Valproic Acid-Associated Acute Pancreatitis: Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources—Searches
2.2. Article Selection
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Data Extraction
2.6. Comprehensiveness of Reporting
2.7. Analysis
3. Results
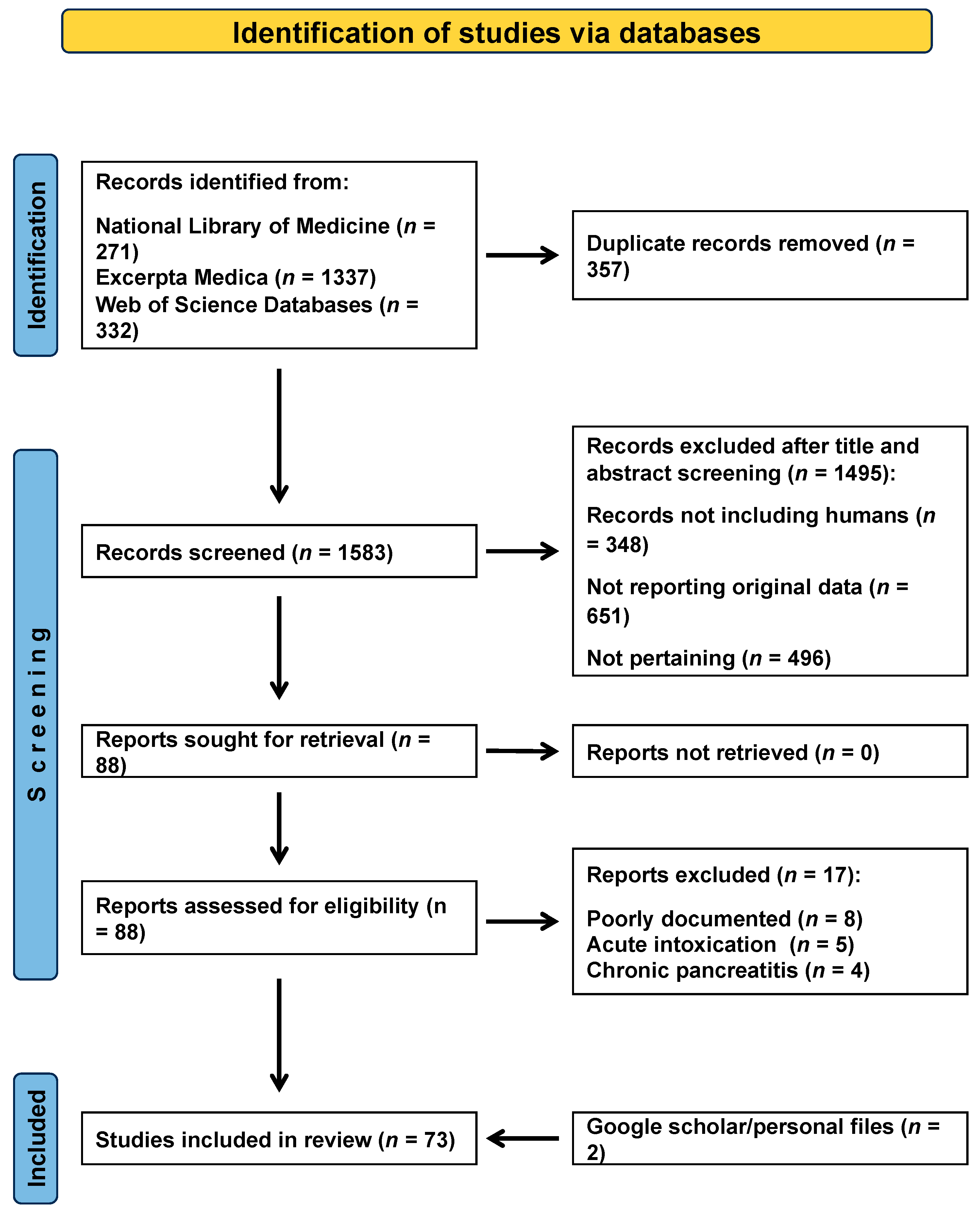
3.1. Search Output
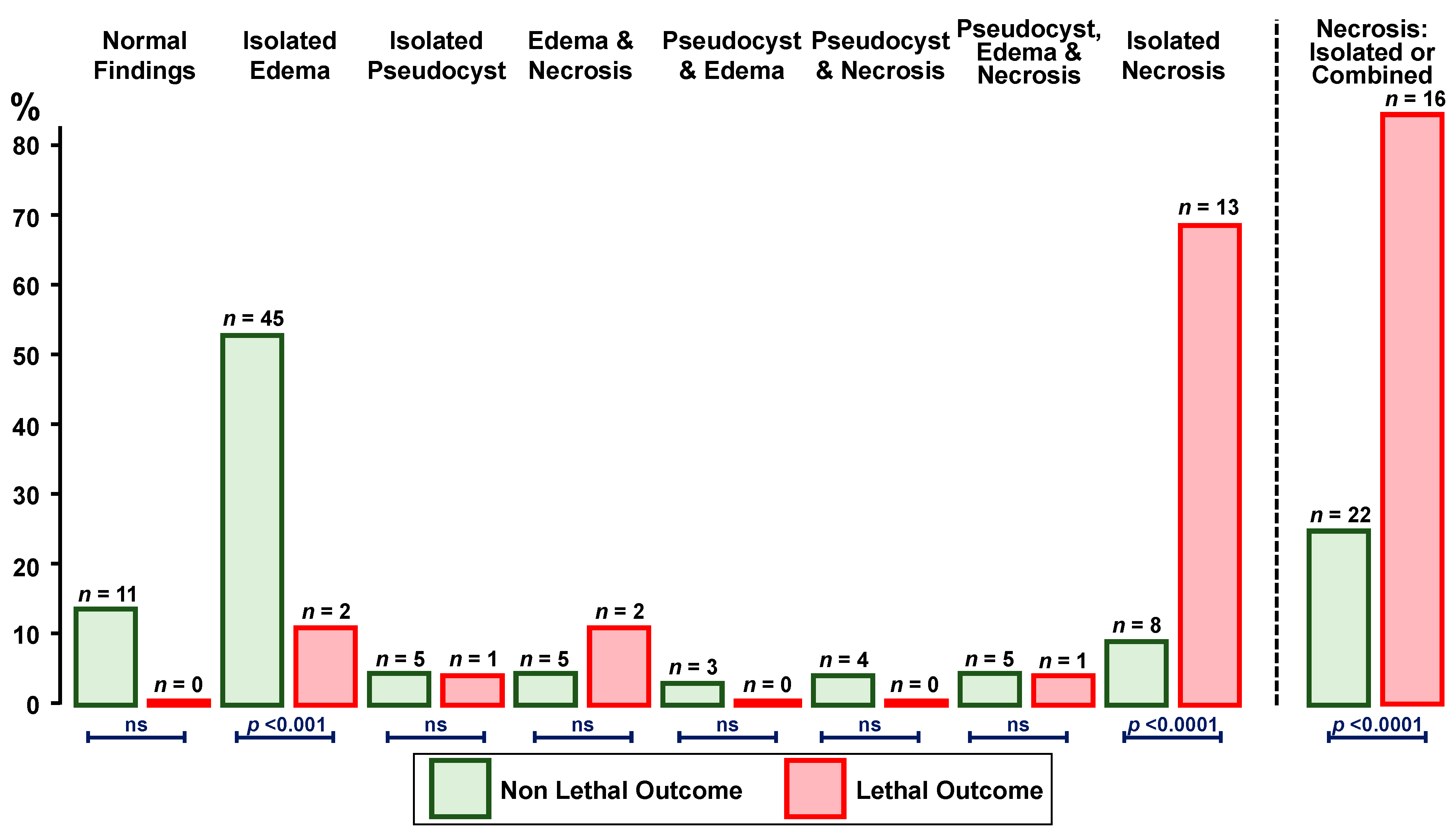
3.2. Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardner, T.B. Acute pancreatitis. Ann. Intern. Med. 2021, 174, ITC17–ITC32. [Google Scholar] [CrossRef] [PubMed]
- Walkowska, J.; Zielinska, N.; Karauda, P.; Tubbs, R.S.; Kurtys, K.; Olewnik, Ł. The pancreas and known factors of acute pancreatitis. J Clin. Med. 2022, 11, 5565. [Google Scholar] [CrossRef] [PubMed]
- Graf, G.; Vassalli, G.A.M.; Kottanattu, L.; Bianchetti, M.G.; Agostoni, C.; Milani, G.P.; Lava, S.A.G.; Faré, P.B.; Janett, S. Acute pancreatitis associated with atypical bacterial pneumonia: Systematic literature review. J. Clin. Med. 2022, 11, 7248. [Google Scholar] [CrossRef]
- Barakat, M.T.; Abu-El-Haija, M.; Husain, S.Z. Clinical insights into drug-associated pancreatic injury. Curr. Opin. Gastroenterol. 2022, 38, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.M.; Naunton, M. Valproate: A simple chemical with so much to offer. J. Clin. Pharm. Ther. 2005, 30, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wartman, C.; VandenBerg, A. Valproate: Not all boxed warnings are created equal. Ann. Pharmacother. 2022, 56, 1349–1355. [Google Scholar] [CrossRef]
- Anguissola, G.; Leu, D.; Simonetti, G.D.; Simonetti, B.G.; Lava, S.A.G.; Milani, G.P.; Bianchetti, M.G.; Scoglio, M. Kidney tubular injury induced by valproic acid: Systematic literature review. Pediatr. Nephrol. 2023, 38, 1725–1731. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The role of Google Scholar in evidence reviews and its applicability to grey literature searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef]
- Papageorgiou, G.; Grant, S.W.; Takkenberg, J.J.M.; Mokhles, M.M. Statistical primer: How to deal with missing data in scientific research? Interact. Cardiovasc. Thorac. Surg. 2018, 27, 153–158. [Google Scholar] [CrossRef]
- Moses, L.E.; Emerson, J.D.; Hosseini, H. Analyzing data from ordered categories. N. Engl. J. Med. 1984, 311, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.W.; Hayden, G.F. Nonparametric methods. Clinical applications. Clin. Pediatr. 1985, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Batalden, P.B.; Van Dyne, B.J.; Cloyd, J. Pancreatitis associated with valproic acid therapy. Pediatrics 1979, 64, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Camfield, P.R.; Bagnell, P.; Camfield, C.S.; Tibbles, J.A. Pancreatitis due to valproic acid. Lancet 1979, 313, 1198–1199. [Google Scholar] [CrossRef]
