Abstract
Obesity increases the risk of developing chronic kidney disease (CKD), which has a major negative impact on global health. Bariatric surgery (BS) has demonstrated a substantial improvement of obesity-related comorbidities and thus, it has emerged as a potential therapeutic tool in order to prevent end-stage renal disease. A limited number of publications to date have examined the beneficial effects and risks of BS in patients with non-advanced stages of CKD. We aimed to investigate the safety of BS in patients with CKD stages 3–4 (directly related or not to obesity) and both the metabolic/renal outcomes post-BS. A total of 57 individuals were included (n = 19 for CKD-group; n = 38 for patients with obesity, but normal eGFR [control-group]). Weight loss and obesity comorbidities resolution after BS were similar in both groups. Renal function (eGFR [CKD-EPI]) improved significantly at the 1-year follow-up: Δ10.2 (5.2–14.9) (p < 0.001) for CKD-group and Δ4.0 (−3.9–9.0) mL/min/1.73 m2 (p = 0.043) for controls. Although this improvement tended to decrease in the 5-year follow-up, eGFR remained above its basal value for the CKD-group. Noteworthy, eGFR also improved in those patients who presented CKD not directly attributed to obesity. For patients with CKD, BS appears to be safe and effective regarding weight loss and obesity comorbidities resolution, irrespective of the main cause of CKD (related or not to obesity).
1. Introduction
Obesity has become a major global health challenge and part of its costs are due to obesity comorbidities, including chronic kidney disease (CKD) [1,2,3,4]. Patients with severe obesity present distinct glomerular morphologic changes (glomerulomegaly, expansion and proliferation of mesangial matrix, podocyte hypertrophy, and glomerular sclerosis). These lesions are independent from other obesity-associated diseases, such as diabetes or hypertension. Altogether, these lead to a clinical pattern of proteinuria and progressive renal failure [3]. Moreover, obesity has been associated with a more rapid progression of CKD to end-stage renal disease (ESRD) in patients with pre-existing CKD [5]. It should also be highlighted that CKD has a major negative effect on global health, both as a direct cause of morbidity and mortality and as an important risk factor for cardiovascular (CV) disease [6].
Bariatric surgery (BS) in individuals with severe obesity is associated with significant and sustained weight loss, substantial improvement of obesity-related comorbidities, and increased life expectancy [7]. Therefore, it emerges as a potential therapeutic tool in order to prevent CKD progression to ESRD [8,9]. An improvement in albuminuria and proteinuria after BS has been extensively reported, supporting its beneficial effect on renal function [10,11,12,13]. However, a limited number of publications thus far have examined the beneficial effects of weight loss surgery in patients with CKD. In addition, the reported study groups were clearly dissimilar and yet not comparable, including patients with different stages of CKD (non-advanced stages to ESRD), and even patients who have had kidney transplantation with subsequent BS [5,14,15,16,17,18,19,20,21,22,23]. However, no study has directly assessed if the renal benefits of BS are exclusive of those patients with CKD directly related to obesity, without considering other etiologies. Moreover, there are renal risks related to BS which include perioperative acute kidney injury (AKI), with reports ranging from 2.9 to 8.5% [24,25,26].
Considering this background, our study primarily aimed at exploring BS safety in patients with CKD stages 3–4, and evaluating both renal and metabolic outcomes in the short (1 year) and mid-term (5 years) after BS. As a secondary aim, we assessed BS renal outcomes in patients with CKD not directly related to obesity.
2. Materials and Methods
We retrospectively evaluated all patients followed at the Obesity Unit of our institution and eligible for BS (laparoscopic Roux-en-Y Gastric Bypass [RYGB] or sleeve gastrectomy [SG]) between January 2005 to January 2018. During this study period, a total of 2298 BS were performed (revisional surgeries were not considered). Eligibility criteria for BS were age between 18–70 years and a body mass index (BMI) above 40 Kg/m2 or above 35 Kg/m2 in the presence of obesity-related comorbidities [27]. The technical aspects and the selection criteria for RYGB or SG at our institution have previously been reported [28].
The inclusion criteria for the study group (CKD-group) were the presence of CKD, defined as an estimated glomerular filtration rate (eGFR [CKD-EPI]) < 60 mL/min/1.73 m2 for at least 3 months, irrespective of the cause, and severe obesity eligible for BS. Patients with CKD stage 5 or on dialysis were excluded from the analysis. Patients in the CKD-group were paired 1:2 with patients who also suffered from obesity and were eligible for BS but had normal renal function (control-group). An eGFR > 60 mL/min/1.73 m2 and the absence of albuminuria were required for patients in the control-group. The matching criteria included year and type of BS, age, BMI, sex, and major comorbidities (diabetes and hypertension diagnosis as well as years of evolution). Using this propensity index score, 19 patients with obesity and CKD (CKD-group) were matched with 38 subjects without CKD but also entitled to BS (control-group).
