Postesophagectomy Diaphragmatic Prolapse after Robot-Assisted Minimally Invasive Esophagectomy (RAMIE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Management of Esophageal Cancer Patients
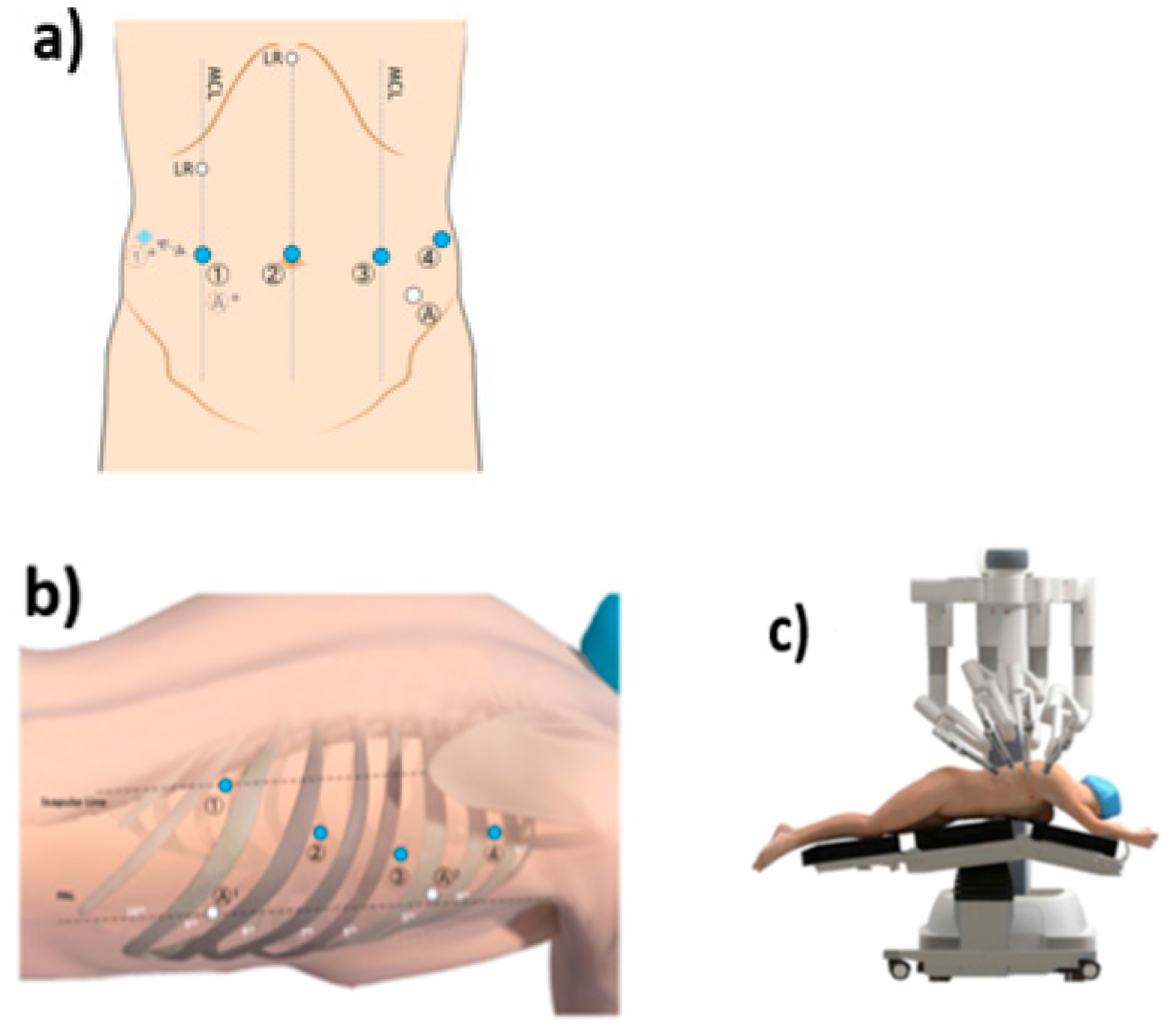
2.3. Technique Description for IL-RAMIE
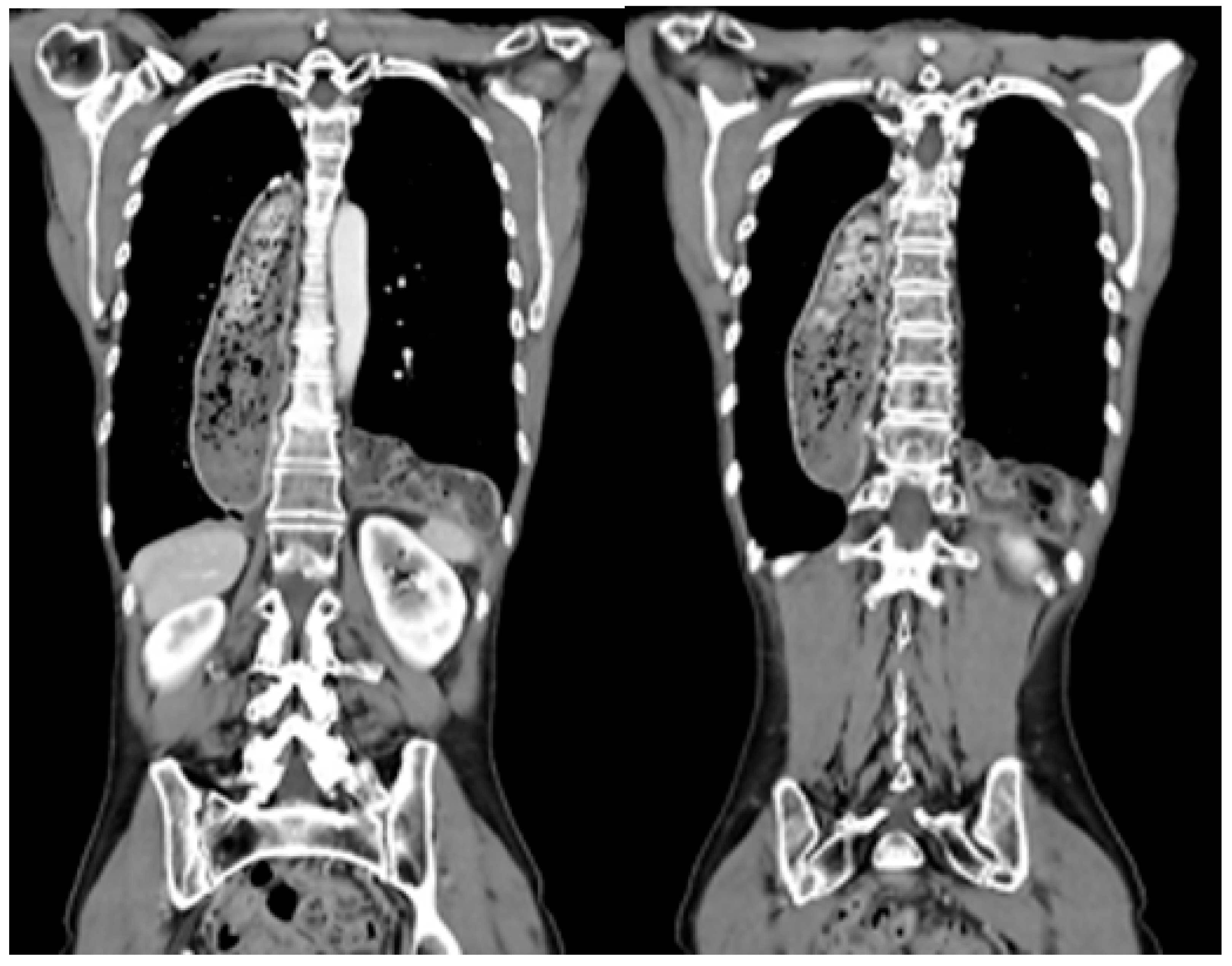
2.4. Management of Patients with Postesophagectomy Diaphragmatic Prolapse
2.5. Statistical Analysis
2.6. Study Outcome
2.7. Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.C.M.; van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; RichMeiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar]
- An, J.Y.; Baik, Y.H.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. The prognosis of gastric cardia cancer after R0 resection. Am. J. Surg. 2010, 199, 725–729. [Google Scholar] [CrossRef]
- Hayashi, T.; Yoshikawa, T. Optimal surgery for esophagogastric junctional cancer. Langenbeck’s Arch. Surg. 2022, 407, 1399–1407. [Google Scholar] [CrossRef]
- Nimptsch, U.; Haist, T.; Krautz, C.; Grützmann, R.; Mansky, T.; Lorenz, D. Hospital Volume, In-Hospital Mortality, and Failure to Rescue in Esophageal Surgery. Dtsch. Arztebl. Int. 2018, 115, 793–800. [Google Scholar] [CrossRef]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Vallböhmer, D.; Hölscher, A.H.; Herbold, T.; Gutschow, C.; Schröder, W. Diaphragmatic hernia after conventional or laparoscopic-assisted transthoracic esophagectomy. Ann. Thorac. Surg. 2007, 84, 1847–1852. [Google Scholar] [CrossRef]
- Fuchs, H.F.; Knepper, L.; Müller, D.T.; Bartella, I.; Bruns, C.J.; Leers, J.M.; Schröder, W. Transdiaphragmatic herniation after transthoracic esophagectomy: An underestimated problem. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2020, 33, doaa024. [Google Scholar] [CrossRef]
- Price, T.N.; Allen, M.S.; Nichols, F.C., 3rd; Cassivi, S.D.; Wigle, D.A.; Shen, K.R.; Deschamps, C. Hiatal hernia after esophagectomy: Analysis of 2,182 esophagectomies from a single institution. Ann. Thorac. Surg. 2011, 92, 2041–2045. [Google Scholar] [CrossRef]
- Hölscher, A.H.; Fetzner, U.K. Paraconduit hiatal hernia after esophagectomy. Prevention—Indication for surgery—Surgical technique. Dis. Esophagus 2021, 34, doab025. [Google Scholar] [CrossRef]
