54,56]. In this regard, 10% of MCDA twin pregnancies develop twin–twin transfusion syndrome (TTTS), and 5% develop twin anemia polycythemia sequence (TAPS) [54,56].
ART increases the rate of monochorionic twin pregnancies [41,47], and MCDA gestations are at a particularly elevated risk of adverse outcomes due to placental vascular anastomoses [54,56]. Thus, the present study assessed whether perinatal outcomes are more adverse in MCDA twin pregnancies conceived through ART than in those naturally conceived. Not surprisingly, patients with an MCDA pregnancy who conceived through ART displayed a significantly higher maternal age and an elevated rate of nulliparity. Accordingly, Simoes et al. compared MCDA twins conceived naturally and through ART and revealed that women pregnant through ART had a significantly more advanced maternal age and were more often nulliparous [48].
Concerning pregnancy complications, the present study shows that MCDA twin pregnancies through ART were associated with significantly higher incidences of gestational diabetes, hypertensive disorders during pregnancy, and other pregnancy complications such as threatened preterm labor or preterm prelabor rupture of membranes than naturally conceived MCDA twin pregnancies. Nonetheless, when adjusting for confounding factors, MCDA twin pregnancies conceived via ART were only more likely to develop gestational diabetes than naturally conceived MCDA twin pregnancies. These results are in line with those in the available literature. Prats et al. performed a retrospective cohort study and revealed that MCDA twin pregnancies conceived through ART had a heightened risk of gestational diabetes than naturally conceived MCDA twin gestations [52]. No differences were found between groups regarding gestational age at delivery, onset of labor, preterm birth, or intrauterine growth restriction [52]. Similarly, a recent meta-analysis did not find significant differences regarding hypertensive disorders of pregnancy, very preterm delivery, risk of intrauterine death, and small for gestational age fetuses between MCDA twin pregnancies conceived naturally and through ART [49]. Identically, a study conducted by Tronjer-Bregar et al. concluded that MCDA twins conceived through ART were not associated with an increased risk of adverse perinatal outcomes compared with spontaneous MCDA twins [51]. Nevertheless, other authors have reported that MCDA twin pregnancies conceived through ART display more adverse perinatal outcomes than naturally conceived MCDA pregnancies [47,57]. Couck et al. carried out a retrospective cohort study of MCDA twin pregnancies conceived after ART or naturally and concluded that MCDA twins by ART displayed reduced survival rates and larger rates of second-trimester miscarriage than the naturally conceived MCDA twins [47]. Similarly, Sun et al. conducted a retrospective review and described an increased risk of preterm premature rupture of membranes in MCDA twin pregnancies conceived via ART compared with in those naturally conceived [57]. Hence, it remains controversial whether the mode of conception of MCDA twin pregnancies has a negative impact on pregnancy complications. However, the updated evidence reveals that MCDA twin pregnancies conceived via ART only have a higher risk of gestational diabetes and not of other pregnancy complications compared with naturally conceived MCDA twin pregnancies.
Noticeably, no differences were found in the incidence of twin-to-twin transfusion syndrome between naturally conceived MCDA twin pregnancies and those conceived through ART in the present study. Accordingly, both the retrospective cohort study of Couck et al. and the meta-analysis of Wang et al. have revealed that the mode of conception of MCDA twin pregnancies had no impact on the risk of TTTS [47,49]. Thus, the available evidence shows no differences in the incidence of TTTS between MCDA twin pregnancies conceived naturally and through ART.
Regarding the mode of delivery, naturally conceived MCDA pregnancies in the present study showed a greater rate of vaginal delivery than MCDA conceived through ART. Nonetheless, when adjusted by confounding factors including maternal age, nulliparity, body mass index, and smoking habit, no statistical differences were found regarding the mode of delivery between naturally conceived MCDA twin pregnancies and those through ART. Similarly, neither Couck et al. nor Prats et al. found differences with respect to the incidence of cesarean delivery between MCDA twin pregnancies conceived after ART or naturally [47,52]. Nonetheless, a meta-analysis regarding monochorionic twin pregnancies conceived by ART vs. naturally revealed that monochorionic twin pregnancies conceived through ART display a higher risk of cesarean section [49]. Hence, when adjusted by confounding factors, the mode of conception in MCDA twin pregnancies does not appear to affect the mode of delivery.