In order to assess BS renal benefits according to CKD etiology, two subgroups were designed: obesity-CKD (n = 10) and non-obesity-CKD (n = 9). In the first group, patients with CKD and no other cause identified beyond obesity and its comorbidities were included. The second group comprised those patients with CKD in which a specific etiology had been reported: nephrectomy (n = 3), amyloidosis (n = 1), lupus nephritis (n = 1), and polycystic kidney disease (n = 2). In two patients, no specific cause was registered in their medical records. However, as renal function impairment was previous to the overweight/obesity diagnosis, they were classified in the non-obesity-CKD subgroup.
All subjects who fulfilled inclusion criteria and had no exclusion criteria were followed for a minimum of 5 years. In order to establish mortality and longer-term (>5 years) renal outcomes after BS, all subjects’ vital status, and creatinine levels and/or need for renal replacement therapy were revised until their last contact with the medical care system during the study period (end 1 June 2023), being the median follow-up of 11.3 (9.0–14.9) years.
2.1. Clinical and Laboratory Measures
Demographic (sex, age), anthropometric (height, weight), and both clinical and analytical data were recorded. The use of specific pharmacological treatments was also documented. The existence of heart disease, obstructive sleep apnea-hypopnea syndrome (OSAHS), hypertension (defined as taking antihypertensive drugs or repeated clinical systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg), diabetes (diagnosed according to ADA criteria [29]) and dyslipidemia (defined as taking lipid-lowering drugs or LDL-cholesterol > 160 mg/dL) was registered.
Standardized assays were used to measure blood glucose, HbA1c, lipid profile, and serum creatinine; eGFR was obtained with the Chronic Kidney Disease-Epidemiology Collaboration equation (CKD-EPI) [30].
2.2. Metabolic and Kidney-Related Outcomes following BS
In order to assess metabolic outcomes, the improvement and/or resolution of obesity-related comorbidities and body weight trajectory following BS were recorded. Weight loss was expressed as the percentage of total weight loss (TWL) and the percentage of excess weight loss EWL) [31]. Weight regain (WR) was calculated as the percentage of maximum weight lost [100*(post-nadir weight − nadir weight)]/(pre-surgery weight − nadir weight) [32]. Kidney outcomes evaluation included the assessment of renal function through eGFR (CKD-EPI).
2.3. Hospitalization, Surgical Complications and Mortality
Both early and late surgical complications (<30 or >30 days after BS), as well as the number of deaths by cause, were assessed. The existence of AKI in the post-operatory period along with the need for hemodialysis was specifically revised. Hospitalization parameters, such as length of stay and surgical time were also recorded.
2.4. Statistical Analyses
Data are presented as median and 25th and 75th percentiles, mean ± standard deviation (SD) or number (%) unless otherwise indicated. The normal distribution of continuous variables was evaluated with the Shapiro–Wilk test in addition to normal P-P plots. Inter-group differences in quantitative variables were assessed using Student’s t-test or Mann-Whitney U, as appropriate. The chi-square test was used to evaluate between-group differences in qualitative variables. McNemar’s test was used on paired nominal data. Paired t-tests or Wilcoxon tests were used to evaluate within-group differences in quantitative variables.
IBM SPSS Statistics 23.0 (SPSS Inc.; Chicago, IL, USA) and STATA/IC 15.0 (StataCorp.; College Station, TX, USA) for Windows were used to perform the statistical analysis. The significance level was defined as a p-value < 0.05, with 2-sided tests.
3. Results
3.1. Subjects’ Characteristics
Participants’ basal characteristics are shown in Table 1. On average, patients were about 57 years-old and 70% were women. The median BMI at baseline was 45.3 Kg/m2. A total of 19 (33%) patients had type 2 diabetes (T2D) and 49 (86%), had hypertension. As expected, CKD and control groups were well-balanced regarding age, sex, BMI, major comorbidities, and type of BS. Dyslipidemia was more common in the CKD-group than in the control-group (84.2% vs. 47.4%, p = 0.008). However, the percentage of patients under statin treatment was similar (68.7% vs. 61.1%, p = 0.642).

Table 1.
Baseline characteristics of the CKD and control groups.
