The Symptoms of Pulmonary Sarcoidosis
Abstract
1. Introduction
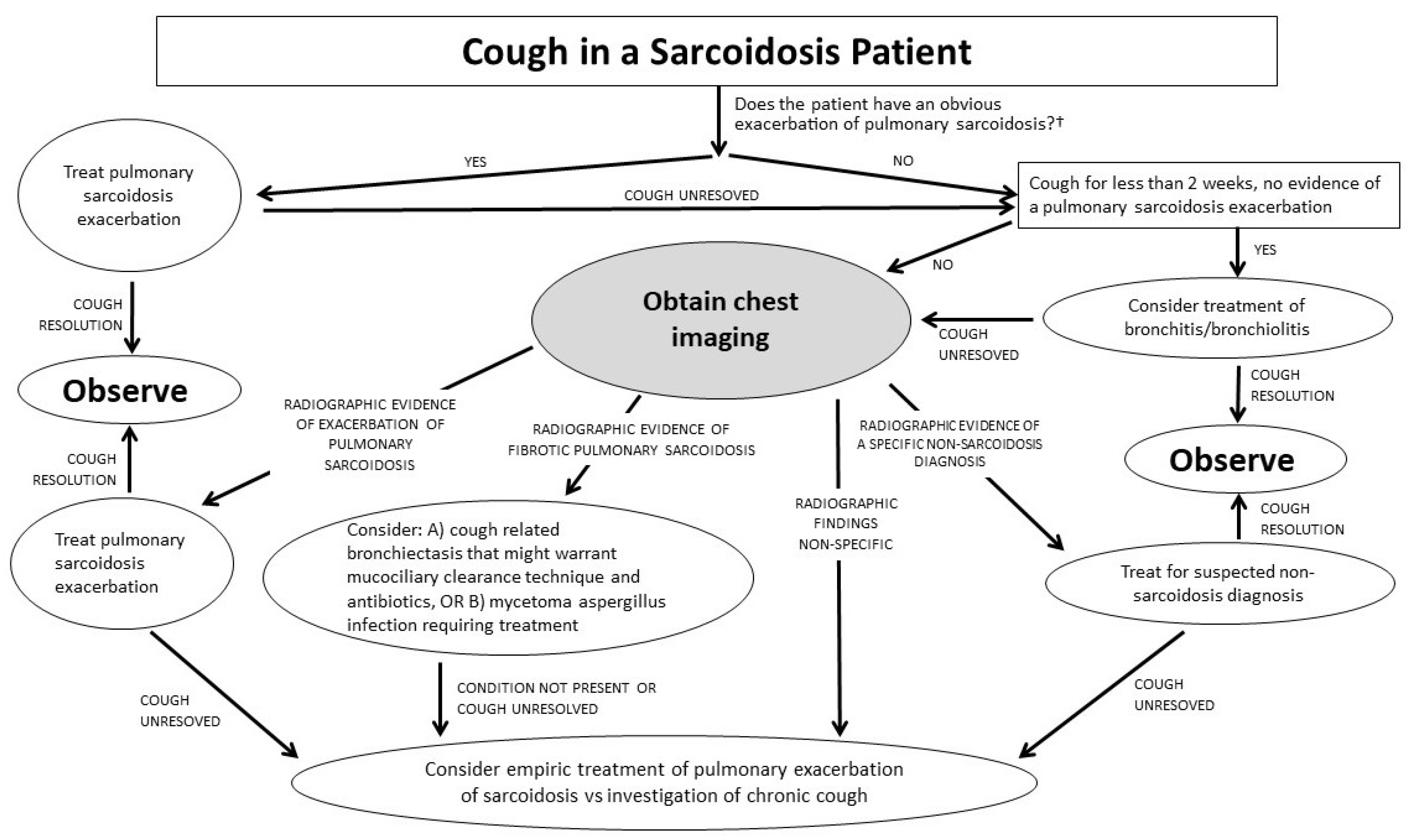
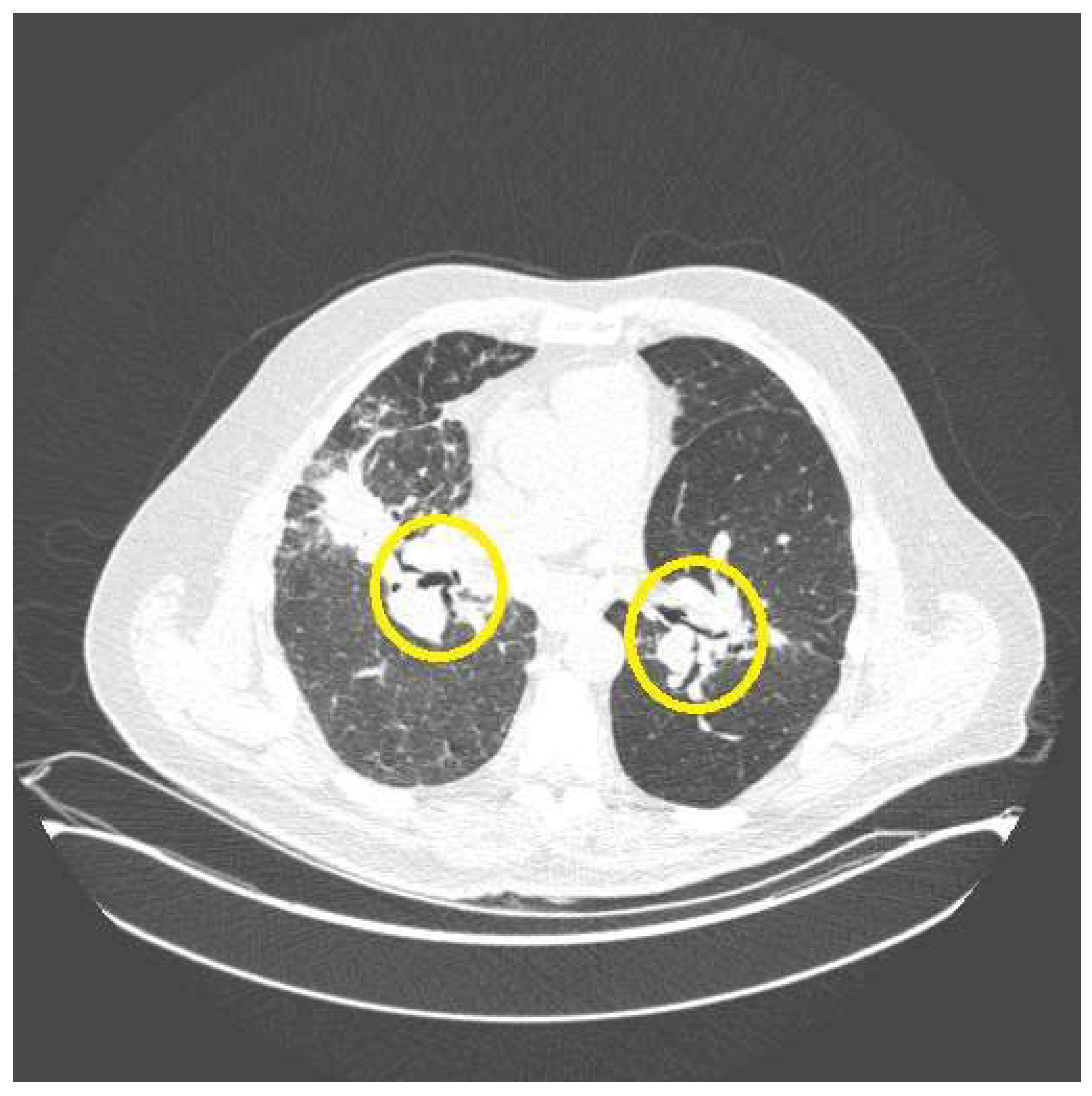
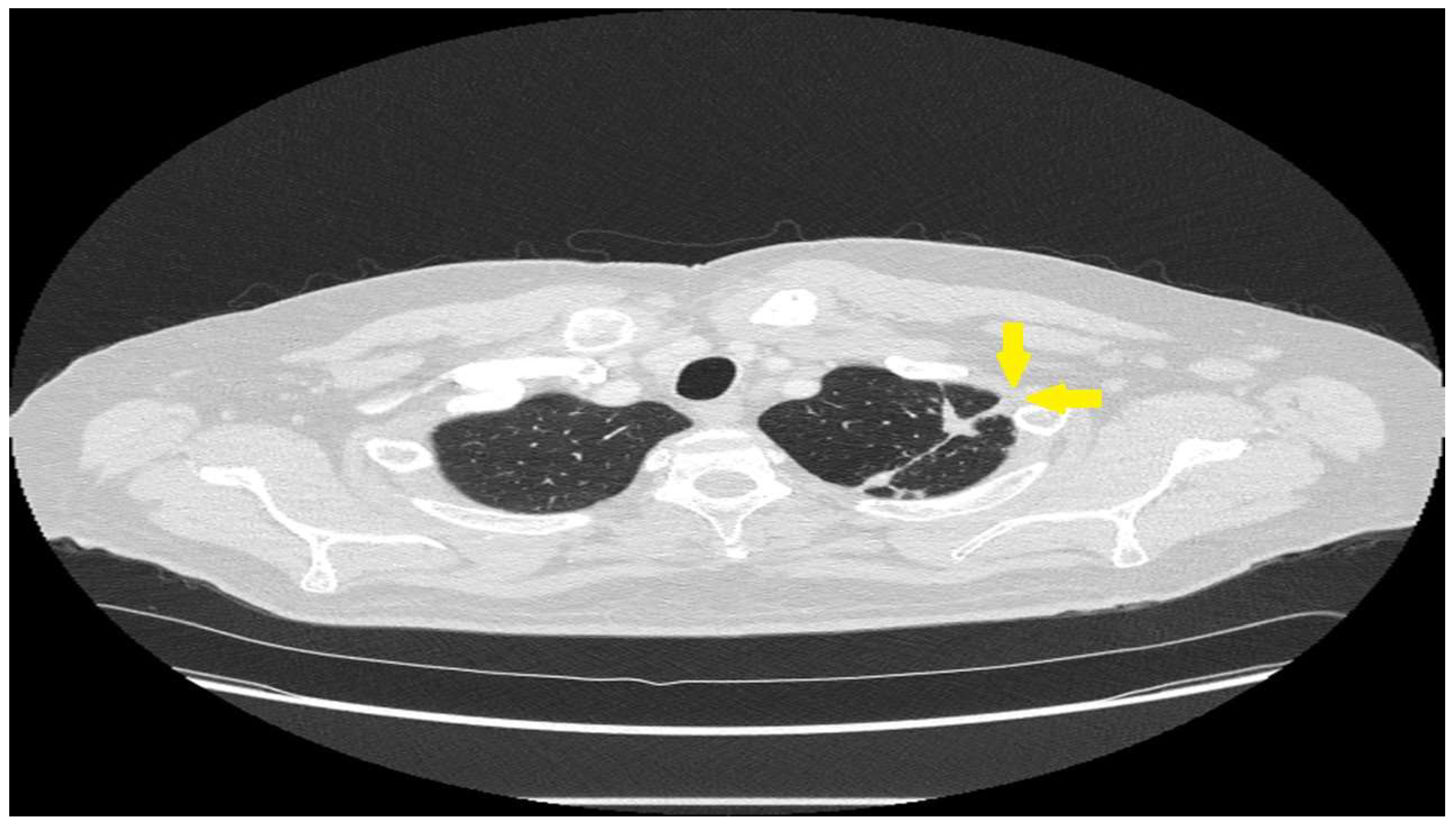
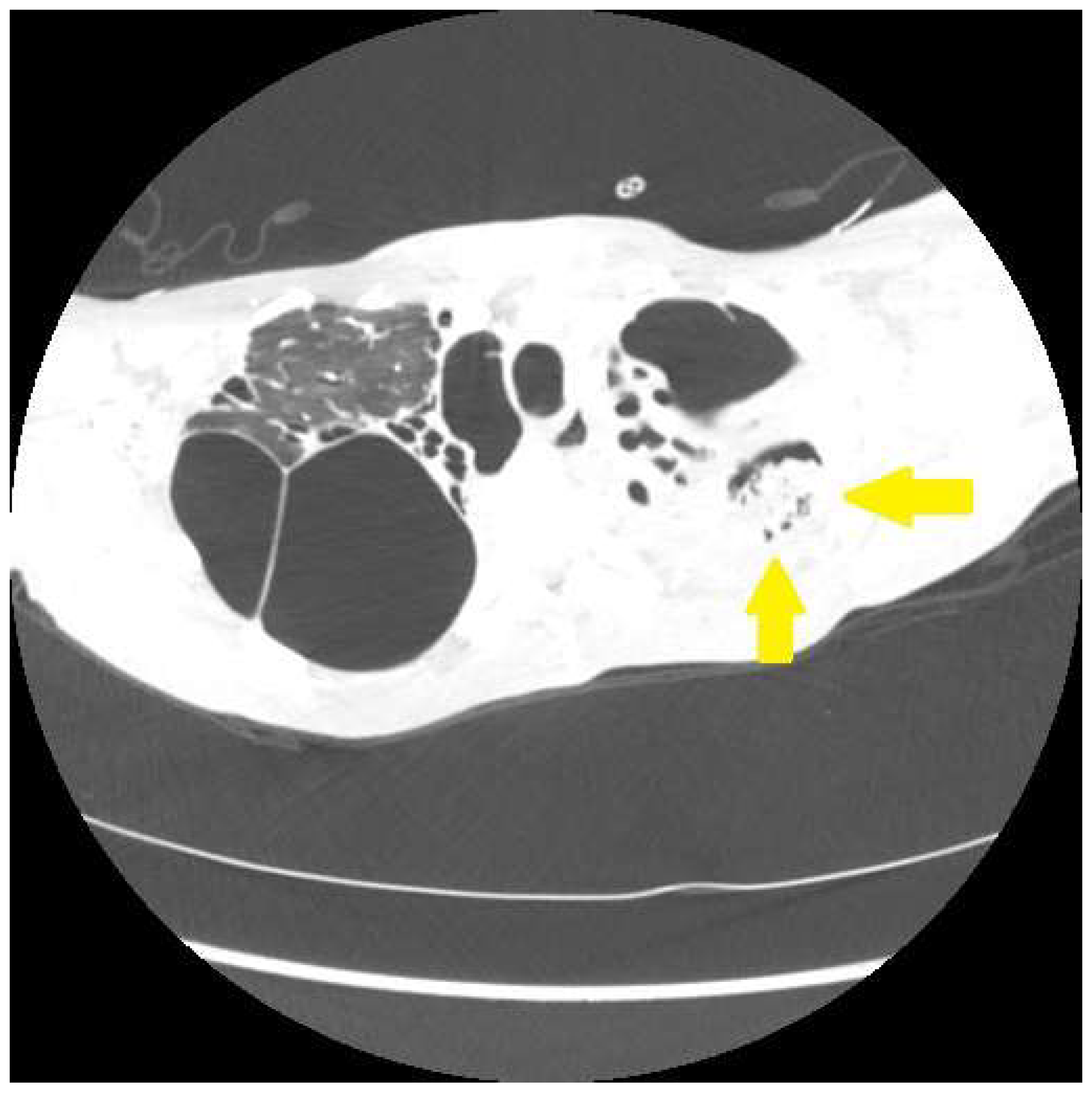
2. Cough
3. Wheezing
4. Dyspnea
5. Chest Pain
6. Hemoptysis
7. Pulmonary Sarcoidosis without Pulmonary Symptoms
8. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rossman, M.D.; Yeager, H., Jr.; Bresnitz, E.A.; Depalo, L.; Hunninghake, G.; Lannuzzi, M.C.; Johns, C.J.; et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Boan, A.D.; Lackland, D.T. The clinical course of sarcoidosis: Presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc. Diffus. Lung Dis. 2012, 29, 119–127. [Google Scholar]
- Polverino, F.; Balestro, E.; Spagnolo, P. Clinical Presentations, Pathogenesis, and Therapy of Sarcoidosis: State of the Art. J. Clin. Med. 2020, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P.; Drent, M.; Culver, D.A.; Grutters, J.C.; Handa, T.; Humbert, M.; Judson, M.A.; Lower, E.E.; Mana, J.; Pereira, C.A.; et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2012, 29, 90–98. [Google Scholar]
- Chopra, A.; Kalkanis, A.; Judson, M.A. Biomarkers in sarcoidosis. Expert. Rev. Clin. Immunol. 2016, 12, 1191–1208. [Google Scholar] [CrossRef]
- Chu, B.; O’Connor, D.M.; Wan, M.; Barnett, I.; Shou, H.; Judson, M.; Rosenbach, M. Quality of Life and Physical Activity in 629 Individuals with Sarcoidosis: Prospective, Cross-sectional Study Using Smartphones (Sarcoidosis App). JMIR Mhealth Uhealth 2022, 10, e38331. [Google Scholar] [CrossRef]
- Baughman, R.P.; Judson, M.A.; Wells, A.U. The indications for the treatment of sarcoidosis: Well’s Law. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 280–282. [Google Scholar]
- Sinha, A.; Lee, K.K.; Rafferty, G.F.; Yousaf, N.; Pavord, I.D.; Galloway, J.; Birring, S.S. Predictors of objective cough frequency in pulmonary sarcoidosis. Eur. Respir. J. 2016, 47, 1461–1471. [Google Scholar] [CrossRef]
- Harrison, N.K. Cough, sarcoidosis and idiopathic pulmonary fibrosis: Raw nerves and bad vibrations. Cough 2013, 9, 9. [Google Scholar] [CrossRef]
- Judson, M.A.; Chopra, A.; Conuel, E.; Koutroumpakis, E.; Schafer, C.; Austin, A.; Zhang, R.; Cao, K.; Berry, R.; Khan, M.M.H.S.; et al. The Assessment of Cough in a Sarcoidosis Clinic Using a Validated instrument and a Visual Analog Scale. Lung 2017, 195, 587–594. [Google Scholar] [CrossRef]
- McKinzie, B.P.; Bullington, W.M.; Mazur, J.E.; Judson, M.A. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am. J. Med. Sci. 2010, 339, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.; Uzunhan, Y.; Gille, T.; Lamberto, C.; Valeyre, D.; Brillet, P.Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur. Respir. J. 2012, 40, 750–765. [Google Scholar] [CrossRef] [PubMed]
- Drent, M.; Mansour, K.; Linssen, C. Bronchoalveolar lavage in sarcoidosis. Semin. Respir. Crit. Care Med. 2007, 28, 486–495. [Google Scholar] [CrossRef]
- Vorselaars, A.D.; Crommelin, H.A.; Deneer, V.H.; Meek, B.; Claessen, A.M.E.; Keijers, R.G.M.; van Moorsel, C.H.M.; Grutters, J.C. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur. Respir. J. 2015, 46, 175–185. [Google Scholar] [CrossRef]