- Coulter, D.L.; Allen, R.J. Pancreatitis associated with valproic acid therapy for epilepsy. Ann. Neurol. 1980, 7, 92. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Criée, C.P. Akute Pankreatitis unter antikonvulsiver Therapie mit Natriumvalproinat (Ergenyl®) [Acute pancreatitis during anticonvulsant therapy using sodium valproinate (Ergenyl®)]. Dtsch. Med. Wochenschr. 1980, 105, 905. [Google Scholar]
- Sasaki, M.; Tonoda, S.; Aoki, Y.; Katsumi, M. Pancreatitis due to valproic acid. Lancet 1980, 315, 1196. [Google Scholar] [CrossRef]
- Murphy, M.J.; Lyon, I.W.; Taylor, J.W.; Mitts, G. Valproic acid associated pancreatitis in an adult. Lancet 1981, 317, 41–42. [Google Scholar] [CrossRef]
- Parker, P.H.; Helinek, G.L.; Ghishan, F.K.; Greene, H.L. Recurrent pancreatitis induced by valproic acid. A case report and review of the literature. Gastroenterology 1981, 80, 826–828. [Google Scholar] [CrossRef]
- Ng, J.Y.; Disney, A.P.; Jones, T.E.; Purdie, G. Acute pancreatitis and sodium valproate. Med. J. Aust. 1982, 2, 362. [Google Scholar] [CrossRef]
- Williams, L.H.; Reynolds, R.P.; Emery, J.L. Pancreatitis during sodium valproate treatment. Arch. Dis. Child. 1983, 58, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Baskies, A.M. Case report: Pancreatic pseudocyst associated with valproic acid therapy. J. Med. Soc. N. J. 1984, 81, 399–400. [Google Scholar] [PubMed]
- Wyllie, E.; Wyllie, R.; Cruse, R.P.; Erenberg, G.; Rothner, A.D. Pancreatitis associated with valproic acid therapy. Am. J. Dis. Child. 1984, 138, 912–914. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.G.; Bassett, M.L.; Searle, J.; Tyrer, J.H.; Eadie, M.J. Valproate hepatotoxicity: A review and report of two instances in adults. Clin. Exp. Neurol. 1985, 21, 79–91. [Google Scholar]
- Rosenberg, H.K.; Ortega, W. Hemorrhagic pancreatitis in a young child following valproic acid therapy. Clinical and ultrasonic assessment. Clin. Pediatr. 1987, 26, 98–101. [Google Scholar] [CrossRef]
- Boudailliez, B.; André, J.L.; Broyer, M.; Davin, J.C.; Landthaler, G.; Palcoux, J.B. Acute pancreatitis in six non-transplanted uraemic children. A co-operative study from the French Society of Paediatric Nephrology. Pediatr. Nephrol. 1988, 2, 431–435. [Google Scholar] [CrossRef]
- Scheffner, D.; König, S.; Rauterberg-Ruland, I.; Kochen, W.; Hofmann, W.J.; Unkelbach, S. Fatal liver failure in 16 children with valproate therapy. Epilepsia 1988, 29, 530–542. [Google Scholar] [CrossRef]
- Bouget, J.; Deugnier, Y.; Camus, C.; Thoreux, P.H.; Letulzo, Y.; Thomas, R.; Ramée, M.P. Acide valproïque: Association d’une hépatite aiguë mortelle et d’une pancréatite [Valproic acid: Association of a fatal acute hepatitis and pancreatitis]. Ann. Med. Interne 1990, 141, 491–493. [Google Scholar]