In the CKD-group, most patients were classified as stage 3 (6 [31.6%] for stage 3a and 7 [36.8%] for stage 3b), and only 6 (31.6%) were classified as stage 4. CKD etiology was established according to medical clinical records. Obesity was the most frequently reported cause (31.5%), followed by a tie between hypertension (15.8%), nephrectomy (15.8%), and others (10.5%), including lupus nephritis and amyloidosis. Among those patients classified in the obesity etiologic division, renal biopsy was performed just in one case and informed as focal segmental glomerulosclerosis. Polycystic kidney disease accounted for 10.8% of CKD causes and T2D accounted for 5.3%. CKD etiology could not certainly be established in 2 patients (10.5%).
3.2. Metabolic and Kidney Related Outcomes
Participant’s metabolic and kidney-related outcomes at 1 and 5 years after BS are presented in Table 2 and Table 3, respectively.

Table 2.
1-year follow-up post-surgical characteristics of study groups.

Table 3.
5-year follow-up post-surgical characteristics of study groups.
The weight loss achieved following BS, both at the 1-year and 5-year follow-up, was comparable between groups and successful according to the classical Reinhold’s criteria (EWL > 50% and/or BMI < 35 Kg/m2). Five years after BS, WR was similar, but with a wide range of variation: 16.2 (6.3–28.2)% vs. 19.7 (11.7–25.7)% for the CKD vs. control-group (p = 0.439). It has to be mentioned that three subjects in the control-group required revisional surgery (conversion of SG to RYGB) because of gastroesophageal reflux disease (GERD). None of the patients included in the CKD-group required revisional surgery.
Attending obesity comorbidities, in the CKD-group the prevalence of hypertension remained mainly unchanged following BS, but the number of anti-hypertensive drugs could be reduced. The percentage of T2D resolution at 1 year following BS [33] was similar in both groups (42.9% for CKD-group and 50% for controls; p = 0.764). The metabolic improvements reached at 1-year follow-up remained stable at the 5-year re-evaluation (Table 3). Regarding renal function, at the 1-year follow-up, eGFR clearly upgraded: Δ10.2 (5.2–14.9) (p < 0.001) for CKD-group and Δ4.0 (−3.9–9.0) mL/min/1.73 m2 (p = 0.043) for control-group. Although this improvement tended to decrease, eGFR remained above its basal value for the CKD-group: Δ3.8 (−1.7–9.7) mL/min/1.73 m2 (p = 0.031). Only one patient required hemodialysis before the completion of the 5-year follow-up period.
Figure 1 shows all the details regarding eGFR evolution post-BS in the longer-term. Close to the 5-year follow-up, two patients required renal replacement therapy (5.7- and 5.5-years post-BS, respectively). Specifically, one of them was placed on the national kidney transplant waiting list; the other one was excluded because of the coexistence of prostatic adenocarcinoma. Except for these two cases, eGFR remained stable for both study groups until the 10-year follow-up. After this period, eGFR tended to decrease without reaching a value inferior to 30 mL/min/1.73 m2 in any case.
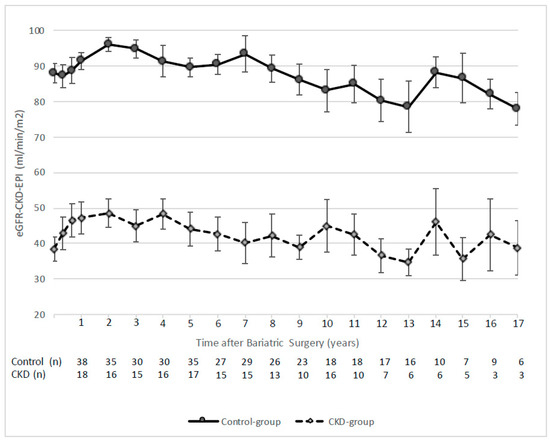
Figure 1.
eGFR evolution after bariatric surgery for study groups. Data are shown as mean ± standard error.
As depicted in Figure 2, when assessing eGFR depending on the CKD stage, subjects classified in the higher CKD stages tended to worsen in the long follow-up. In fact, both patients requiring hemodialysis in the extended follow-up (>5 years) had been classified in CKD stage 4 baseline.
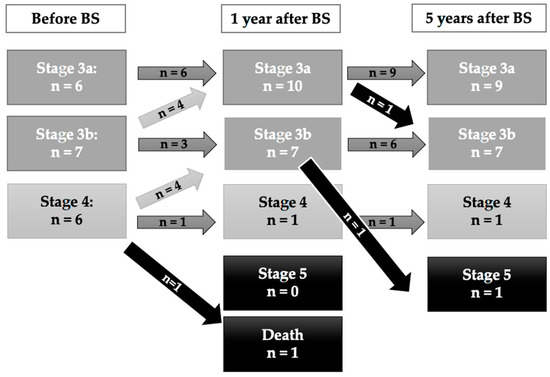
Figure 2.