- van der Horst, S.; Weijs, T.J.; Braunius, W.W.; Mook, S.; Mohammed, N.H.; Brosens, L.; van Rossum, P.S.N.; Weusten, B.L.A.M.; Ruurda, J.P.; van Hillegersberg, R. ASO Visual Abstract: Safety and Feasibility of Robot-Assisted Minimally Invasive Esophagectomy (RAMIE) with Three-Field Lymphadenectomy and Neoadjuvant Chemoradiotherapy in Patients with Resectable Esophageal Cancer and Cervical Lymph Node Metastasis. Ann. Surg. Oncol. 2023, 30, 2755–2756. [Google Scholar] [CrossRef]
- Biere, S.S.A.Y.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Szakó, L.; Németh, D.; Farkas, N.; Kiss, S.; Dömötör, R.Z.; Engh, M.A.; Hegyi, P.; Eross, B.; Papp, A. Network meta-analysis of randomized controlled trials on esophagectomies in esophageal cancer: The superiority of minimally invasive surgery. World J. Gastroenterol. 2022, 28, 4201–4210. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.; Mohammad, N.H.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef]
- De Silva, I.; Wee, M.; Cabalag, C.S.; Fong, R.; Tran, K.; Wu, M.; Schloithe, A.; Bright, T.; Duong, C.P.; Watson, D.I. Para-conduit diaphragmatic hernia following esophagectomy-the new price of minimally invasive surgery? Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2023, 36, doad011. [Google Scholar] [CrossRef]
- Lee, A.H.H.; Oo, J.; Cabalag, C.S.; Link, E.; Duong, C.P. Increased risk of diaphragmatic herniation following esophagectomy with a minimally invasive abdominal approach. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2022, 35, doab066. [Google Scholar] [CrossRef]
- Oor, J.E.; Wiezer, M.J.; Hazebroek, E.J. Hiatal Hernia After Open versus Minimally Invasive Esophagectomy: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2016, 23, 2690–2698. [Google Scholar] [CrossRef]