- Ford, D.M.; Portman, R.J.; Lum, G.M. Pancreatitis in children on chronic dialysis treated with valproic acid. Pediatr. Nephrol. 1990, 4, 259–261. [Google Scholar] [CrossRef]
- Lott, J.A.; Bond, L.W.; Bobo, R.C.; McClung, H.J.; Murray, R.D. Valproic acid-associated pancreatitis: Report of three cases and a brief review. Clin. Chem. 1990, 36, 395–397. [Google Scholar] [CrossRef]
- Binek, J.; Hany, A.; Heer, M. Valproic-acid-induced pancreatitis. Case report and review of the literature. J. Clin. Gastroenterol. 1991, 13, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kayemba Kay’s Kabangu, S.; Bovier Lapierre, M.; Jalaguier, E. Pancréatite aiguë et acide valproïque [Acute pancreatitis and valproic acid]. Pédiatrie 1991, 46, 839–843. [Google Scholar] [PubMed]
- Rose, E.; de Miscault, G.; Thome, M.; Boussard, N. Pancréatite aiguë au valproate de sodium. Revue de la littérature à propos d’un cas chez l’enfant [Acute pancreatitis caused by sodium valproate. Review of the literature apropos of a case in a child]. Pédiatrie 1991, 46, 831–837. [Google Scholar] [PubMed]
- Ayoola, E.A.; Dahmash, N.S.; Ajarim, D.; Al-Murgairin, S.M. Delayed multiple toxic reactions possibly related to valproate therapy in a Saudi patient. Ann. Saudi Med. 1994, 14, 163–164. [Google Scholar] [CrossRef][Green Version]
- Croizet, O.; Louvel, D.; Teulière, J.P.; Buscail, L.; Escourrou, J.; Frexinos, J. Pancréatite aiguë induite par l’acide valproïque [Acute pancreatitis induced by valproic acid]. Gastroenterol. Clin. Biol. 1994, 18, 910–911. [Google Scholar]
- Talwar, D. Valproate-associated acute pancreatitis in a child with neuronal ceroid lipofuscinosis. J. Child. Neurol. 1994, 9, 36–37. [Google Scholar] [CrossRef]
- Buzan, R.D.; Firestone, D.; Thomas, M.; Dubovsky, S.L. Valproate-associated pancreatitis and cholecystitis in six mentally retarded adults. J. Clin. Psychiatry 1995, 56, 529–532. [Google Scholar]
- Engelmann, M.D.; Henriksen, S.D.; Tingsgaard, L.K. Letal pancreatitis associeret med valproatbehandling [Fatal pancreatitis associated with valproate therapy]. Ugeskr. Laeger. 1995, 157, 4357–4358. [Google Scholar]
- Evans, R.J.; Miranda, R.N.; Jordan, J.; Krolikowski, F.J. Fatal acute pancreatitis caused by valproic acid. Am. J. Forensic. Med. Pathol. 1995, 16, 62–65. [Google Scholar] [CrossRef]
- Otusbo, S.; Huruzono, T.; Kobae, H.; Yoshimi, S.; Miyata, K. Pancreatitis with normal serum amylase associated with sodium valproate: A case report. Brain Dev. 1995, 17, 219–221. [Google Scholar] [CrossRef]
- Tobias, J.D.; Capers, C.; Sims, P.; Holcomb, G.W., 3rd. Necrotizing pancreatitis after 10 years of therapy with valproic acid. Clin. Pediatr. 1995, 34, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Zenker, M.; Metzker, M.; Wegener, E.; Heidemann, P.H. Valproat-induzierte Pankreatitis. Monatsschr. Kinderheilkd. 1995, 143, 843–846. [Google Scholar]