CKD stages for the CKD-group during the 5-year follow-up.
When specifically assessing BS benefits on renal function regarding the main etiology of CKD, eGFR improved at the 1-year follow-up in both subgroups. For the obesity-CKD group (n = 10), baseline eGFR increased from 48.6 (39.2–52.6) to 53.34 (46.7–57.9) mL/min/1.73 m2 at the 1-year follow-up (p = 0.009); similarly for the non-obesity CKD group (n = 8 since one patient died in the first follow-up), from a baseline value of 28.6 (25.4–32.5) to 40.6 (33.0–47.9) mL/min/1.73 m2 (p = 0.017).
At the 5-year follow-up, eGFR remained stable for the obesity-CKD division (n = 9 since one patient died in the second follow-up; 54.1 [48.0–54.4] mL/min/1.73 m2 vs. 1-year eGFR, p = 0.594). However, eGFR slightly deteriorated in the non-obesity CKD subgroup (n = 8; 35.2 [29.4–40.1] mL/min/1.73 m2 vs. 1-year eGFR, p = 0.036). As it has been previously stated, one male patient in the obesity-CKD division (obesity comorbidities) and one male patient in the non-obesity CKD subgroup (polycystic kidney disease) required hemodialysis in the extended follow-up at ages 60.5 and 67.7, respectively. Further details concerning the characteristics of the CKD subgroups can be found in Table 4.

Table 4.
Characteristics of the CKD subgroups.
3.3. Post-Operative Complications and Mortality
No major clinically significant differences regarding length of stay, surgical time, or postoperative complications were observed between the study groups. Two patients presented AKI, but none of them needed hemodialysis in the recent post-operative period (<30 days). All the details are presented in Table 5.

Table 5.
Post-operative complications and mortality after bariatric surgery.
Regarding mortality, one patient in the control group (at age 73), and five patients in the CKD-group (at ages 27, 56, 61, 73, and 80) died. The most frequent causes were CV disease (myocardial infarction) and septic shock, being the source of infection the gastrointestinal tract and jugular endovascular catheter, respectively.
The median interval between the initial surgery and death was 2.9 (0.6–9.9) years in the CKD-group and, 12.1 years in the control-group. One patient in the CKD-group died shortly after having initiated renal replacement therapy (3 months from BS) because of an endovascular catheter infection.
4. Discussion
Our study primarily aimed to assess BS safety along with long-term renal and metabolic outcomes in patients with CKD. We have shown that BS appears to be safe in patients with CKD (stages 3–4). In fact, these patients are the ones whose renal function is susceptible to recovering after BS. Additionally, our results point out that BS is effective for achieving/maintaining weight loss and for improving obesity comorbidities independently of the presence of CKD. Although previous authors have also approached these objectives, this is the first long-term follow-up study applying a propensity-score matching method so as to correct for sample selection bias. In addition, it specifically considers BS renal and metabolic benefits attending CKD etiology in a binary approach (directly related or not to obesity).
Current evidence supports the use of BS to treat patients with obesity and CKD. Actually, several observational studies have analyzed kidney outcomes in patients with or without CKD who undergo BS [9,34,35,36]. Overall, results have consistently shown that BS is associated with slower eGFR decline [8,9,37,38,39]. In line with these findings, patients in our cohort experienced an improvement in eGFR in the short and medium term. Furthermore, it should be stressed that this improvement was irrespective of the main cause of CKD. Multiple mechanisms may be involved in this improvement. Recent studies point to a connection between lipotoxicity and CKD. Moreover, adipose tissue depots directly related to the kidney (renal sinus fat) might also play a role [39].
The last guidelines for the management of CKD emphasize the importance of controlling those risk factors associated with CKD progression such as hypertension, diabetes, and dyslipidemia, which are also widely recognized classical CV risk factors [40]. BS has demonstrated a marked reduction in CV morbidity/mortality and a significant increase in life expectancy [41,42,43] and thus, it should be considered as a useful, safe, and valid therapeutic tool in the CKD setting.
In agreement with previous observational studies in the field [14,36,37], we have shown an improvement of hypertension in the CKD-group reflected by a decreased need of anti-hypertensive medications at both the 1-year and 5-year follow-up. However, a resolution of hypertension following BS could not be demonstrated. Regarding T2D, although its prevalence remained almost the same at 1-year follow-up for patients in the CKD-group, HbA1c levels clearly improved. A similar situation occurred when considering the prevalence of dyslipidemia and the laboratory lipid profile. The levels of total cholesterol, non-HDL-cholesterol, and triglycerides improved, without any significant change in the use of statins. It must be underlined that these advances in both glycemic and lipid control persisted at the 5-year follow-up. Previous works and metanalyses have also reported BS effectiveness in order to achieve significant weight loss in patients with CKD, both in the short and medium-term. Our results reinforce these aforementioned findings and extend them to the long term [44,45].