Importantly, no differences regarding neonatal outcomes were found in the present study between MCDA twin pregnancies conceived naturally and through ART. Similarly, Couck et al. and Prats et al. did not find differences regarding weight discordance [47,52], birth weight [47], and admission to the NICU [52] between MCDA twin pregnancies conceived after ART or naturally. Nevertheless, Simoes et al. revealed that monochorionic twins conceived via ART had a lower mean birth weight than those naturally conceived [48]. Additionally, the meta-analysis by Wang et al. and a retrospective cohort study by Hack et al. have described that MCDA twin pregnancies conceived via ART displayed a heightened risk of neonatal deaths compared to those naturally conceived [49,50]. Therefore, it is still unclear whether the mode of conception of MCDA twin pregnancies has a negative impact on neonatal outcomes.
The primary importance of this study is that it adds to the scientific evidence regarding the perinatal outcomes of MCDA twin pregnancies according to the mode of conception. Drawbacks of the present work include the limited sample size. Further studies should be carried out in order to clarify whether the mode of conception of MCDA twin pregnancies has an impact on perinatal outcomes.
In conclusion, the present study shows that, when adjusting for confounding factors, MCDA twin pregnancies conceived through ART have a significantly higher incidence of gestational diabetes than naturally conceived MCDA twin gestations. Noticeably, no differences regarding other pregnancy complications, mode of delivery, and neonatal outcomes were found between naturally conceived MCDA twin pregnancies and those through ART. These findings are reassuring for both healthcare professionals and patients. Nonetheless, additional studies are required to confirm these findings and to appropriately counsel pregnant women with MCDA twin pregnancies.
Author Contributions
A.M.-V., J.M.-R. and V.D.-A. performed the conceptualization of the work. A.M.-V. carried out the methodology and design of the work. A.M.-V. supervised the research work. M.M.-G. and B.N. contributed to the investigation by acquiring data for the study. J.D. performed the formal analysis. J.D. and A.M.-V. analyzed and interpreted the study’s data. A.M.-V. wrote the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol was reviewed and approved by the Ethics Committee of the Health Research Institute Hospital La Fe (IIS La Fe), approval Ref: 2018/0318, P.I. Exp. 2018_0318_PP_MARTINEZ VARE.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data in this study were obtained from the clinical program of the University and Polytechnic Hospital La Fe. Such a dataset may be completely available on request to the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandra, A.; Copen, C.E.; Stephen, E.H. Infertility and impaired fecundity in the United States, 1982–2010: Data from the National Survey of Family Growth. Natl. Health Stat. Rep. 2013, 14, 1–8. [Google Scholar]
- Marques-Pinto, A.; Carvalho, D. Human infertility: Are endocrine disruptors to blame? Endocr. Connect. 2013, 2, R15–R29. [Google Scholar] [CrossRef] [PubMed]
- Gimenes, F.; Souza, R.P.; Bento, J.C.; Teixeira, J.J.; Maria-Engler, S.S.; Bonini, M.G.; Consolaro, M.E. Male infertility: A public health issue caused by sexually transmitted pathogens. Nat. Rev. Urol. 2014, 11, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Parekh, N.; Henkel, R. A Schematic Overview of the Current Status of Male Infertility Practice. World J. Mens. Health 2020, 38, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia 2021, 53, e13614. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Borumandnia, N.; Alavi Majd, H.; Khadembashi, N.; Alaii, H. Worldwide trend analysis of primary and secondary infertility rates over past decades: A cross-sectional study. Int. J. Reprod. Biomed. 2022, 20, 37–46. [Google Scholar] [CrossRef]