- Kovacova, E.; Buday, T.; Vysehradsky, R.; Plevkova, J. Cough in sarcoidosis patients. Respir. Physiol. Neurobiol. 2018, 257, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Birring, S.S.; Prudon, B.; Carr, A.J.; Singh, S.J.; Morgan, M.D.; Pavord, I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003, 58, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.C.; Fichtner, U.A.; Frye, B.C.; Heyduck-Weides, K.; Birring, S.S.; Windisch, W.; Criee, C.; Muller-Quernheim, J.; Farin, E. Psychometric properties of the German version of the Leicester Cough Questionnaire in sarcoidosis. PLoS ONE 2018, 13, e0205308. [Google Scholar] [CrossRef]
- Baughman, R.P.; Drent, M.; Kavuru, M.; Judson, M.A.; Costabel, U.; du Bois, R.; Albera, C.; Brutsche, M.; Davis, G.; Donohue, J.F.; et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006, 174, 795–802. [Google Scholar] [CrossRef]
- Cox, C.E.; Donohue, J.F.; Brown, C.D.; Kataria, Y.P.; Judson, M.A. The Sarcoidosis Health Questionnaire: A new measure of health-related quality of life. Am. J. Respir. Crit. Care Med. 2003, 168, 323–329. [Google Scholar] [CrossRef]
- Shorr, A.F.; Torrington, K.G.; Hnatiuk, O.W. Endobronchial biopsy for sarcoidosis: A prospective study. Chest 2001, 120, 109–114. [Google Scholar] [CrossRef]
- Shorr, A.F.; Torrington, K.G.; Hnatiuk, O.W. Endobronchial involvement and airway hyperreactivity in patients with sarcoidosis. Chest 2001, 120, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Xin-Min, D.; Qin-Zhi, X.; Yun-You, D.; Zhang, C.; Pen, C.; Nig, H.; Li, Y.; Meng, J.; Nie, Z.; Feng, H. Impact of tracheal mucosa involvement on clinical characteristics of sarcoidosis. South. Med. J. 2011, 104, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Kalkanis, A.; Judson, M.A. Distinguishing asthma from sarcoidosis: An approach to a problem that is not always solvable. J. Asthma 2013, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tully, T.; Birring, S.S. Cough in Sarcoidosis. Lung 2016, 194, 21–24. [Google Scholar] [CrossRef]
- Carvalho, S.R.; Dias, R.M.; Neves, D.D. Airway responsiveness in sarcoidosis. Rev. Port. Pneumol. 2005, 11, 97–110. [Google Scholar]
- Ohrn, M.B.; Skold, C.M.; van Hage-Hamsten, M.; Sigurdardottir, O.; Zetterstrom, O.; Eklund, A. Sarcoidosis patients have bronchial hyperreactivity and signs of mast cell activation in their bronchoalveolar lavage. Respir. Int. Rev. Thorac. Dis. 1995, 62, 136–142. [Google Scholar]
- Marcias, S.; Ledda, M.A.; Perra, R.; Severino, C.; Rosetti, L. Aspecific bronchial hyperreactivity in pulmonary sarcoidosis. Sarcoidosis 1994, 11, 118–122. [Google Scholar]
- Hattori, T.; Konno, S.; Shigemura, M.; Matsuno, K.; Shimizu, C.; Shigehara, K.; Shijubo, N.; Hizawa, N.; Yamaguchi, E.; Nishimura, M. Total serum IgE levels and atopic status in patients with sarcoidosis. Allergy Asthma Proc. Off. J. Reg. State Allergy Soc. 2012, 33, 90–94. [Google Scholar] [CrossRef]
- Morice, A.H.; Millqvist, E.; Belvisi, M.G.; Bieksiene, K.; Birring, S.S.; Chung, K.F.; Dal Negro, R.W.; Dicpinigaitis, P.; Kantar, A.; McGarvey, L.P.; et al. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur. Respir. J. 2014, 44, 1132–1148. [Google Scholar] [CrossRef]
- Gupta, R.; Judson, M.A.; Baughman, R.P. Management of Advanced Pulmonary Sarcoidosis. Am. J. Respir. Crit. Care Med. 2022, 205, 495–506. [Google Scholar] [CrossRef]