- Benzie, A.L.; Darwish, M.B.; Basta, A.; McLaren, P.J.; Cho, E.E.; Osman, H.; Jay, J.; Jeyarajah, D.R. Hiatal Hernia After Esophagectomy: A Single-Center Retrospective Analysis of a Rare Postoperative Phenomenon. Am. Surg. 2023, 89, 2820–2823. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Porschen, R.; Fischbach, W.; Gockel, I.; Hollerbach, S.; Hölscher, A.; Jansen, P.L.; Miehlke, S.; Pech, O.; Stahl, M.; Thuss-Patience, P.; et al. S3-Leitlinie—Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des ösophagus. Z. Gastroenterol. 2022, 57, 336–418. [Google Scholar]
- Fuchs, H.F.; Müller, D.T.; Leers, J.M.; Schröder, W.; Bruns, C.J. Modular step-up approach to robot-assisted transthoracic esophagectomy—Experience of a German high volume center. Transl. Gastroenterol. Hepatol. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Stier, R.; Straatman, J.; Babic, B.; Schiffmann, L.; Eckhoff, J.; Schmidt, T.; Bruns, C.; Fuchs, H.F. ICG lymph node mapping in cancer surgery of the upper gastrointestinal tract. Chirurgie 2022, 93, 925–933. [Google Scholar] [CrossRef]
- Müller, D.T.; Babic, B.; Herbst, V.; Gebauer, F.; Schlößer, H.; Schiffmann, L.; Chon, S.-H.; Schröder, W.; Bruns, C.J.; Fuchs, H.F. Does Circular Stapler Size in Surgical Management of Esophageal Cancer Affect Anastomotic Leak Rate? 4-Year Experience of a European High-Volume Center. Cancers 2020, 12, 3474. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, H.; Järvinen, T.; Ilonen, I.; Räsänen, J. Paraconduit hernia after minimally invasive esophagectomy–incidence and risk factors. Scand. J. Gastroenterol. 2023, 58, 764–770. [Google Scholar] [CrossRef]
- Hanna, A.N.; Guajardo, I.; Williams, N.; Kucharczuk, J.; Dempsey, D.T. Hiatal Hernia after Esophagectomy: An Underappreciated Complication? J. Am. Coll. Surg. 2020, 230, 700–707. [Google Scholar] [CrossRef]
- Ganeshan, D.M.; Correa, A.M.; Bhosale, P.; Vaporciyan, A.A.; Rice, D.; Mehran, R.J.; Walsh, G.L.; Iyer, R.; Roth, J.A.; Swisher, S.G.; et al. Diaphragmatic hernia after esophagectomy in 440 patients with long-term follow-up. Ann. Thorac. Surg. 2013, 96, 1138–1145. [Google Scholar] [CrossRef]
- Benjamin, G.; Ashfaq, A.; Chang, Y.H.; Harold, K.; Jaroszewski, D. Diaphragmatic hernia post-minimally invasive esophagectomy: A discussion and review of literature. Hernia 2015, 19, 635–643. [Google Scholar] [CrossRef]