- Kundu, S.; Ali, B.; Dhillon, P. Surging trends of infertility and its behavioural determinants in India. PLoS ONE 2023, 18, e0289096. [Google Scholar] [CrossRef]
- Kyrgiafini, M.A.; Mamuris, Z. Male Infertility: From Genes to Genomes 2022. Genes 2023, 14, 959. [Google Scholar] [CrossRef]
- Sang, Q.; Ray, P.F.; Wang, L. Understanding the genetics of human infertility. Science 2023, 380, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef] [PubMed]
- Chiware, T.M.; Vermeulen, N.; Blondeel, K.; Farquharson, R.; Kiarie, J.; Lundin, K.; Matsaseng, T.C.; Ombelet, W.; Toskin, I. IVF and other ART in low- and middle-income countries: A systematic landscape analysis. Hum. Reprod. Update 2021, 27, 213–228. [Google Scholar] [CrossRef]
- Niederberger, C.; Pellicer, A.; Cohen, J.; Gardner, D.K.; Palermo, G.D.; O’Neill, C.L.; Chow, S.; Rosenwaks, Z.; Cobo, A.; Swain, J.E.; et al. Forty years of IVF. Fertil Steril 2018, 110, 185–324.e5. [Google Scholar] [CrossRef] [PubMed]
- de Ziegler, D.; Toner, J.P. Fertility workups: The times they are a-changin’. Fertil Steril 2022, 118, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Folger, S.G.; Boulet, S.L.; Warner, L.; Callaghan, W.M.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2016. MMWR Surveill. Summ. 2019, 68, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, S.C.; Wickins-Drazilova, D.; Wickins, J. The ethics of fertility treatment for same-sex male couples: Considerations for a modern fertility clinic. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 244, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Hemalal, S.; Yee, S.; Ross, L.; Loutfy, M.; Librach, C. Same-sex male couples and single men having children using assisted reproductive technology: A quantitative analysis. Reprod. Biomed. Online 2021, 42, 1033–1047. [Google Scholar] [CrossRef]
- Brandao, P.; de Pinho, A.; Ceschin, N.; Sousa-Santos, R.; Reis-Soares, S.; Bellver, J. ROPA—Lesbian shared in vitro fertilization—Ethical aspects. Eur. J. Obs. Gynecol. Reprod. Biol. 2022, 272, 230–233. [Google Scholar] [CrossRef]
- Brandao, P.; Ceschin, N.; Cruz, F.; Sousa-Santos, R.; Reis-Soares, S.; Bellver, J. Similar reproductive outcomes between lesbian-shared IVF (ROPA) and IVF with autologous oocytes. J. Assist. Reprod. Genet. 2022, 39, 2061–2067. [Google Scholar] [CrossRef]
- Wrande, T.; Kristjansdottir, B.H.; Tsiartas, P.; Hadziosmanovic, N.; Rodriguez-Wallberg, K.A. Live birth, cumulative live birth and perinatal outcome following assisted reproductive treatments using donor sperm in single women vs. women in lesbian couples: A prospective controlled cohort study. J. Assist. Reprod. Genet. 2022, 39, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.; Gonzalez, M.; Morgado, B. Single mothers by choice in Spain: Parenting and psychosocial adjustment in adopted and ART children. J. Fam. Psychol. 2021, 35, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Volgsten, H.; Schmidt, L. Exploring Swedish single women’s decision to choose motherhood through medically assisted reproduction—A qualitative study. Hum. Fertil. (Camb.) 2023, 26, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; Garcia-Velasco, J.A.; Remohi, J.; Pellicer, A. Oocyte vitrification for fertility preservation for both medical and nonmedical reasons. Fertil Steril 2021, 115, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Bakkensen, J.B.; Goldman, K.N. After the thaw: When patients return to use cryopreserved oocytes. Fertil Steril 2021, 115, 1437–1438. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M. Fertility preservation in men and women: Where are we in 2021? Are we rising to the challenge? Fertil Steril 2021, 115, 1089–1090. [Google Scholar] [CrossRef] [PubMed]