- Baughman, R.P.; Lower, E.E. Frequency of acute worsening events in fibrotic pulmonary sarcoidosis patients. Respir. Med. 2013, 107, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A. Sarcoidosis of the upper and lower airways. In Diseases of the Central Airways; Mehta, A.C., Jain, P., Gildea, T.R., Eds.; Springer: Cham, Switzerland, 2016; pp. 71–85. [Google Scholar]
- Birring, S.S.; Matos, S.; Patel, R.B.; Prudon, B.; Evans, D.H.; Pavord, I.D. Cough frequency, cough sensitivity and health status in patients with chronic cough. Respir. Med. 2006, 100, 1105–1109. [Google Scholar] [CrossRef]
- Gabaldón-Figueira, J.C.; Keen, E.; Rudd, M.; Orrilo, V.; Blavia, I.; Chaccour, J.; Glavosas, M.; Small, P.; Lapierre, S.G.; Chaccour, C. Longitudinal passive cough monitoring and its implications for detecting changes in clinical status. ERJ Open Res. 2022, 8, 00001–2022. [Google Scholar] [CrossRef] [PubMed]
- French, C.T.; Irwin, R.S.; Fletcher, K.E.; Adams, T.M. Evaluation of a cough-specific quality-of-life questionnaire. Chest 2002, 121, 1123–1131. [Google Scholar] [CrossRef]
- Alberts, C.; van der Mark, T.W.; Jansen, H.M. Inhaled budesonide in pulmonary sarcoidosis: A double-blind, placebo-controlled study. Dutch Study Group on Pulmonary Sarcoidosis. Eur. Respir. J. 1995, 8, 682–688. [Google Scholar] [CrossRef] [PubMed]
- du Bois, R.M.; Greenhalgh, P.M.; Southcott, A.M.; Johnson, N.M.; Harris, T.A. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: A pilot study. Eur. Respir. J. 1999, 13, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Paramothayan, S.; Jones, P.W. Corticosteroid therapy in pulmonary sarcoidosis: A systematic review. Jama 2002, 287, 1301–1307. [Google Scholar] [CrossRef]
- Paramothayan, N.S.; Lasserson, T.J.; Jones, P.W. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst. Rev. 2005, 2, CD001114. [Google Scholar] [CrossRef]
- Thillai, M.; Potiphar, L.; Eberhardt, C.; Pareek, M.; Dhawan, R.; Kon, O.M.; Wickremasinghe, M.; Wells, A.; Mitchell, D.; Lalvani, A. Obstructive lung function in sarcoidosis may be missed, especially in older white patients. Eur. Respir. J. 2012, 39, 775–777. [Google Scholar] [CrossRef]
- Polychronopoulos, V.S.; Prakash, U.B. Airway involvement in sarcoidosis. Chest 2009, 136, 1371–1380. [Google Scholar] [CrossRef]
- Broos, C.E.; Wapenaar, M.; Looman, C.W.N.; In ‘t Veen, J.C.C.M.; van den Toorn, L.M.; Overbeek, M.J.; Grootenboers, M.J.J.H.; Heller, R.; Mostard, R.L.; Poell, L.H.C.; et al. Daily home spirometry to detect early steroid treatment effects in newly treated pulmonary sarcoidosis. Eur. Respir. J. 2018, 51, 1702089. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Fingerlin, T.E.; Mroz, M.; Barkes, B.; Hamzeh, H.; Maier, L.A.; Carlsen, N.E. Radiomic measures from chest high-resolution computed tomography associated with lung function in sarcoidosis. Eur. Respir. J. 2019, 54, 1900371. [Google Scholar] [CrossRef] [PubMed]
- Verleden, S.E.; Vanstapel, A.; De Sadeleer, L.; Dubbeldam, A.; Goos, T.; Gyselinck, I.; Geudens, V.; Kaes, J.; Van Raemdonck, D.E.; Ceulemans, L.J. Distinct Airway Involvement in Subtypes of End-Stage Fibrotic Pulmonary Sarcoidosis. Chest 2021, 160, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Okuzumi, S.; Kakimoto, T.; Hamabe, K.; Morinaga, S.; Minematsu, N. Long-term Findings in Bullous Sarcoidosis: A Case Report and Literature Review. Intern. Med. 2023, 62, 2395–2400. [Google Scholar] [CrossRef]
