Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
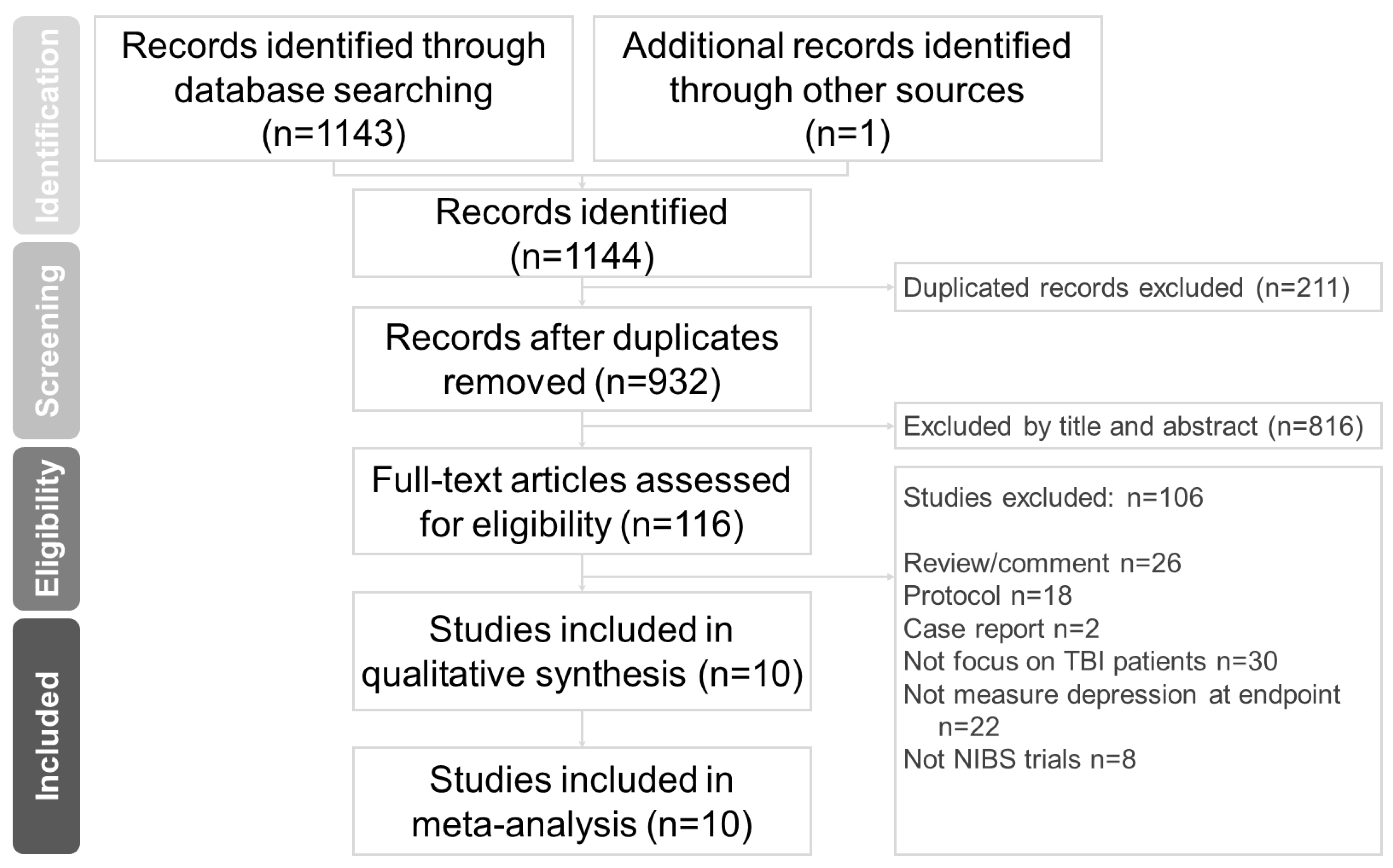
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
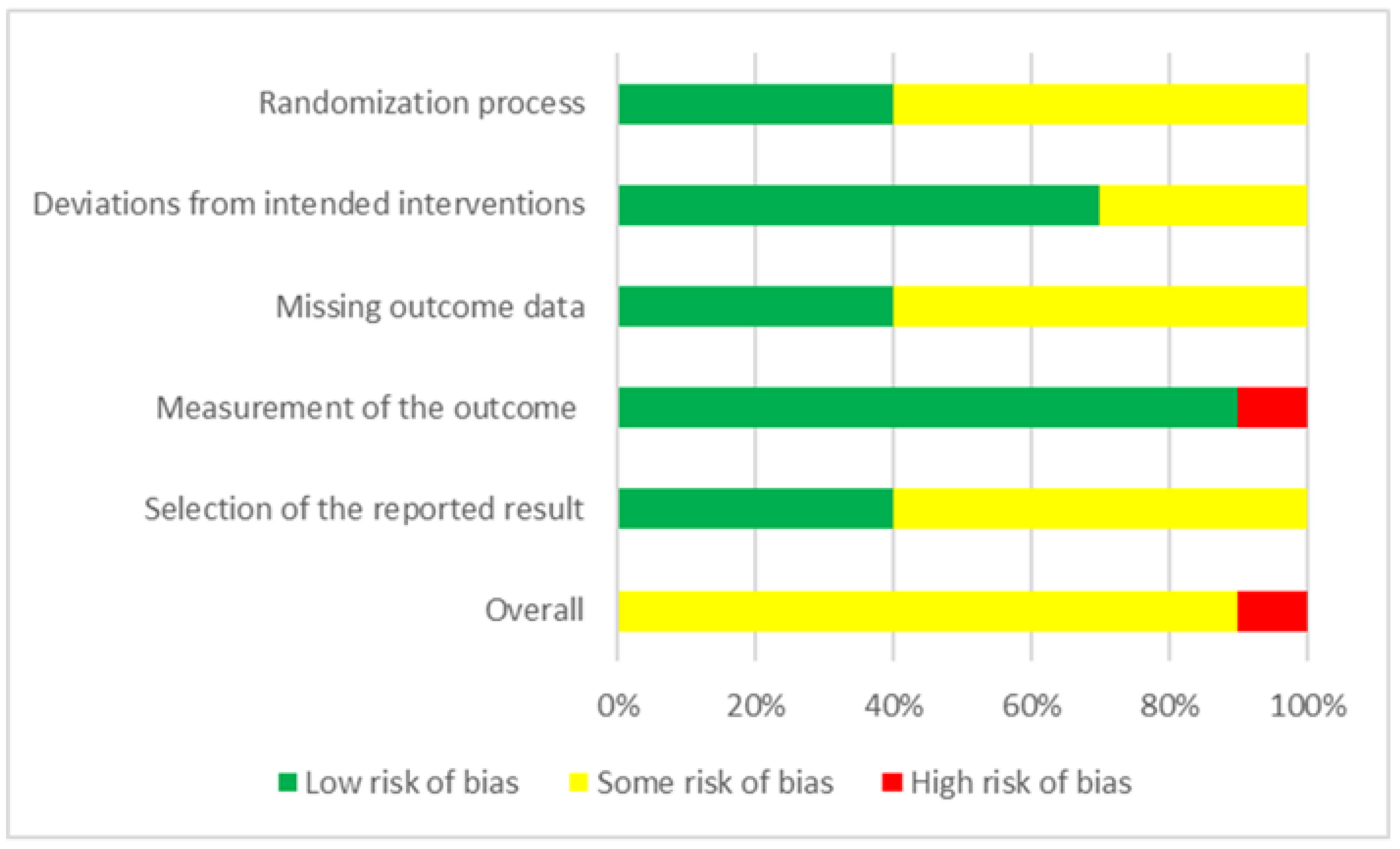
2.4. Methodological Quality Appraisal
2.5. Primary Outcome (Changes in Depression Scores)
2.6. Secondary Outcomes (Adverse Effect Rates, Seizures, and Suicide)
2.7. Data Integration and Statistical Evaluation
3. Results
3.1. Characteristics of the Included Studies