A relatively small number of studies to date have tested the impact of renal function on the early postoperative outcomes following BS, suggesting an increased risk for complications in patients with more advanced CKD stages, especially with ESRD, due to the inherent associated negative health consequences of an additional concomitant chronic disease [5,23,46,47]. Although a higher rate of complications was also observed in our cohort, it did not result in an increased length of stay or what is more relevant, it did not have a negative impact on the immediate postsurgical mortality rate. Nevertheless, and as it could be expected, the presence of AKI after BS was only present in the CKD-group [24,25,26]. Renal replacement therapy was not needed immediately after BS, but 3 months after the surgical procedure one patient started on hemodialysis. Unfortunately, the patient suffered an endovascular catheter infection because of multidrug-resistant bacteria and died shortly after.
It should be strengthened that although the mortality rate was clearly higher in the CKD group, it was not an immediate consequence of BS. It is well-known that life expectancy is reduced for all levels of renal function below an eGFR of 60 mL/min/1.73 m2. Actually, CKD has been referred to as a silent and poorly known killer [48]. This higher risk of premature death is principally related to an increase in CV morbidity, as observed in our cohort [49].
Our study has strengths and limitations. The exhaustive short (1-year) and mid-term (5-year) follow-up, along with the comprehensive clinical evaluation (both renal and metabolic outcomes), of patients with CKD eligible for BS is one of its key strengths. Moreover, it includes a well-paired group of controls attending BMI, sex, age, main comorbidities, and year and type of BS. Finally, it precisely assesses BS renal benefits according to CKD etiology.
However, limitations should also be acknowledged. Firstly, the main drawback of our study is its small sample, especially attending subgroups (CKD directly related or not to obesity). Consequently, extrapolating our results to other populations has to be taken with great caution. Although the exact prevalence of obesity among patients with CKD can vary widely based on the criteria used to define both obesity and CKD, in Spain, it has been set at around 22.6% [50]. Therefore, a higher percentage of patients with CKD and obesity eligible for BS could be expected. Nevertheless, the specific characteristics of our Renal Unit, in which highly specialized dietitians offer specific dietary advice through regular visits might have contributed to a lower proportion of severe obesity and thus, explain our relatively small sample. Secondly, our control-group was not the most suitable one as it consisted of subjects with obesity entitled to BS, but normal kidney function. Although we aimed to include individuals with CKD and obesity who were also candidates for BS, but refused it, the number of eligible individuals was clearly insufficient, and the existence of multiple confounders (age, sex, comorbidities) could not be ruled out. Therefore, we opted to apply a propensity index score. Thirdly, regarding the assessment of renal function, the CKD-EPI equation with creatinine rather than cystatin C was used, thus possibly affecting the precision of eGFR. As cystatin C is not routinely collected in our institution, using the CKD-EPI was considered the most accurate choice [15]. Other important indicators of kidney function, such as proteinuria, were not widely available in our cohort. Nevertheless, the existence of proteinuria was carefully revised and excluded before including any patient in the control-group. Finally, the CKD etiology was established according to the available medical records. As a result, misclassifications cannot certainly be discarded.
5. Conclusions
Weight loss surgery in patients with CKD improves short-, medium-, and long-term kidney outcomes as well as obesity comorbidities. In addition, its benefits seem irrespective of CKD main cause considered in a binary approach (related or not to obesity). Consequently, BS could be considered as a renoprotective intervention in patients with pre-existing CKD and it should not be discouraged depending on CKD etiology. Nevertheless, these potential benefits must always be counterbalanced with eventual adverse events in multidisciplinary teams. Additional surgical risks should always be considered beforehand in order to be prepared and therefore avoid a negative impact not only on hospital length of stay but also complications and survival rate.
Author Contributions
All authors have discussed the results and commented on the final version of the manuscript. A.P., M.C., A.M.-A., R.O., E.M.-M., J.V. (Judith Viaplana), B.R.-A. and A.I. acquired and processed all the clinical data. A.P., A.J., A.J.A., P.V.-A., A.d.H. and L.F. participated in data analysis and interpretation. P.V.-A., J.V. (Josep Vidal), A.d.H. and L.F. contributed to the study concept and design. A.d.H. and L.F. supervised the study and participated in data analysis and interpretation. A.P., M.C., L.B. and A.d.H. wrote the manuscript and had final responsibility for the decision to submit it for publication. A.P. and A.d.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Pla Estratègic de Recerca i Innovació en Salut” (PERIS) (SLT008/18/00127 to A.J.) and by the “Ajut a la Recerca Josep Font, 2018” (Hospital Clinic, Barcelona) to A.P.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Hospital Clínic de Barcelona, Spain (Reg. HCB/2019/0324).