- Sekiguchi, H.; Suzuki, J.; Utz, J.P. Sarcoidosis with major airway, vascular and nerve compromise. Can. Respir. J. 2013, 20, 256–258. [Google Scholar] [CrossRef][Green Version]
- Jones, P.W. St. George Respiratory Questionnaire Manual: A Manual; St. George’s, University of London: London, UK, 2008. [Google Scholar]
- Vizel, A.A.; Islamova, L.V.; Guryleva, M.E.; Amirov, N.B.; Dmitriev, E.G.; Bondarev, A.V.; Nasretdinova, G.R. Comparative evaluation of effects of various bronchodilators in sarcoidosis. Probl. Tuberk. Bolezn. Legk. 2003, 6, 33–36. [Google Scholar]
- Young, R.C., Jr.; Sahetya, G.K.; Hassan, S.N.; Cobb, W.B.; Kumar, B.; Facen, H.T. Chronic airways obstruction in pulmonary sarcoidosis: Its poor response to bronchodilators. J. Natl. Med. Assoc. 1980, 72, 965–972. [Google Scholar]
- Bloem, A.E.M.; Houben-Wilke, S.; Mostard, R.L.M.; Stoot, N.; Janssen, D.J.A.; Franssen, F.M.E.; Custers, J.W.H.; Spruit, M.A. Respiratory and non-respiratory symptoms in patients with IPF or sarcoidosis and controls. Heart Lung 2023, 61, 136–146. [Google Scholar] [CrossRef]
- Obi, O.N.; Judson, M.A.; Birring, S.S.; Maier, L.A.; Wells, A.U.; Lower, E.E.; Baughman, R.P. Assessment of dyspnea in sarcoidosis using the Baseline Dyspnea Index (BDI) and the Transition Dyspnea Index (TDI). Respir. Med. 2022, 191, 106436. [Google Scholar] [CrossRef]
- de Boer, S.; Kolbe, J.; Wilsher, M.L. The relationships among dyspnoea, health-related quality of life and psychological factors in sarcoidosis. Respirology 2014, 19, 1019–1024. [Google Scholar] [CrossRef]
- De Vries, J.; Drent, M. Quality of life and health status in sarcoidosis: A review of the literature. Clin. Chest Med. 2008, 29, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Thunold, R.F.; Løkke, A.; Cohen, A.L.; Ole, H.; Bendstrup, E. Patient reported outcome measures (PROMs) in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2017, 34, 2–17. [Google Scholar]
- Gvozdenovic, B.S.; Mihailovic-Vucinic, V.; Ilic-Dudvarski, A.; Zugic, V.; Judson, M.A. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Respir. Med. 2008, 102, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Sweiss, N.J.; Noth, I.; Mirsaeidi, M.; Zhang, W.; Naureckas, E.T.; Hogarth, D.K.; Strek, M.; Caligiuri, P.; Machado, R.F.; Niewold, T.B.; et al. Efficacy Results of a 52-week Trial of Adalimumab in the Treatment of Refractory Sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2014, 31, 46–54. [Google Scholar]
- Strookappe, B.; Swigris, J.; De Vries, J.; Elfferich, M.; Knevel, T.; Drent, M. Benefits of Physical Training in Sarcoidosis. Lung 2015, 193, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Karadallı, M.N.; Boşnak-Güçlü, M.; Camcıoğlu, B.; Kokturk, N.; Türktaş, H. Effects of Inspiratory Muscle Training in Subjects with Sarcoidosis: A Randomized Controlled Clinical Trial. Respir. Care 2016, 61, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Chappell, A.G.; Cheung, W.Y.; Hutchings, H.A. Sarcoidosis: A long-term follow up study. Sarcoidosis Vasc. Diffus. Lung Dis. 2000, 17, 167–173. [Google Scholar]