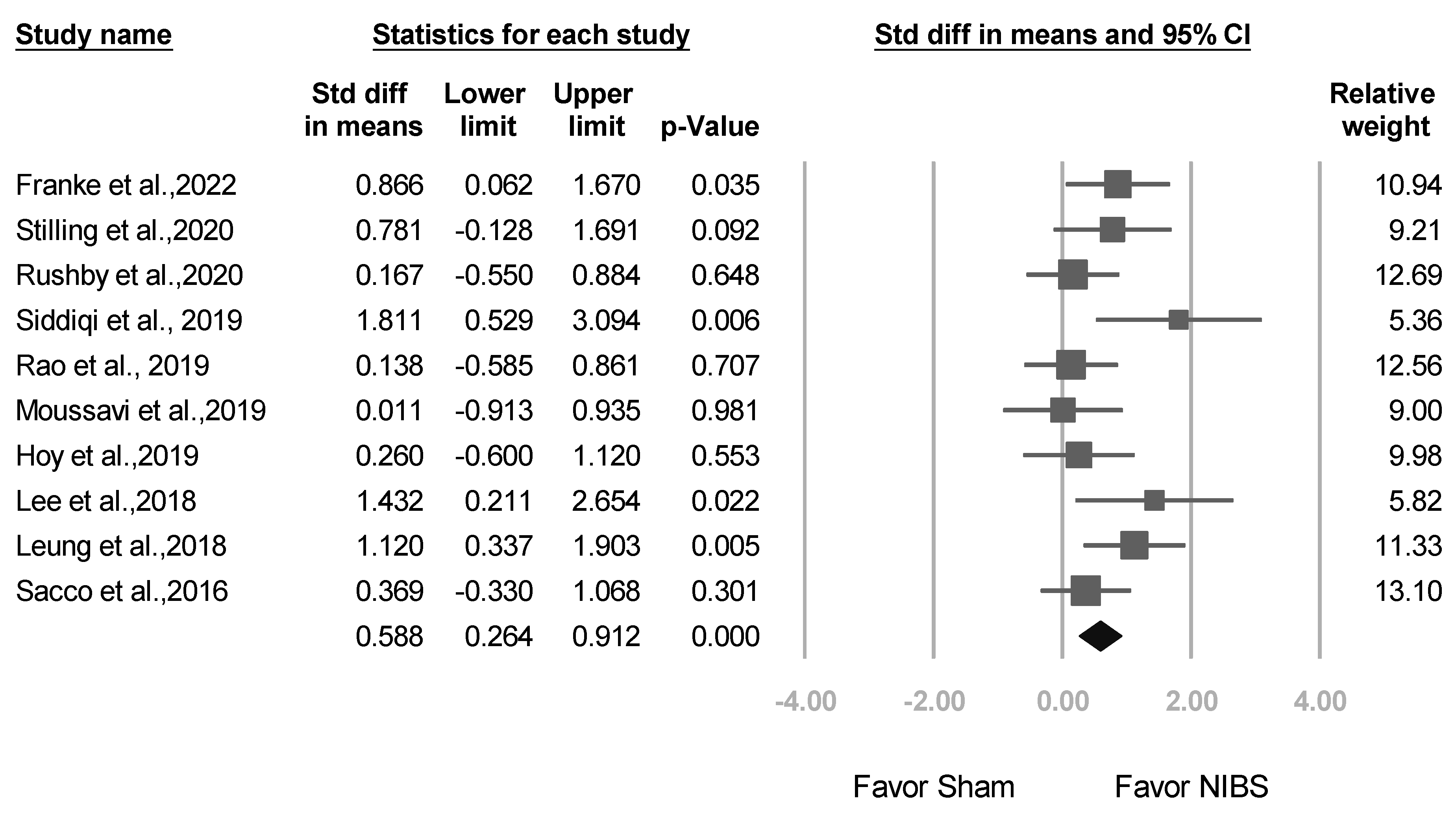
3.2. Meta-Analysis Results of the Overall Effects of NIBS
3.3. Meta-Analysis Results of Studies Stratified by Different Factors
3.3.1. Studies Stratified by NIBS Type
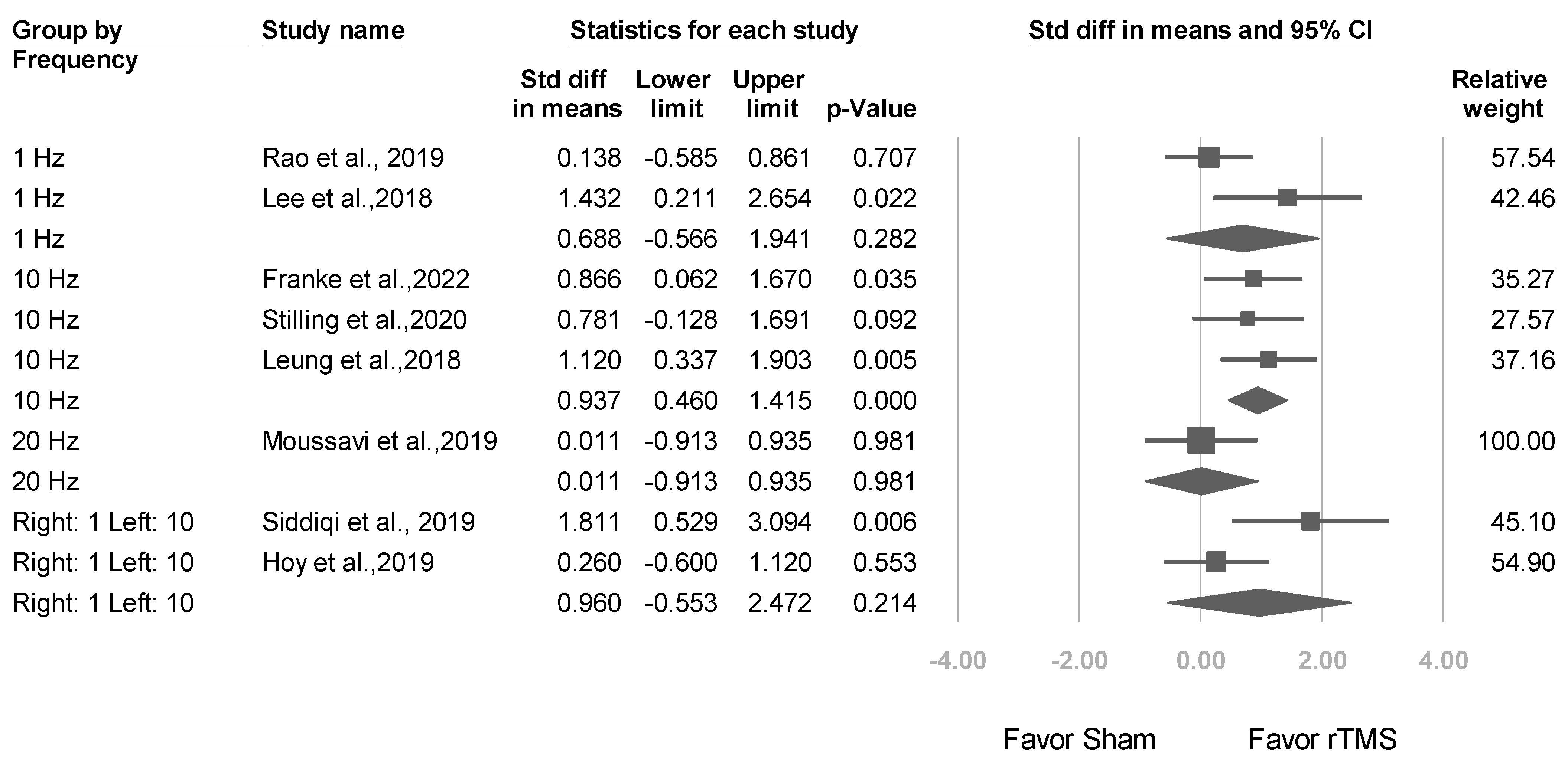
3.3.2. Studies Stratified by Stimulation Frequency
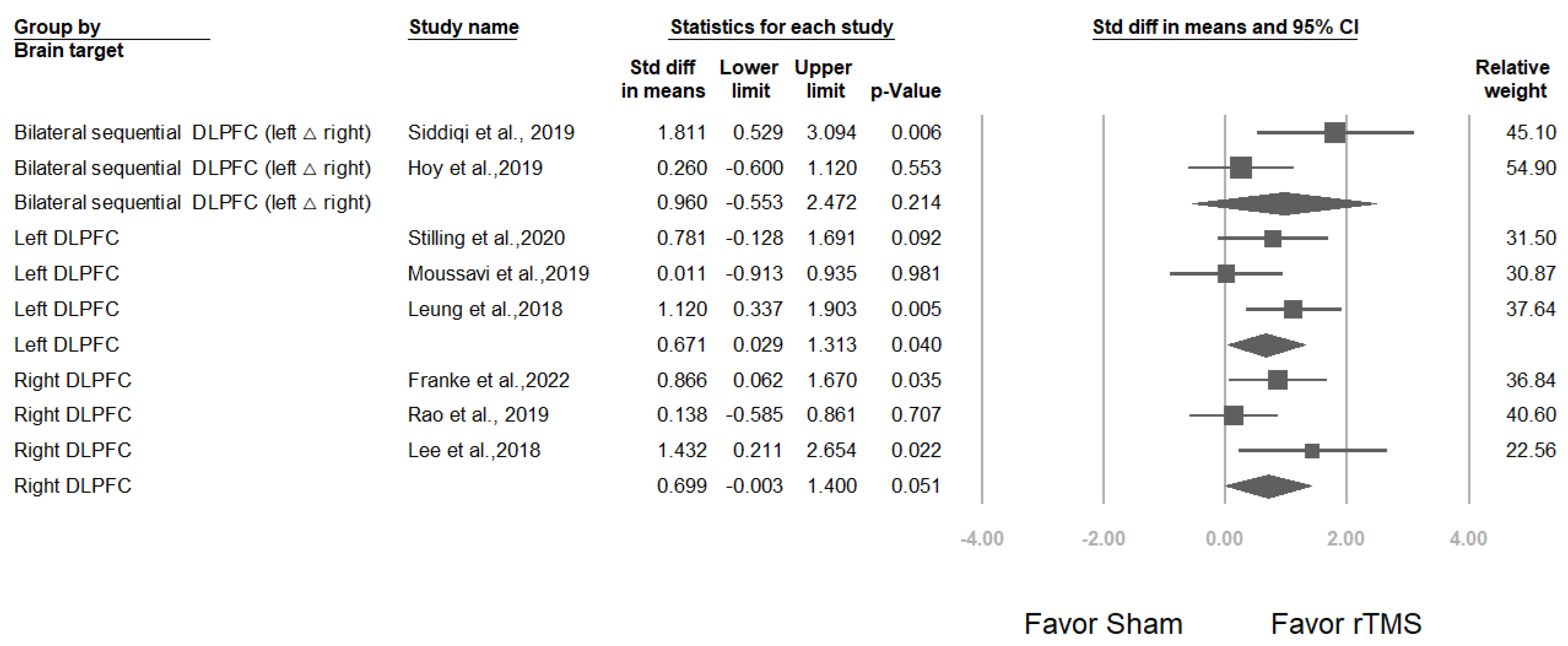
3.3.3. Studies Stratified by Brain Target
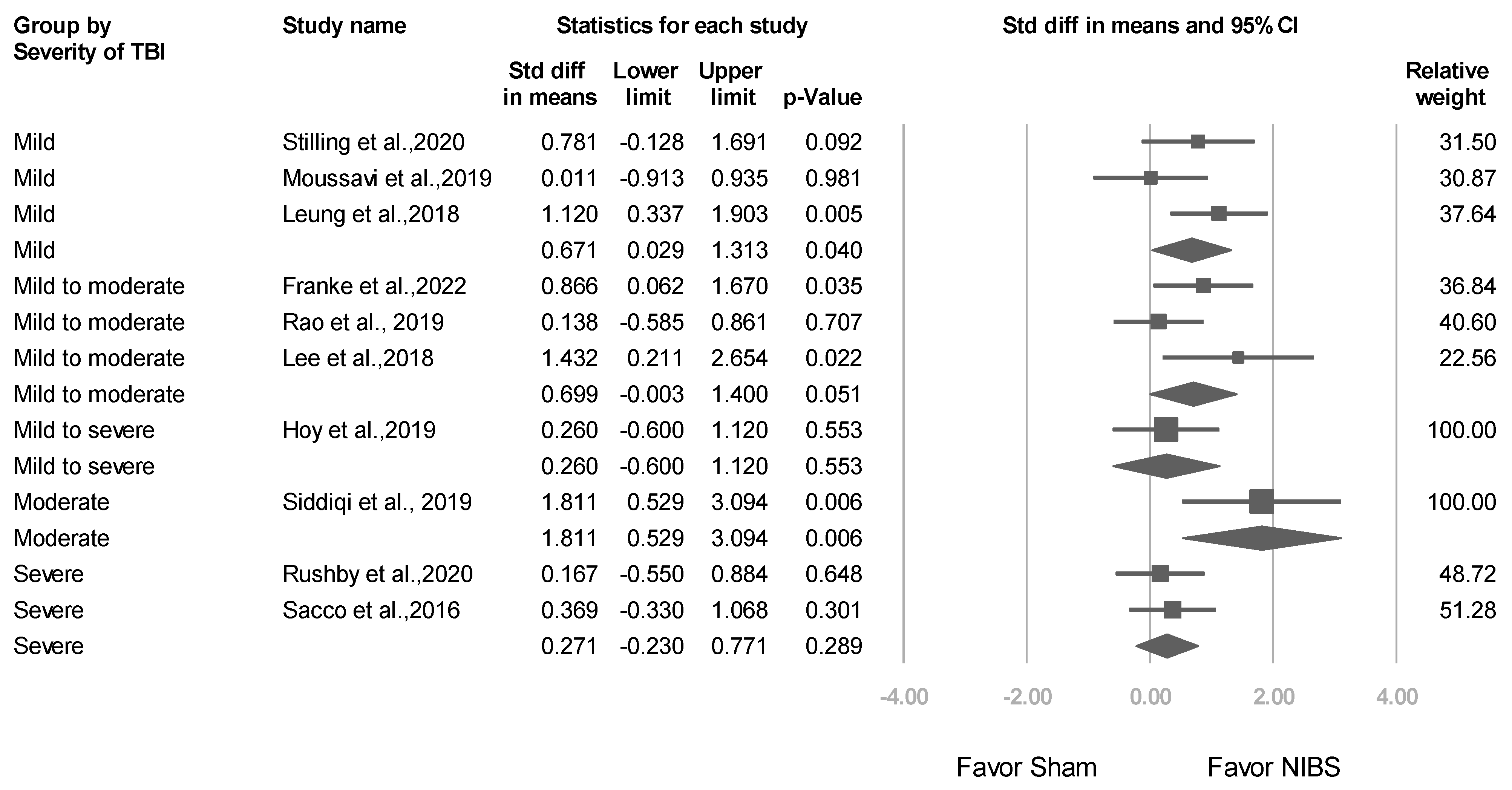