- Bahamonde Carrasco, A.; Morán Blanco, A.; Olcoz Goñi, J.L. Pancreatitis aguda por ácido valproico: A propósito de un caso [Acute pancreatitis caused by valproic acid: Apropos a case]. Gastroenterol. Hepatol. 1996, 19, 253–254. [Google Scholar]
- Levin, T.L.; Berdon, W.E.; Seigle, R.R.; Nash, M.A. Valproic-acid-associated pancreatitis and hepatic toxicity in children with endstage renal disease. Pediatr. Radiol. 1997, 27, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.F. Severe acute necrotising pancreatitis caused by sodium valproate: A case report. Crit. Care Resusc. 1999, 1, 366–367. [Google Scholar] [PubMed]
- Fecik, S.E.; Stoner, S.C.; Raphael, J.; Lindsey, C. Recurrent acute pancreatitis associated with valproic acid use for mood stabilization. J. Clin. Psychopharmacol. 1999, 19, 483–484. [Google Scholar] [CrossRef]
- Moreiras Plaza, M.; Rodríguez Goyanes, G.; Cuiña, L.; Alonso, R. On the toxicity of valproic-acid. Clin. Nephrol. 1999, 51, 187–189. [Google Scholar]
- Munhoz, R.P.; dos Santos, M.L.; Hernández-Fustes, O.J. Pancreatite necro-hemorrágica fatal associada ao uso de valproato de sódio: Relato de caso [Fatal necro-hemorrhagic pancreatitis related to sodium valproate: Case report]. Arq. Neuropsiquiatr. 2001, 59, 821–823. [Google Scholar] [CrossRef]
- Vaca CZHarris, P.D.; Barriga, F.C.; Castillo, A.M.; Mesa, T.L.; García, C.B.; Varela, C. Pancreatitis aguda grave y pseudoquiste pancreático por uso de drogas en niños. Presentación de tres casos clínicos y revisión de la literatura [Severe acute pancreatitis and pancreatic pseudocyst formation caused by drugs in children. Presentation of three clinical cases and review of the literature]. Rev. Chil. Pediatr. 2001, 72, 235–243. [Google Scholar] [CrossRef]
- Battillocchi, B.; Diana, M.; Dandolo, R.; Stefanini, S.; D’Amore, L.; Negro, P. Pancreatite acuta da farmaci: Contributo personale [Drug-induced acute pancreatitis: A personal contribution]. Chir. Ital. 2002, 54, 605–612. [Google Scholar]
- Mileusnic, D.; Donoghue, E.R.; Lifschultz, B.D. Pathological case of the month: Sudden death in a child as a result of pancreatitis during valproic acid therapy. Pediatr. Pathol. Mol. Med. 2002, 21, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, K.; Lippmann, M.; Gala, I. Fatal pancreatitis associated with valproic acid: Review of the literature. Medicine 2002, 81, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Grauso-Eby, N.L.; Goldfarb, O.; Feldman-Winter, L.B.; McAbee, G.N. Acute pancreatitis in children from valproic acid: Case series and review. Pediatr. Neurol. 