Informed Consent Statement
In accordance with the Ethics Committee of Hospital Clínic de Barcelona, patient consent was waived due to the retrospective nature of the research.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors, A.D.H. and A.P., upon reasonable request.
Acknowledgments
The authors would like to express their most sincere gratitude to Sara Caelles for her support and guidance in the design of figures and preparation of tables; to Marty Hall for his help in writing and editing the manuscript; to Tania Arellano and Natalia Garcia for her extremely valuable aid and cooperation to recover all the necessary medical records; and to all the nephrologists, nurses and nurse’s technicians of the Nephrology and Kidney Transplantation Department (Hospital Clinic, Barcelona, Spain) who have encouraged us to share our clinical experience in the management of obesity among patients with CKD. The images presented in the visual abstract were designed by Freepik (http://www.freepik.com) and also acquired through Shutterstock (https://www.shutterstock.com) accessed on 2 August 2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L.J. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Morales, E. The Fatty Kidney: Obesity and Renal Disease. Nephron 2017, 136, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Navarro Díaz, M. Consequences of morbid obesity on the kidney. Where are we going? Clin. Kidney J. 2016, 9, 782. [Google Scholar] [CrossRef]
- Nelson, R.G.; Grams, M.E.; Ballew, S.H.; Sang, Y.; Azizi, F.; Chadban, S.J.; Chaker, L.; Dunning, S.C.; Fox, C.; Hirakawa, Y.; et al. Development of Risk Prediction Equations for Incident Chronic Kidney Disease. JAMA-J. Am. Med. Assoc. 2019, 322, 2104–2114. [Google Scholar] [CrossRef]
- Turgeon, N.A.; Perez, S.; Mondestin, M.; Scott Davis, S.; Lin, E.; Tata, S.; Kirk, A.D.; Larsen, C.P.; Pearson, T.C.; Sweeney, J.F. The Impact of Renal Function on Outcomes of Bariatric Surgery. J. Am. Soc. Nephrol. 2012, 23, 885–894. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, C.N.; Goss, L.E.; Almehmi, A.; Grams, J.M.; Corey, B.L. Bariatric surgery is associated with renal function improvement. Surg. Endosc. 2017, 32, 276–281. [Google Scholar] [CrossRef]
- Friedman, A.N.; Cohen, R.V. Bariatric surgery as a renoprotective intervention. Curr. Opin. Nephrol. Hypertens. 2019, 28, 537–544. [Google Scholar] [CrossRef]
- Afshinnia, F.; Wilt, T.J.; Duval, S.; Esmaeili, A.; Ibrahim, H.N. Weight loss and proteinuria: Systematic review of clinical trials and comparative cohorts. Nephrol. Dial. Transplant. 2010, 25, 1173–1183. [Google Scholar] [CrossRef]
- Li, K.; Zou, J.; Ye, Z.; Di, J.; Han, X.; Zhang, H.; Liu, W.; Ren, Q.; Zhang, P. Effects of bariatric surgery on renal function in obese patients: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0163907. [Google Scholar] [CrossRef] [PubMed]
- Shulman, A.; Andersson-Assarsson, J.C.; Sjöström, C.D.; Jacobson, P.; Taube, M.; Sjöholm, K.; le Roux, C.W.; Peltonen, M.; Carlsson, L.M.S.; Svensson, P.A. Remission and progression of pre-existing micro- and macroalbuminuria over 15 years after bariatric surgery in Swedish Obese Subjects study. Int. J. Obes. 2020, 45, 535–546. [Google Scholar] [CrossRef]
- Amor, A.; Jiménez, A.; Moizé, V.; Ibarzabal, A.; Flores, L.; Lacy, A.M.; Vidal, J. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg. Endosc. 2013, 27, 2046–2051. [Google Scholar] [CrossRef]
- Kassam, A.F.; Mirza, A.; Kim, Y.; Hanseman, D.; Woodle, E.S.; Quillin, R.C.; Johnson, B.L.; Govil, A.; Cardi, M.; Schauer, D.P.; et al. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am. J. Transplant. 2020, 20, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Imam, T.H.; Fischer, H.; Jing, B.; Burchette, R.; Henry, S.; DeRose, S.F.; Coleman, K.J. Estimated GFR Before and After Bariatric Surgery in CKD. Am. J. Kidney Dis. 2017, 69, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W.; Goodman, H.R.; Gersin, K.; Cardi, M.; Austin, J.; Goel, S.; Safdar, S.; Huang, S.; Woodle, E.S. Gastric bypass in morbidly obese patients with chronic renal failure and kidney transplant. Transplantation 2004, 78, 469–474. [Google Scholar] [CrossRef]