- Multifetal Gestations: Twin, Triplet, and Higher-Order Multifetal Pregnancies: ACOG Practice Bulletin, Number 231. Obs. Gynecol 2021, 137, e145–e162. [CrossRef]
- Bonnelykke, B. Maternal age and parity as predictors of human twinning. Acta Genet. Med. Gemellol. 1990, 39, 329–334. [Google Scholar] [CrossRef]
- Bortolus, R.; Parazzini, F.; Chatenoud, L.; Benzi, G.; Bianchi, M.M.; Marini, A. The epidemiology of multiple births. Hum Reprod Update 1999, 5, 179–187. [Google Scholar] [CrossRef]
- McLennan, A.S.; Gyamfi-Bannerman, C.; Ananth, C.V.; Wright, J.D.; Siddiq, Z.; D’Alton, M.E.; Friedman, A.M. The role of maternal age in twin pregnancy outcomes. Am. J. Obs. Gynecol. 2017, 217, 80.e1–80.e8. [Google Scholar] [CrossRef]
- Bulmer, M.G. The Biology of Twinning in Man; Clarendon Press: Oxford, UK, 1970. [Google Scholar]
- Crawford, N.M.; Steiner, A.Z. Age-related infertility. Obs. Gynecol. Clin. N. Am. 2015, 42, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Attali, E.; Yogev, Y. The impact of advanced maternal age on pregnancy outcome. Best Pract. Res. Clin. Obs. Gynaecol. 2021, 70, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Rodgers, M.; Baschat, A.; Bhide, A.; Gratacos, E.; Hecher, K.; Kilby, M.D.; Lewi, L.; Nicolaides, K.H.; Oepkes, D.; et al. ISUOG Practice Guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obs. Gynecol. 2016, 47, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Styer, A.K.; Wright, D.L.; Wolkovich, A.M.; Veiga, C.; Toth, T.L. Single-blastocyst transfer decreases twin gestation without affecting pregnancy outcome. Fertil Steril 2008, 89, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Hughes, E.; Young, D.; Joint Sogc-Cfas Clinical Practice Guidelines, C.; Reproductive, E.; Infertility, C. Elective single embryo transfer following in vitro fertilization. J. Obs. Gynaecol. Can. 2010, 32, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Tobias, T.; Sharara, F.I.; Franasiak, J.M.; Heiser, P.W.; Pinckney-Clark, E. Promoting the use of elective single embryo transfer in clinical practice. Fertil Res. Pract. 2016, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, K.K.; Berglund, L.; Tilly, J.; Hadziosmanovic, N.; Brodin, T.; Holte, J. Construction and validation of a prediction model to minimize twin rates at preserved high live birth rates after IVF. Reprod. Biomed. Online 2019, 38, 22–29. [Google Scholar] [CrossRef]
- Huang, X.; Liu, R.; Shen, W.; Cai, Y.; Ding, M.; Sun, H.; Zhou, J. An elective single cleavage embryo transfer strategy to minimize twin live birth rate based on a prediction model from double cleavage embryos transfer patients. J. Matern. Fetal. Neonatal. Med. 2022, 35, 1775–1782. [Google Scholar] [CrossRef]
- Kawachiya, S.; Bodri, D.; Shimada, N.; Kato, K.; Takehara, Y.; Kato, O. Blastocyst culture is associated with an elevated incidence of monozygotic twinning after single embryo transfer. Fertil Steril 2011, 95, 2140–2142. [Google Scholar] [CrossRef]
- Knopman, J.M.; Krey, L.C.; Oh, C.; Lee, J.; McCaffrey, C.; Noyes, N. What makes them split? Identifying risk factors that lead to monozygotic twins after in vitro fertilization. Fertil Steril 2014, 102, 82–89. [Google Scholar] [CrossRef]