- Murad, H.; Huang, B.; Ndegwa, N.; Rouvelas, I.; Klevebro, F. Postoperative hiatal herniation after open vs. minimally invasive esophagectomy; a systematic review and meta-analysis. Int. J. Surg. 2021, 93, 106046. [Google Scholar] [CrossRef]
- Puccetti, F.; Cossu, A.; Parise, P.; Barbieri, L.; Elmore, U.; Carresi, A.; De Pascale, S.; Romario, U.F.; Rosati, R. Diaphragmatic hernia after Ivor Lewis esophagectomy for cancer: A retrospective analysis of risk factors and post-repair outcomes. J. Thorac. Dis. 2021, 13, 160–168. [Google Scholar] [CrossRef]
- Ulloa Severino, B.; Fuks, D.; Christidis, C.; Denet, C.; Gayet, B.; Perniceni, T. Laparoscopic repair of hiatal hernia after minimally invasive esophagectomy. Surg. Endosc. 2016, 30, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Carrera Ceron, R.E.; Oelschlager, B.K. Management of Recurrent Paraesophageal Hernia. J. Laparoendosc. Adv. Surg. Tech. Part A 2022, 32, 1148–1155. [Google Scholar] [CrossRef]
- Messenger, D.E.; Higgs, S.M.; Dwerryhouse, S.J.; Hewin, D.F.; Vipond, M.N.; Barr, H.; Wadley, M.S. Symptomatic diaphragmatic herniation following open and minimally invasive oesophagectomy: Experience from a UK specialist unit. Surg. Endosc. 2015, 29, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kent, M.S.; Luketich, J.D.; Tsai, W.; Churilla, P.; Federle, M.; Landreneau, R.; Alvelo-Rivera, M.; Schuchert, M. Revisional surgery after esophagectomy: An analysis of 43 patients. Ann. Thorac. Surg. 2008, 86, 974–975. [Google Scholar] [CrossRef] [PubMed]
| Variable | N |
|---|---|
| Gender (male) | 9 (81.81%) |
| Age (years) | 56 (43.65%) |
| ASA | |
| I | 3 (27.27%) |
| II | 6 (54.55%) |
| III | 2 (18.18%) |
| BMI (kg/m2) | 25.51 (18–35.35) |
| Preoperative comorbidities | |
| Cardiac | 1 (9.09%) |
| Pulmonary | 2 (18.18%) |
| Vascular | 5 (45.45%) |
| Tumor histology | |
| AC | 10 (90.91%) |
| SCC | 1 (9.09%) |
| Tumor localization | |
| Distal esophagus | 11 (100%) |
| Middle esophagus | 0 (0%) |
| Neoadjuvant treatment | |
| Radiochemotherapy | 7 (63.64%) |
| Chemotherapy | 4 (36.36%) |
| Variable | N |
|---|---|
| Time to diagnosis (days, range) | 241 (49–394) |
| Symptoms | |
| Asymptomatic | 4 (36.36%) |