- Al-Bahri, S.; Fakhry, T.K.; Gonzalvo, J.P.; Murr, M.M. Bariatric Surgery as a Bridge to Renal Transplantation in Patients with End-Stage Renal Disease. Obes. Surg. 2017, 13, 336–2955. [Google Scholar] [CrossRef]
- Lin, M.Y.C.; Tavakol, M.M.; Sarin, A.; Amirkiai, S.M.; Rogers, S.J.; Carter, J.T.; Posselt, A.M. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg. Obes. Relat. Dis. 2013, 9, 653–658. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Waits, S.A.; Dimick, J.B.; Telem, D.A. Risks of Bariatric Surgery among Patients with End-stage Renal Disease. JAMA Surg. 2019, 154, 1160–1162. [Google Scholar] [CrossRef]
- Gheith, O.; Al-Otaibi, T.; Halim, M.A.; Mahmoud, T.; Mosaad, A.; Yagan, J.; Zakaria, Z.; Rida, S.; Nair, P.; Hassan, R. Bariatric Surgery in Renal Transplant Patients. Exp. Clin. Transplant. 2017, 15, 164–169. [Google Scholar] [CrossRef]
- MacLaughlin, H.L.; Hall, W.L.; Patel, A.G.; MacDougall, I.C. Laparoscopic sleeve gastrectomy is a novel and effective treatment for obesity in patients with chronic kidney disease. Obes. Surg. 2012, 22, 119–123. [Google Scholar] [CrossRef]
- Cohen, R.V.; Pereira, T.V.; Aboud, C.M.; Petry, T.B.Z.; Lopes Correa, J.L.; Schiavon, C.A.; Pompílio, C.E.; Pechy, F.N.Q.; Da Costa Silva, A.C.C.; De Melo, F.L.G.; et al. Effect of Gastric Bypass vs. Best Medical Treatment on Early-Stage Chronic Kidney Disease in Patients with Type 2 Diabetes and Obesity: A Randomized Clinical Trial. JAMA Surg. 2020, 155, e200420. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, E.; Aminizadeh, E.; Dooghaie Moghadam, A.; Sabetkish, N.; Abbasi Dezfouli, S.; Morath, C.; Zeier, M.; Nickel, F.; Billeter, A.T.; Müller-Stich, B.P.; et al. Bariatric surgery in patients with obesity and end-stage renal disease. Surg. Obes. Relat. Dis. 2023, 19, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Kharat, V.; Blanck, S.; Leonard, A.C. Acute kidney injury after gastric bypass surgery. Clin. J. Am. Soc. Nephrol. 2007, 2, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, T.N.; Gurrieri, C.; McCaffrey, J.M.; Ricter, S.J.; Hilgeman, M.L.; Schroeder, D.R.; Kendrick, M.L.; Greene, E.L.; Sprung, J. Acute kidney injury following bariatric surgery. Obes. Surg. 2013, 23, 64–70. [Google Scholar] [CrossRef]
- Abdullah, H.R.; Tan, T.P.; Vaez, M.; Deb, C.; Farag, N.; Jackson, T.D.; Wong, D.T. Predictors of Perioperative Acute Kidney Injury in Obese Patients Undergoing Laparoscopic Bariatric Surgery: A Single-Centre Retrospective Cohort Study. Obes. Surg. 2016, 26, 1493–1499. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar] [CrossRef]
- Jiménez, A.; Ibarzabal, A.; Moizé, V.; Pané, A.; Andreu, A.; Molero, J.; de Hollanda, A.; Flores, L.; Ortega, E.; Lacy, A.; et al. Ten-year outcomes after Roux-en-Y gastric bypass and sleeve gastrectomy: An observational nonrandomized cohort study. Surg. Obes. Relat. Dis. 2019, 15, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Kim, J.; El Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Hinerman, A.S.; Belle, S.H.; Wahed, A.S.; Courcoulas, A.P. Comparison of the Performance of Common Measures of Weight Regain after Bariatric Surgery for Association with Clinical Outcomes. JAMA-J. Am. Med. Assoc. 2018, 320, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef]