3.3.4. Studies Stratified by Baseline TBI Severity
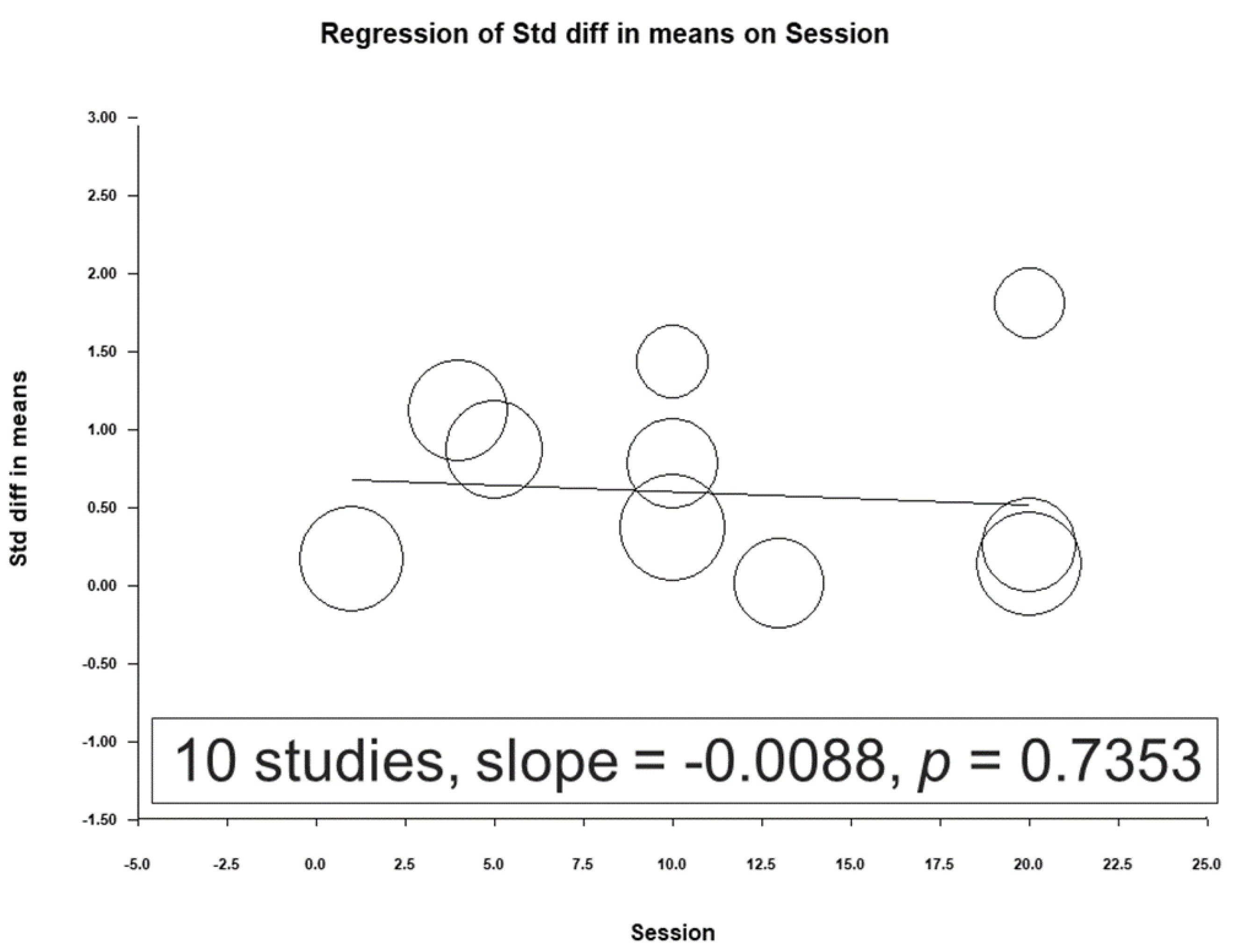
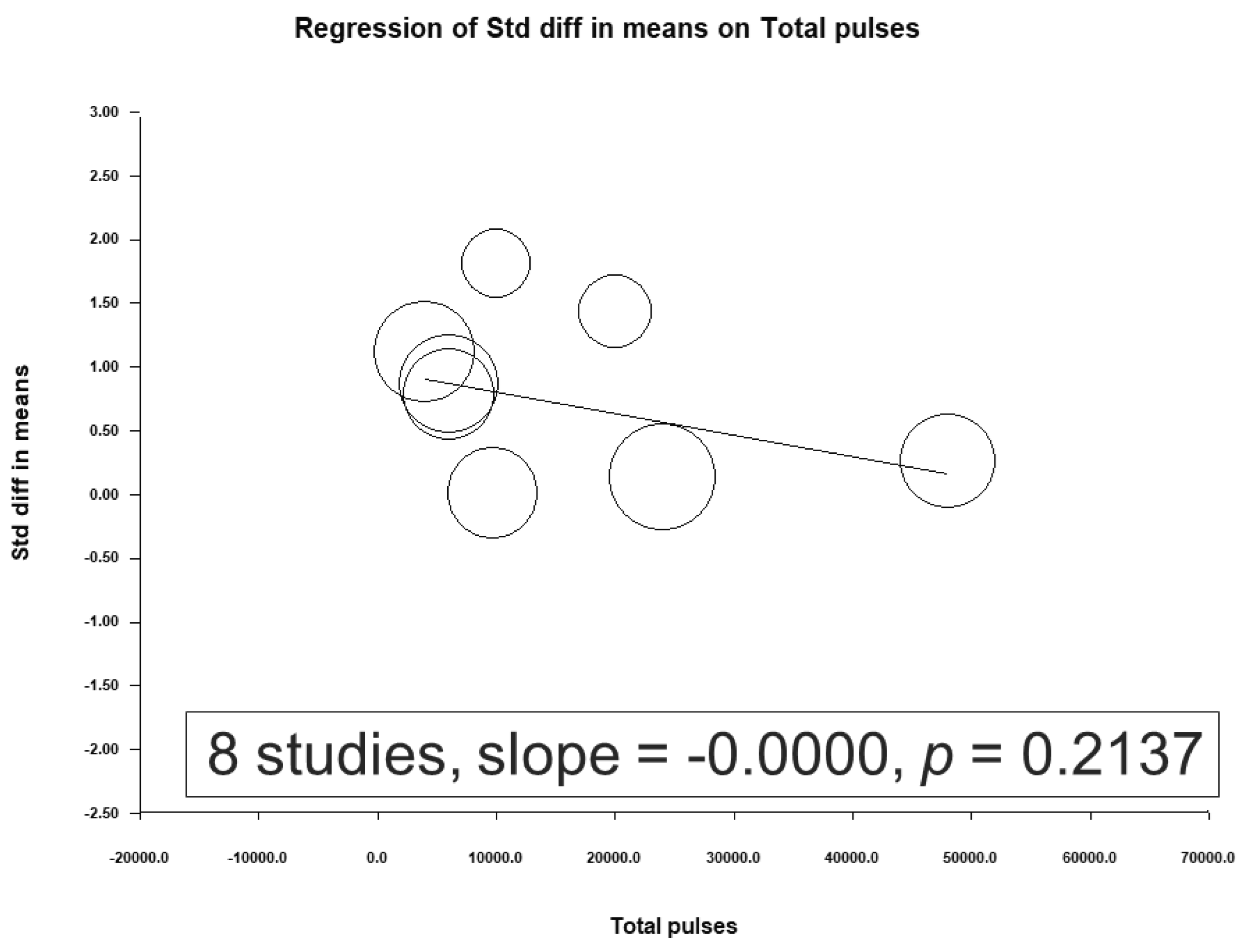
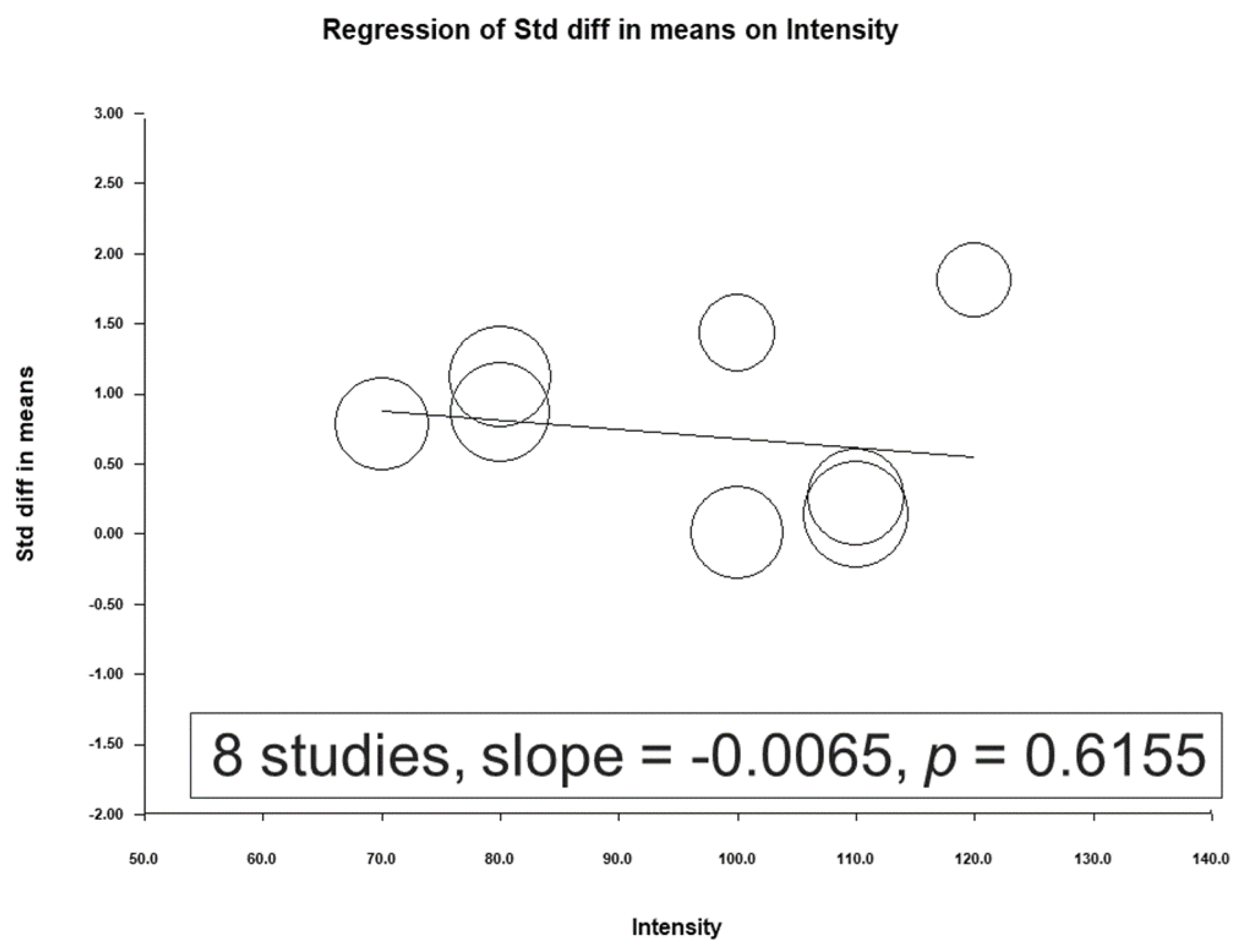
3.4. Meta-Regression Analysis Results
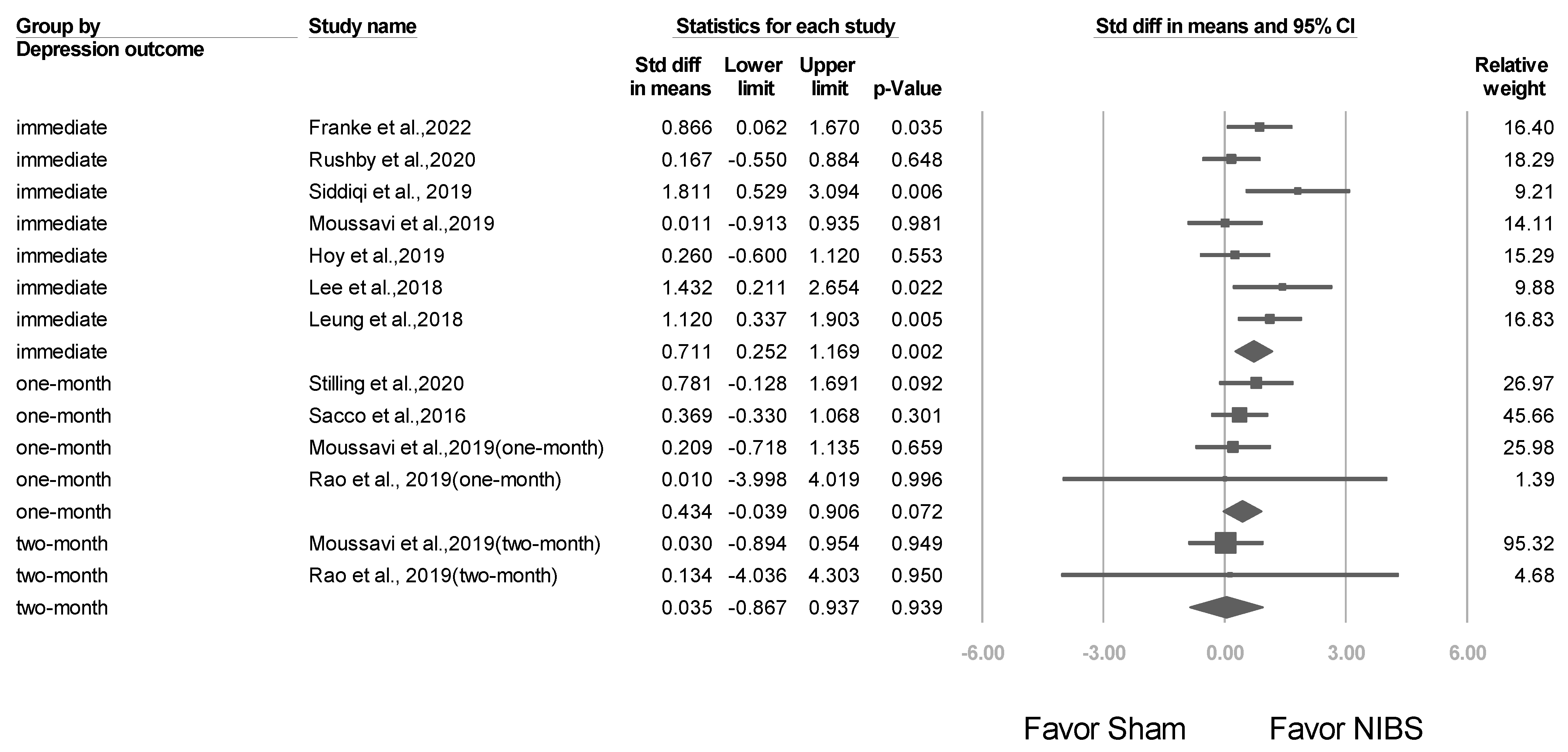
3.5. Short- and Long-Term Effects after NIBS Treatment
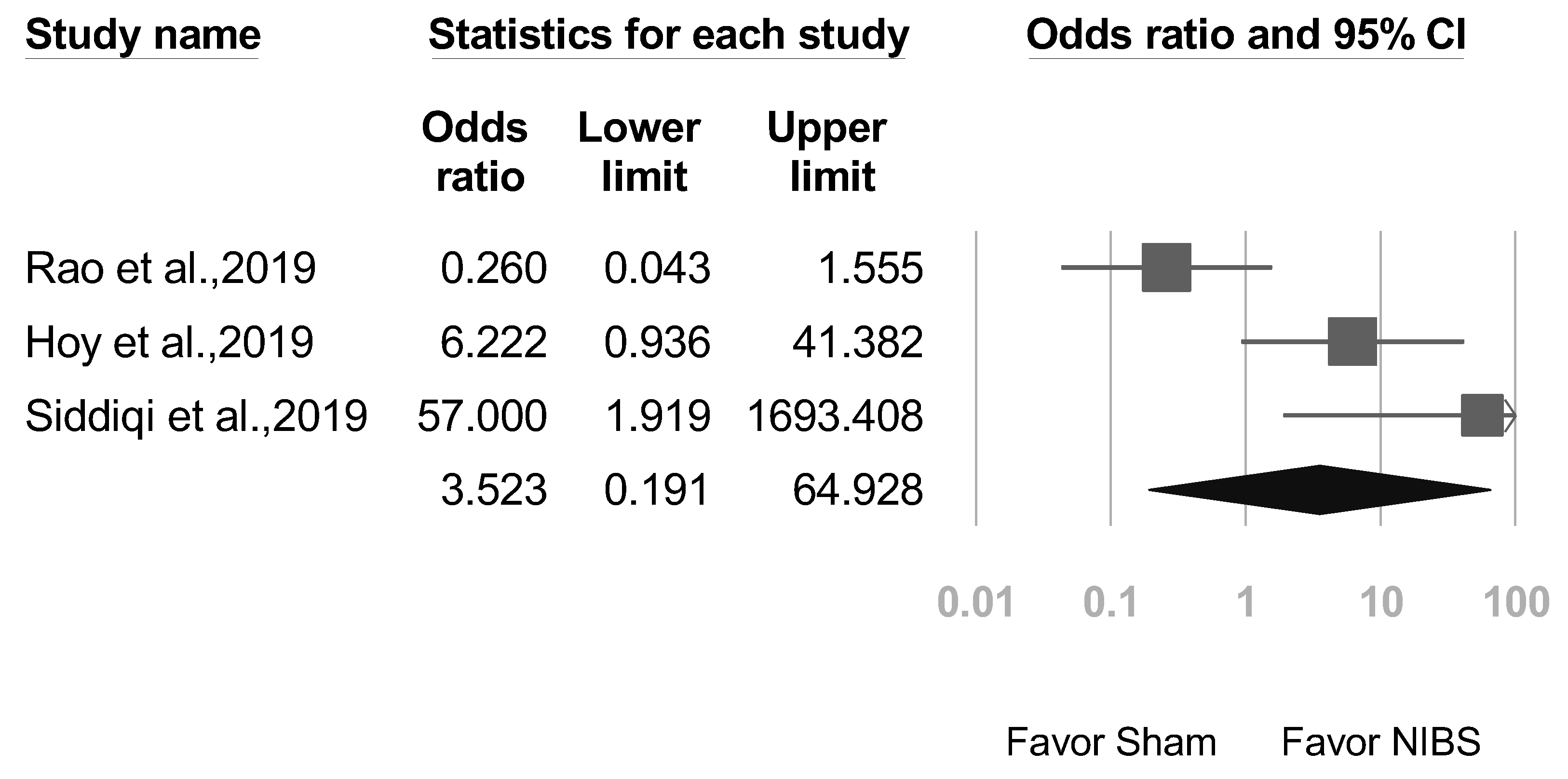
3.6. Secondary Outcomes: Adverse Event Rates, Seizures, and Suicide
3.7. Heterogeneity and Publication Bias