- Hoitsma, E.; De Vries, J.; van Santen-Hoeufft, M.; Faber, C.G.; Drent, M. Impact of pain in a Dutch sarcoidosis patient population. Sarcoidosis Vasc. Diffus. Lung Dis. 2003, 20, 33–39. [Google Scholar]
- Highland, K.B.; Retalis, P.; Coppage, L.; Schabel, S.I.; Judson, M.A. Is there an anatomic explanation for chest pain in patients with pulmonary sarcoidosis? South. Med. J. 1997, 90, 911–914. [Google Scholar] [CrossRef]
- Tavee, J.; Culver, D. Sarcoidosis and small-fiber neuropathy. Curr. Pain. Headache Rep. 2011, 15, 201–206. [Google Scholar] [CrossRef]
- Crawshaw, A.P.; Wotton, C.J.; Yeates, D.G.; Goldacre, M.J.; Ho, L.P. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax 2011, 66, 447–448. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, P.; Santagiuliana, M.; Wade, B.; Baratella, E.; Kodric, M.; Berria, M.; Jaber, M.; Torregiani, C.; Bruni, C.; et al. Correlation between Potential Risk Factors and Pulmonary Embolism in Sarcoidosis Patients Timely Treated. J. Clin. Med. 2021, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Manika, K.; Kioumis, I.; Zarogoulidis, K.; Kougioumtzi, I.; Dryllis, G.; Pitsiou, G.; Machairiotis, N.; Katsikogiannis, N.; Lampaki, S.; Papaiwannou, A.; et al. Pneumothorax in sarcoidosis. J. Thorac. Dis. 2014, 6 (Suppl. S4), S466–S469. [Google Scholar] [PubMed]
- Chopra, A.; Foulke, L.; Judson, M.A. Sarcoidosis associated pleural effusion: Clinical aspects. Respir. Med. 2022, 191, 106723. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Driver, A.G.; Townsend, C.A.; Kataria, Y.P. Hemoptysis in sarcoidosis. Sarcoidosis 1987, 4, 49–54. [Google Scholar] [PubMed]
- Devine, S.T.; Delgado, J.A.; Lippmann, M.L. Hemoptysis as an initial presentation of sarcoidosis: How common is it? Clin. Pulm. Med. 2007, 14, 76–81. [Google Scholar] [CrossRef]
- Rubinstein, I.; Baum, G.L.; Hiss, Y.; Solomon, A. Hemoptysis as the presenting manifestation of sarcoidosis. Chest 1987, 91, 931. [Google Scholar] [CrossRef]
- Nazemiyeh, M.; Rashidi, F.; Gharemohammadlou, R. A rare presentation of pulmonary sarcoidosis with massive hemoptysis. Arch. Iran. Med. 2014, 17, 84–85. [Google Scholar]
- Lewis, M.M.; Mortelliti, M.P.; Yeager, H., Jr.; Tsou, E. Clinical bronchiectasis complicating pulmonary sarcoidosis: Case series of seven patients. Sarcoidosis Vasc. Diffus. Lung Dis. 2002, 19, 154–159. [Google Scholar]
- Judson, M.A. Strategies for identifying pulmonary sarcoidosis patients at risk for severe or chronic disease. Expert Rev. Respir. Med. 2017, 11, 111–118. [Google Scholar] [CrossRef]
- Culver, D.A.; Judson, M.A. New advances in the management of pulmonary sarcoidosis. BMJ 2019, 367, l5553. [Google Scholar] [CrossRef] [PubMed]
- Uzunhan, Y.; Nunes, H.; Jeny, F.; LaCroix, M.; Brun, S.; Brillit, P.; Martinoid, E.; Carette, M.; Bouvry, D.; Charlier, C.; et al. Chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2017, 49, 1602396. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, J.N.; Berry, M.W.; Schabel, S.I.; Judson, M.A. A modern series of percutaneous intracavitary instillation of amphotericin B for the treatment of severe hemoptysis from pulmonary aspergilloma. Chest 2013, 143, 1414–1421. [Google Scholar] [CrossRef]