- Docherty, N.G.; le Roux, C.W. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat. Rev. Nephrol. 2020, 16, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Raveendran, L.; Lovrics, O.; Tian, C.; Khondker, A.; Koyle, M.A.; Farcas, M.; Doumouras, A.G.; Hong, D. The role of bariatric surgery on kidney transplantation: A systematic review and meta-analysis. Can. Urol. Assoc. J. 2021, 15, E553–E562. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Porrini, E.; Martin-Taboada, M.; Luis-Lima, S.; Vila-Bedmar, R.; de Pablos, I.G.; Gómez, P.; Rodríguez, E.; Torres, L.; Lanzón, B.; et al. Renoprotective role of bariatric surgery in patients with established chronic kidney disease. Clin. Kidney J. 2021, 14, 2037–2046. [Google Scholar] [CrossRef]
- Friedman, A.N.; Moe, S.; Fadel, W.F.; Inman, M.; Mattar, S.G.; Shihabi, Z.; Quinney, S.K.; Cheng, Y.L.; Elli, E.F.; de Hollanda, A.; et al. Outcomes of Bariatric Surgery after Solid Organ Transplantation. Obes. Surg. 2017, 27, 4899–4904. [Google Scholar] [CrossRef]
- Huang, H.; Lu, J.; Dai, X.; Li, Z.; Zhu, L.; Zhu, S.; Wu, L. Improvement of Renal Function After Bariatric Surgery: A Systematic Review and Meta-analysis. Obes. Surg. 2021, 31, 4470–4484. [Google Scholar] [CrossRef]
- Sandino, J.; Martín-Taboada, M.; Medina-Gómez, G.; Vila-Bedmar, R.; Morales, E. Novel Insights in the Physiopathology and Management of Obesity-Related Kidney Disease. Nutrients 2022, 14, 3937. [Google Scholar] [CrossRef]
- Official Journal of the International Society of Nephrology KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Available online: www.publicationethics.org (accessed on 1 December 2020).
- Syn, N.L.; Cummings, D.E.; Wang, L.Z.; Lin, D.J.; Zhao, J.J.; Loh, M.; Koh, Z.J.; Chew, C.A.; Loo, Y.E.; Tai, B.C.; et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: A one-stage meta-analysis of matched cohort and prospective controlled studies with 174,772 participants. Lancet 2021, 397, 1830–1841. [Google Scholar] [CrossRef]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of Metabolic Surgery with Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes and Obesity. JAMA-J. Am. Med. Assoc. 2019, 322, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Zoccali, C. Effects of weight loss on renal function in obese CKD patients: A systematic review. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S4), iv82–iv98. [Google Scholar] [CrossRef] [PubMed]
- Abou-Mrad, R.M.; Abu-Alfa, A.K.; Ziyadeh, F.N. Effects of weight reduction regimens and bariatric surgery on chronic kidney disease in obese patients. Am. J. Physiol. Renal Physiol. 2013, 305, F613–F617. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.R.; Cohen, J.A.; Brown, C.S.; Sheetz, K.H.; Chao, G.F.; Waits, S.A.; Telem, D.A. Perioperative risks of bariatric surgery among patients with and without history of solid organ transplant. Am. J. Transplant. 2020, 20, 2530–2539. [Google Scholar] [CrossRef]
- Cohen, J.B.; Tewksbury, C.M.; Torres Landa, S.; Williams, N.N.; Dumon, K.R. National Postoperative Bariatric Surgery Outcomes in Patients with Chronic Kidney Disease and End-Stage Kidney Disease. Obes. Surg. 2019, 29, 975–982. [Google Scholar] [CrossRef]
- Ortiz, A.; Roger, M.; Jiménez, V.M.; Perez, J.C.R.; Furlano, M.; Atxer, L.S.; Zurro, D.G.; Casabona, C.M.R.; Gómez, C.G.; Bermúdez, P.P.; et al. RICORS2040: The need for collaborative research in chronic kidney disease. Clin. Kidney J. 2021, 15, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Turin, T.C.; Tonelli, M.; Manns, B.J.; Ravani, P.; Ahmed, S.B.; Hemmelgarn, B.R. Chronic kidney disease and life expectancy. Nephrol. Dial. Transplant. 2012, 27, 3182–3186. [Google Scholar] [CrossRef]
- Gorostidi, M.; Sánchez-Martínez, M.; Ruilope, L.M.; Graciani, A.; de la Cruz, J.J.; Santamaría, R.; del Pino, M.D.; Guallar-Castillón, P.; de Álvaro, F.; Rodríguez-Artalejo, F.; et al. Chronic kidney disease in Spain: Prevalence and impact of accumulation of cardiovascular risk factors. Nefrologia 2018, 38, 606–615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).