| Pain and nausea | 4 (36.36%) |
| Acute abdomen | 1 (9.09%) |
| Dysphagia and reflux | 2 (18.18%) |
| Localization | |
| Left hemithorax | 7 (36.64%) |
| Right hemithorax | 1 (9.09%) |
| Bilateral | 3 (27.27%) |
| Prolapsed organs | |
| Colon | 10 (90.91%) |
| Small intestine, colon, pancreas, greater omentum | 1 (9.09%) |
| Variable | N |
|---|---|
| Surgical indication | |
| Emergency surgery | 1 (9%) |
| Elective surgery | 10 (91%) |
| Surgical approach | |
| Open | 1 (9%) |
| Laparoscopic | 9 (82%) |
| Conversion from laparoscopic to open | 1 (9%) |
| Surgical procedure | |
| Hiatoplasty | 11 (100%) |
| Thoracic drainage | 4 (36.4) |
| Diaphragmatic fixation of prolapsed organ | 4 (36.4%) |
| Length of hospital stay (days) | 12 (5–23) |
| In-hospital mortality | 1 (9%) |
| Recurrent PDP | 4 (36%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunner, S.; Müller, D.T.; Eckhoff, J.A.; Lange, V.; Chon, S.-H.; Schmidt, T.; Schröder, W.; Bruns, C.J.; Fuchs, H.F. Postesophagectomy Diaphragmatic Prolapse after Robot-Assisted Minimally Invasive Esophagectomy (RAMIE). J. Clin. Med. 2023, 12, 6046. https://doi.org/10.3390/jcm12186046
Brunner S, Müller DT, Eckhoff JA, Lange V, Chon S-H, Schmidt T, Schröder W, Bruns CJ, Fuchs HF. Postesophagectomy Diaphragmatic Prolapse after Robot-Assisted Minimally Invasive Esophagectomy (RAMIE). Journal of Clinical Medicine. 2023; 12(18):6046. https://doi.org/10.3390/jcm12186046
Chicago/Turabian StyleBrunner, Stefanie, Dolores T. Müller, Jennifer A. Eckhoff, Valentin Lange, Seung-Hun Chon, Thomas Schmidt, Wolfgang Schröder, Christiane J. Bruns, and Hans F. Fuchs. 2023. "Postesophagectomy Diaphragmatic Prolapse after Robot-Assisted Minimally Invasive Esophagectomy (RAMIE)" Journal of Clinical Medicine 12, no. 18: 6046. https://doi.org/10.3390/jcm12186046
APA StyleBrunner, S., Müller, D. T., Eckhoff, J. A., Lange, V., Chon, S.-H., Schmidt, T., Schröder, W., Bruns, C. J., & Fuchs, H. F. (2023). Postesophagectomy Diaphragmatic Prolapse after Robot-Assisted Minimally Invasive Esophagectomy (RAMIE). Journal of Clinical Medicine, 12(18), 6046. https://doi.org/10.3390/jcm12186046