- Baughman, R.P.; Shlobin, O.A.; Wells, A.U.; Alhamad, E.H.; Culver, D.A.; Barney, J.; Cordova, F.C.; Scholand, M.; Wijsenbeek, M.; Ganesh, S. Clinical features of sarcoidosis associated pulmonary hypertension: Results of a multi-national registry. Respir. Med. 2018, 139, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Cantu, J.; Wang, D.; Safdar, Z. Clinical implications of haemoptysis in patients with pulmonary arterial hypertension. Int. J. Clin. Pract. 2012, 177, 5–12. [Google Scholar] [CrossRef][Green Version]
- Chun, J.Y.; Belli, A.M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur. Radiol. 2010, 20, 558–565. [Google Scholar] [CrossRef]
- Loh, G.A.; Lettieri, C.J.; Shah, A.A. Bronchial arterial embolisation for massive haemoptysis in cavitary sarcoidosis. BMJ Case Rep. 2013, 2013, br2012008268. [Google Scholar] [CrossRef]
- Alman, K.J.; Sadd, C.J.; Reif, M.M.; Kanne, J.P.; Runno, J.R. PH Grand Rounds: An interesting case of sarcoidosis-associated pulmonary hypertension. Adv. Pulm. Hypertens. 2021, 20, 60–65. [Google Scholar]
- Liebow, A.A. The J. Burns Amberson lecture—Pulmonary angiitis and granulomatosis. Am. Rev. Respir. Dis. 1973, 108, 1–18. [Google Scholar]
- Rosen, Y. Four decades of necrotizing sarcoid granulomatosis: What do we know now? Arch. Pathol. Lab. Med. 2015, 139, 252–262. [Google Scholar] [CrossRef]
- Quaden, C.; Tillie-Leblond, I.; Delobbe, A.; Delaunois, L.; Verstraeten, A.S.; Demedts, M.; Wallaert, B. Necrotising sarcoid granulomatosis: Clinical, functional, endoscopical and radiographical evaluations. Eur. Respir. J. 2005, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Dawson, A.; Jenkins, G.H.; Nicholson, A.G.; Hansell, D.M.; Harrison, N.K. Sarcoidosis-related pulmonary veno-occlusive disease presenting with recurrent haemoptysis. Eur. Respir. J. 2009, 34, 517–520. [Google Scholar] [CrossRef]
- Swigris, J.J.; Olson, A.L.; Huie, T.J.; Fernandez-Perez, E.R.; Solomon, J.J.; Sprunger, D.; Brown, K.K. Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007. Chest 2011, 140, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Goljan-Geremek, A.; Geremek, M.; Puscinska, E.; Sliwinski, P. Venous thromboembolism and sarcoidosis: Co-incidence or coexistence? Cent. Eur. J. Immunol. 2015, 40, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Askling, J.; Grunewald, J.; Eklund, A.; Hillerdal, G.; Ekbom, A. Increased risk for cancer following sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.K.; Sværke, C.; Thomsen, R.W.; Nørgaard, M. Sarcoidosis and subsequent cancer risk: A Danish nationwide cohort study. Eur. Respir. J. 2015, 45, 269–272. [Google Scholar] [CrossRef]
- Valeyre, D.; Bernaudin, J.F.; Jeny, F.; Duchemann, B.; Freynet, O.; Planes, C.; Kambouchner, M.; Nunes, H. Pulmonary Sarcoidosis. Clin. Chest Med. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Mana, J.; Rubio-Rivas, M.; Villalba, N.; Marcoval, J.; Iriarte, A.; Molina-Molina, M.; Llatjos, R.; Garcia, O.; Martinzez-Yelamos, S.; Vicens-Zygmunt, C.G.; et al. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: Cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine 2017, 96, e7595. [Google Scholar] [CrossRef]
- Sartwell, P.E.; Edwards, L.B. Epidemiology of sarcoidosis in the U.S. Navy. Am. J. Epidemiol. 1974, 99, 250–257. [Google Scholar] [CrossRef]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calander, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef]