2003, 28, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Queizán, A.; Hernández, F.; Rivas, S. Pancreatic pseudocyst caused by valproic acid: Case report and review of the literature. Eur. J. Pediatr. Surg. 2003, 13, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Laghate, V.D.; Gupta, S.B. Acute pancreatitis and diabetic ketoacidosis in non-diabetic person while on treatment with sodium valproate, chlorpromazine and haloperidol. J. Assoc. Physicians. India 2004, 52, 257–258. [Google Scholar]
- Sinclair, D.B.; Berg, M.; Breault, R. Valproic acid-induced pancreatitis in childhood epilepsy: Case series and review. J. Child Neurol. 2004, 19, 498–502. [Google Scholar] [CrossRef]
- Stojanović, M.; Zivanović, D.; Madić, J.; Karadzić, D.; Stanojević, G.; Jovanović, M.; Jeremić Lj Stojanović, M. Akutni pankreatitis kod deteta uzrokovan Na-valproatom [An acute pancreatitis in a child caused by Na-valproate]. Acta Chir. Iugosl. 2004, 51, 125–127. [Google Scholar] [CrossRef]
- Yoshikawa, H. The difficulties of diagnosing VPA-induced pancreatitis in children with severe motor and intellectual disabilities. Eur. J. Paediatr. Neurol. 2004, 8, 109–110. [Google Scholar] [CrossRef]
- Houben, M.L.; Wilting, I.; Stroink, H.; van Dijken, P.J. Pancreatitis, complicated by a pancreatic pseudocyst associated with the use of valproic acid. Eur. J. Paediatr. Neurol. 2005, 9, 77–80. [Google Scholar] [CrossRef]
- Barreda, L.; Rosas, J.; Milian, W.; Valdivia, D.; Targarona, J. Valproato de sodio como causa de pancreatitis aguda: Reporte de un caso [Sodium valproate as a cause of acute pancreatitis: A case report]. Rev. Gastroenterol. Peru 2006, 26, 318–323. [Google Scholar]
- Gerstner, T.; Büsing, D.; Bell, N.; Longin, E.; Kasper, J.M.; Klostermann, W.; Hebing, B.; Hanefeld, F.; Eckel, U.; Hoffmann, R.; et al. Valproic acid-induced pancreatitis: 16 new cases and a review of the literature. J. Gastroenterol. 2007, 42, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Özaydin, E.; Yükselgüngör, H.; Köse, G. Acute hemorrhagic pancreatitis due to the use of valproic acid in a child. Eur. J. Paediatr. Neurol. 2008, 12, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Campos, J.; González-Guevara, L.; Vacaro-Bolívar, I.; Rojas, J.M. Acute pancreatitis associated to the use of valproic acid. Arq. Neuropsiquiatr. 2009, 67, 513–515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dos Santos, B.L.; Fernandes, R.M.; Neves, F.F. Valproic acid-induced pancreatitis in an adult. Arq. Neuropsiquiatr. 2010, 68, 135–136. [Google Scholar] [CrossRef]
- Dinopoulos, A.; Karapanou, O.; Alexopoulou, E.; Tzetis, M.; Attilakos, A.; Fretzayas, A. VPA-induced recurrent pancreatitis in a cystic fibrosis carrier. Eur. J. Paediatr. Neurol. 2011, 15, 453–455. [Google Scholar] [CrossRef]