- Kanter, J.R.; Boulet, S.L.; Kawwass, J.F.; Jamieson, D.J.; Kissin, D.M. Trends and correlates of monozygotic twinning after single embryo transfer. Obs. Gynecol. 2015, 125, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Wei, Z.L.; Xu, X.F.; Wang, X.; He, X.J.; Wu, H.; Zhou, P.; Cao, Y.X. Prevalence and risk factors of monochorionic diamniotic twinning after assisted reproduction: A six-year experience base on a large cohort of pregnancies. PLoS ONE 2017, 12, e0186813. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Chen, S.; Kang, X.; Du, H.; Li, L. Elevated incidence of monozygotic twinning is associated with extended embryo culture, but not with zona pellucida manipulation or freeze-thaw procedure. Fertil Steril 2018, 109, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Dallagiovanna, C.; Reschini, M.; Paffoni, A.; Fedele, L.; Somigliana, E. Risk factors for monozygotic twinning after in vitro fertilization: A systematic review and meta-analysis. Fertil Steril 2019, 111, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Dallagiovanna, C.; Vanni, V.S.; Somigliana, E.; Busnelli, A.; Papaleo, E.; Villanacci, R.; Candiani, M.; Reschini, M. Risk Factors for Monozygotic Twins in IVF-ICSI Cycles: A Case-Control Study. Reprod. Sci. 2021, 28, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Couck, I.; Van Nylen, L.; Deprest, J.; Lewi, L. Monochorionic twins after in-vitro fertilization: Do they have poorer outcomes? Ultrasound Obs. Gynecol. 2020, 56, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Simoes, T.; Queiros, A.; Marujo, A.T.; Valdoleiros, S.; Silva, P.; Blickstein, I. Outcome of monochorionic twins conceived by assisted reproduction. Fertil Steril 2015, 104, 629–632. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Chai, J. Comparison of outcomes of monochorionic twin pregnancies conceived by assisted reproductive technology vs. spontaneous conceptions: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 962190. [Google Scholar] [CrossRef]
- Hack, K.E.A.; Vereycken, M.; Torrance, H.L.; Koopman-Esseboom, C.; Derks, J.B. Perinatal outcome of monochorionic and dichorionic twins after spontaneous and assisted conception: A retrospective cohort study. Acta Obs. Gynecol. Scand. 2018, 97, 717–726. [Google Scholar] [CrossRef]
- Trojner Bregar, A.; Blickstein, I.; Verdenik, I.; Lucovnik, M.; Tul, N. Outcome of monochorionic-biamniotic twins conceived by assisted reproduction: A population-based study. J. Perinat Med. 2016, 44, 881–885. [Google Scholar] [CrossRef]
- Prats, P.; Zarragoitia, J.; Rodriguez, M.A.; Rodriguez, I.; Martinez, F.; Rodriguez-Melcon, A.; Serra, B. Outcome in a series of 1135 twin pregnancies: Does the type of conception play a role? AJOG Glob. Rep. 2022, 2, 100129. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2021. Natl. Vital. Stat. Rep. 2023, 72, 1–53. [Google Scholar]
- Lewi, L. Monochorionic diamniotic twin pregnancies. Am. J. Obs. Gynecol. MFM 2022, 4, 100501. [Google Scholar] [CrossRef] [PubMed]
- Al Riyami, N.; Al-Rusheidi, A.; Al-Khabori, M. Perinatal outcome of monochorionic in comparison to dichorionic twin pregnancies. Oman Med. J. 2013, 28, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Lewi, L.; Jani, J.; Blickstein, I.; Huber, A.; Gucciardo, L.; Van Mieghem, T.; Done, E.; Boes, A.S.; Hecher, K.; Gratacos, E.; et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: A prospective cohort study. Am. J. Obs. Gynecol. 2008, 199, 514.e1–514.e8. [Google Scholar] [CrossRef]
- Sun, L.; Zou, G.; Wei, X.; Chen, Y.; Zhang, J.; Okun, N.; Duan, T. Clinical outcomes after assisted reproductive technology in twin pregnancies: Chorionicity-based comparison. Sci. Rep. 2016, 6, 26869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).