- Grunewald, J.; Eklund, A. Lofgren’s syndrome: Human leukocyte antigen strongly influences the disease course. Am. J. Respir. Crit. Care Med. 2009, 179, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, S.; Lundback, H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Medica Scand. 1952, 142, 265–273. [Google Scholar] [CrossRef]
- Brown, F.; Modi, P.; Tanner, L.S. Lofgren Syndrome. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Pranav Modi declares no relevant financial relationships with ineligible companies. In Disclosure: Laura Tanner Declares No Relevant Financial Relationships with Ineligible Companies; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Iriarte, A.; Rubio-Rivas, M.; Villalba, N.; Corbella, X.; Mañá, J. Clinical features and outcomes of asymptomatic pulmonary sarcoidosis. A comparative cohort study. Respir. Med. 2020, 169, 105998. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, G.; Domvri, K.; Manika, K.; Fouka, E.; Kontakiotis, T.; Papakosta, D. Clinical, imaging and functional determinants of sarcoidosis phenotypes in a Greek population. J. Thorac. Dis. 2022, 14, 1941–1949. [Google Scholar] [CrossRef]
- Judson, M.A.; Preston, S.; Hu, K.; Zhang, R.; Jou, S.; Modi, A.; Sukhu, I.; Ilyas, F.; Rosoklija, R.; Yucel, Y. Quantifying the relationship between symptoms at presentation and the prognosis of sarcoidosis. Respir. Med. 2019, 152, 14–19. [Google Scholar] [CrossRef]
| Cause | Form of Pulmonary Sarcoidosis |
|---|---|
| Endobronchial deposition of granulomas | Active pulmonary sarcoidosis |
| Cough reflex hypersensitivity granulomatous inflammation of airways | Active pulmonary sarcoidosis |
| Bronchospasm from granulomatous inflammation of airways | Active pulmonary sarcoidosis |
| Airway fibrosis from previous granulomatous inflammation of airways | Fibrotic pulmonary sarcoidosis |
| Development of bullous disease/emphysema * | Fibrotic pulmonary sarcoidosis |
| Airway compression from mediastinal lymphadenopathy *,† | mediastinal lymphadenopathy from sarcoidosis |
| Major Category | Mechanism | Mechanism of Dyspnea | Treatment |
|---|---|---|---|
| Intrathoracic deposition of granulomas | Intrapulmonary deposition of granulomas in alveoli/ interstitium | Decreased lung compliance | Anti-granulomatous therapy |
| Intrapulmonary deposition of granulomas in airways | Increased airway resistance | Anti-granulomatous therapy | |
| Asthma/bronchospasm | Increased airway resistance | Anti-granulomatous therapy; anti-asthma therapy | |
| Sarcoidosis-associated pleural effusion (deposition of granulomas in the pleura) * | Decreased lung volume, overdistention of respiratory muscles | Anti-granulomatous therapy | |
| Pulmonary fibrosis | Fibrosis in alveoli/ interstitium | Decreased lung compliance | Anti-fibrotic therapy?; Anti-granulomatous therapy? |
| Fibrosis in airways | Increased airway resistance | Anti-fibrotic therapy?; Anti-granulomatous therapy? | |
| Multiple processes: Intrapulmonary deposition of granulomas, pulmonary fibrosis, hypoxic pulmonary vasoconstriction from parenchymal sarcoidosis | Pulmonary hypertension | Increased pulmonary vascular resistance, hypoxemia | Pulmonary vasodilators; anti-granulomatous therapy? |