- Ali, M.F.; Loh, K.Y. Sodium valproate induced necrotising pancreatitis: A case report. Malays Fam. Physician 2013, 8, 28–30. [Google Scholar]
- Capolongo, G.; Zacchia, M.; Pollastro, R.M.; Radice, L.; Anastasio, P. A case of valproic acid-induced acute pancreatitis in tuberous sclerosis coexisting with end-stage renal disease. J. Nephrol. 2013, 26, 412–416. [Google Scholar] [CrossRef][Green Version]
- Jomli, R.; Nacef, F.; Douki, S. Pancréatite aiguë induite par l’acide valproïque [Acute pancreatitis induced by valproic acid]. Encephale 2013, 39, 292–295. [Google Scholar] [CrossRef]
- Veri, K.; Uibo, O.; Talvik, I.; Talvik, T. Valproic acid-induced pancreatitis in a 15-year-old boy with juvenile myoclonic epilepsy. Medicina 2013, 49, 487–489. [Google Scholar] [CrossRef]
- Yaman, A.; Kendirli, T.; Odek, C.; Bektaş, O.; Kuloğlu, Z.; Koloğlu, M.; Ince, E.; Deda, G. Valproic acid-induced acute pancreatitis and multiorgan failure in a child. Pediatr. Emerg. Care 2013, 29, 659–661. [Google Scholar] [CrossRef]
- Barman, B.; Kalotia, N.; Ete, T. Valproic acid induced pancreatitis: A case report. Int. J. Res. Med. Sci. 2014, 2, 1765–1767. [Google Scholar] [CrossRef]
- Okayasu, H.; Shinozaki, T.; Osone, A.; Ozeki, Y.; Shimoda, K. Development of acute pancreatitis caused by sodium valproate in a patient with bipolar disorder on hemodialysis for chronic renal failure: A case report. BMC Psychiatry 2014, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Cofini, M.; Quadrozzi, F.; Favoriti, P.; Favoriti, M.; Cofini, G. Valproic acid-induced acute pancreatitis in pediatric age: Case series and review of literature. G. Chir. 2015, 36, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Khamrui, S.; Kataria, M.; Biswas, J.; Saha, S. Valproic acid-induced severe acute pancreatitis with pseudocyst formation: Report of a case. Cureus 2015, 7, e297. [Google Scholar] [CrossRef] [PubMed]
- Atam, V.; Singh, J.; Agrawal, K.; Dinkar, A.; Atam, I. A case report of valproate-induced acute pancreatitis. J. Med. Soc. 2017, 31, 48–49. [Google Scholar] [CrossRef]
- Saeed, W.; Standish-Parkin, L.; Gupta, N.; Mendez, M. Drug induced acute pancreatitis in an adolescent with seizure disorder and autism. Res. J. Clin. Pediatr. 2017, 1, 1000106. [Google Scholar]
- Quan, W.; Shao, Q.; Zhang, H.; Liu, F.H.; Zhang, X.H. Acute pancreatitis associated with valproate treatment. Chin. Med. J. 2018, 131, 1889–1890. [Google Scholar] [CrossRef]
- Barbosa, S.C.; Cabrera, P.; Guerra, B.; Roman, C.F. Valproic acid induced necrohemorragic pancreatitis: Case report and diagnostic approach in uncommon pancreatitis. Int. J. Surg. Case Rep. 2019, 60, 126–129. [Google Scholar] [CrossRef]
- Imam, E.A.; Idrees, A.; Ibrahim, M.I.M.S. Valproic acid induced pancreatitis in an Arab male. J. Pharmacol. Pharmacother. 2019, 10, 38–41. [Google Scholar] [CrossRef]
- Jain, A.; Haque, I.; Tayal, V.; Roy, V. Valproic acid-induced acute pancreatitis. Indian J. Psychiatry 2019, 61, 421–422. [Google Scholar] [CrossRef]
- Huang, W.; Ren, X.; Shen, F.; Xing, B. Sodium valproate induced acute pancreatitis in a bipolar disorder patient: A case report. BMC Pharmacol. Toxicol. 2019, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, P.C.; Autmizguine, J.; Major, P.; Kleiber, N. Valproic acid induced pancreatitis presenting with decreased level of consciousness in a child with tuberous sclerosis complex. J. Pediatr. Pharmacol. Ther. 2020, 25, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska-Kamieniak, A.; Krawiec, P.; Pac-Kożuchowska, E. Acute pancreatitis as a complication of antiepileptic treatment: Case series and review of the literature. Pediatr. Rep. 2021, 13, 98–103. [Google Scholar] [CrossRef]
- Chauhan, V.; Sharma, M.; Kapur, A.; Garg, G.K. Valproate induced acute pancreatitis—A unique case report. Curr. Drug Saf. 2022, 17, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, M.; Okamoto, N.; Hashimoto, R.; Igata, R.; Konishi, Y.; Ikenouchi, A.; Isomoto, N.; Shinkai, T.; Harada, M.; Yoshimura, R. Acute pancreatitis during valproic acid administration in a patient with vascular dementia, epileptic seizures, and psychiatric symptoms: A case report. J. Med. Case Rep. 2023, 17, 221. [Google Scholar] [CrossRef]
- Tomson, T.; Battino, D.; Perucca, E. Valproic acid after five decades of use in epilepsy: Time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016, 15, 210–218. [Google Scholar] [CrossRef]
- Kelley, R.I. The role of carnitine supplementation in valproic acid therapy. Pediatrics 1994, 93, 891–892. [Google Scholar] [CrossRef]
- Hall, A.M.; Trepiccione, F.; Unwin, R.J. Drug toxicity in the proximal tubule: New models, methods and mechanisms. Pediatr. Nephrol. 2022, 37, 973–982. [Google Scholar] [CrossRef]
- Chapman, S.A.; Wacksman, G.P.; Patterson, B.D. Pancreatitis associated with valproic acid: A review of the literature. Pharmacotherapy 2001, 21, 1549–1560. [Google Scholar] [CrossRef]
- Patel, J.; Berezowski, I.; Mazer-Amirshahi, M.; Frasure, S.E.; Tran, Q.K.; Pourmand, A. Valproic acid overdose: Case report and literature review. J. Emerg. Med. 2022, 63, 651–655. [Google Scholar] [CrossRef]
- Taira, N.; Nishi, H.; Mano, M.; Waki, N.; Tsugita, Y.; Takashima, S.; Fukuda, K.; Komatsubara, S. Pancreatitis induced by valproic acid: Report of a case. Surg. Today 2001, 31, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
| Outcome | ||||
|---|---|---|---|---|
| All Patients | Non-Lethal | Lethal | p-Value | |
| n (%) | 125 | 105 (84%) | 20 (15%) | N.A. |
| Males, n (%) | 83 (66%) | 72 (69%) | 11 (55%) | ns |