| Complications of treatment | Weight gain from corticosteroids | Restrictive ventilatory defect | Weight loss; reduce corticosteroid dose if possible |
| Pulmonary infection | Immunosuppressive medications | Treat the infectious pathogen; reduce immunosuppression if possible | |
| Respiratory muscle weakness | Corticosteroid myopathy | Reduce corticosteroid dose | |
| Ischemic/hypertensive cardiomyopathy | Corticosteroid-induced hypertension/diabetes | Treatment of hypertension/diabetes and ischemic heart disease; reduce corticosteroid dose if possible | |
| Extrapulmonary sarcoidosis | Cardiac sarcoidosis | Cardiomyopathy | Anti-granulomatous therapy for cardiac sarcoidosis |
| Respiratory muscle involvement with sarcoidosis * | Respiratory muscle failure | Anti-granulomatous therapy for respiratory muscle sarcoidosis | |
| Pulmonary embolism | Hypoxemia, increased pulmonary vascular resistance | Anti-granulomatous therapy for respiratory muscle sarcoidosis | |
| Psychological/emotional/physical state associated with sarcoidosis | Multiple mechanisms: depression, fatigue, cognitive impairment | Increase in the sensation of dyspnea | Treat the underlying mechanism |
| Process unrelated to sarcoidosis | Innumerable mechanisms | Innumerable etiologies | Treat the underlying mechanism |
| Cause | Form of Sarcoidosis | Mechanism |
|---|---|---|
| Granulomatous airway lesions | Active pulmonary sarcoidosis (granulomatous inflammation) | Granulomatous necrosis of an airway lesion |
| Bronchiectasis | Fibrocystic sarcoidosis | Bronchiectasis from airway fibrosis from previous granulomatous inflammation. Hemoptysis from infectious bronchitis/bronchiectasis |
| Aspergilloma/Chronic aspergillus lung infection | Fibrocystic sarcoidosis | Aspergillus colonization of devitalized lung with subsequent locally invasive disease |
| Pulmonary hypertension | Many forms of sarcoidosis, most commonly fibrocystic disease | Pulmonary hypertension leads to vascular engorgement and a hemorrhagic diathesis that may be exacerbated by infection, granulomatous inflammation |
| Necrotizing sarcoid granulomatosis | Necrotizing sarcoid granulomatosis—unclear if this is a form of sarcoidosis or a separate disease entity | Parenchymal necrosis |
| Pulmonary embolism | Associated with sarcoidosis epidemiologically | Pulmonary infarction; pulmonary hypertension |
| Lung cancer | Associated with sarcoidosis epidemiologically | Parenchymal/Airway necrosis |
| Pulmonary infection | Associated with immunosuppressive agents used to treat sarcoidosis | Parenchymal/Airway necrosis |
| Hemoptysis not specifically related to sarcoidosis | Not applicable | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judson, M.A. The Symptoms of Pulmonary Sarcoidosis. J. Clin. Med. 2023, 12, 6088. https://doi.org/10.3390/jcm12186088
Judson MA. The Symptoms of Pulmonary Sarcoidosis. Journal of Clinical Medicine. 2023; 12(18):6088. https://doi.org/10.3390/jcm12186088
Chicago/Turabian StyleJudson, Marc A. 2023. "The Symptoms of Pulmonary Sarcoidosis" Journal of Clinical Medicine 12, no. 18: 6088. https://doi.org/10.3390/jcm12186088
APA StyleJudson, M. A. (2023). The Symptoms of Pulmonary Sarcoidosis. Journal of Clinical Medicine, 12(18), 6088. https://doi.org/10.3390/jcm12186088