| Age | ||||
| years | 13 (7.0–21) | 12 (7.0–19) | 14 (7.1–28) | ns |
| ≤16 years, n (%) | 83 (66) | 70 (67) | 13 (65) | ns |
| Underlying condition, n | 121 | 102 | 19 | ns |
| Epilepsy, n (%) | 112 (93) | 94 (92) | 18 (95) | ns |
| Bipolar disorder, n (%) | 7 (5.7) | 6 (5.8) | 1 (5.3) | ns |
| Migraine headache, n (%) | 2 (1.7) | 2 (2.0) | 0 | ns |
| Intellectual disability, n (%) | 49 (39) | 42 (40) | 7 (35) | ns |
| Further antiepileptic co-medication, n (%) | 54 (43) | 43 (41) | 11 (55) | ns |
| Co-existing chronic kidney disease, n (%) | 14 (11) | 10 (9.5) | 4 (20) | ns |
| Duration of treatment with valproic acid, n | 99 | 80 | 19 | |
| months | 11 (3.0–24) | 11 (3.5–28) | 11 (2.9–21) | ns |
| 4–8 weeks, n (%) | 6 (6.1) | 4 (5.0) | 2 (11) | ns |
| 9–12 weeks, n (%) | 22 (22) | 17 (21) | 5 (26) | |
| >3–12 months, n (%) | 28 (28) | 24 (30) | 4 (21) | |
| >12–24 months, n (%) | 18 (18) | 14 (18) | 4 (21) | |
| >24 months, n (%) | 25 (25) | 21 (26) | 4 (21) | |
| Valproic acid dosage #, n | 87 | 73 | 14 | |
| mg/kg daily | 30 (21–45) | 30 (20–43) | 40 (30–48) | ns |
| ≥50 mg/kg daily, n (%) | 15 (17) | 12 (16) | 3 (21) | ns |
| Valproic acid blood level ∆, n | 71 | 61 | 10 | |
| µmol/L | 520 (411–593) | 520 (436–598) | 420 (245–531) | ns |
| ≥900 µmol/L, n (%) | 2 (2.8) | 2 (3.3) | 0 | ns |
| Pathologically altered liver enzymes *, n (%) | 14 (11) | 10 (9.5) | 4 (20) | ns |
| Recurrent valproic acid-associated pancreatitis, n (%) | 16 (13) | 14 (16) | 2 (11) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischof, M.C.M.; Stadelmann, M.I.E.; Janett, S.; Bianchetti, M.G.; Camozzi, P.; Goeggel Simonetti, B.; Lava, S.A.G.; Milani, G.P. Valproic Acid-Associated Acute Pancreatitis: Systematic Literature Review. J. Clin. Med. 2023, 12, 6044. https://doi.org/10.3390/jcm12186044
Bischof MCM, Stadelmann MIE, Janett S, Bianchetti MG, Camozzi P, Goeggel Simonetti B, Lava SAG, Milani GP. Valproic Acid-Associated Acute Pancreatitis: Systematic Literature Review. Journal of Clinical Medicine. 2023; 12(18):6044. https://doi.org/10.3390/jcm12186044
Chicago/Turabian StyleBischof, Monica C. M., Mariana I. E. Stadelmann, Simone Janett, Mario G. Bianchetti, Pietro Camozzi, Barbara Goeggel Simonetti, Sebastiano A. G. Lava, and Gregorio P. Milani. 2023. "Valproic Acid-Associated Acute Pancreatitis: Systematic Literature Review" Journal of Clinical Medicine 12, no. 18: 6044. https://doi.org/10.3390/jcm12186044
APA StyleBischof, M. C. M., Stadelmann, M. I. E., Janett, S., Bianchetti, M. G., Camozzi, P., Goeggel Simonetti, B., Lava, S. A. G., & Milani, G. P. (2023). Valproic Acid-Associated Acute Pancreatitis: Systematic Literature Review. Journal of Clinical Medicine, 12(18), 6044. https://doi.org/10.3390/jcm12186044