3.8. Sensitivity Analysis Results
4. Discussion
4.1. Comparison with Previous Meta-Analyses
4.2. High-Frequency vs. Low-Frequency rTMS Efficacy
4.3. Brain Targets in NIBS Treatment Studies
4.4. Factors Influencing Clinical Outcomes in TBI Patients
4.5. Possible Pathophysiological Mechanisms
4.6. Safety and Tolerability of NIBS for TBI Patients
4.7. Strengths
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Akerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- Nguyen, R.; Fiest, K.M.; McChesney, J.; Kwon, C.S.; Jette, N.; Frolkis, A.D.; Atta, C.; Mah, S.; Dhaliwal, H.; Reid, A.; et al. The International Incidence of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016, 43, 774–785. [Google Scholar] [CrossRef]
- Bryant, R.A.; O’Donnell, M.L.; Creamer, M.; McFarlane, A.C.; Clark, C.R.; Silove, D. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 2010, 167, 312–320. [Google Scholar] [CrossRef]
- Chen, F.; Chi, J.; Niu, F.; Gao, Q.; Mei, F.; Zhao, L.; Hu, K.; Zhao, B.; Ma, B. Prevalence of suicidal ideation and suicide attempt among patients with traumatic brain injury: A meta-analysis. J. Affect. Disord. 2022, 300, 349–357. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mrudula, K.; Sreepada, S.S.; Sathyaprabha, T.N.; Pal, P.K.; Chen, R.; Udupa, K. An Overview of Noninvasive Brain Stimulation: Basic Principles and Clinical Applications. Can. J. Neurol. Sci. 2022, 49, 479–492. [Google Scholar] [CrossRef]
- O’Leary, G.H.; Jenkins, D.D.; Coker-Bolt, P.; George, M.S.; Kautz, S.; Bikson, M.; Gillick, B.T.; Badran, B.W. From adults to pediatrics: A review noninvasive brain stimulation (NIBS) to facilitate recovery from brain injury. Prog. Brain Res. 2021, 264, 287–322. [Google Scholar] [CrossRef]
- Chou, P.H.; Lin, Y.F.; Lu, M.K.; Chang, H.A.; Chu, C.S.; Chang, W.H.; Kishimoto, T.; Sack, A.T.; Su, K.P. Personalization of Repetitive Transcranial Magnetic Stimulation for the Treatment of Major Depressive Disorder According to the Existing Psychiatric Comorbidity. Clin. Psychopharmacol. Neurosci. 2021, 19, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipovic, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Sandrini, M.; Umilta, C.; Rusconi, E. The use of transcranial magnetic stimulation in cognitive neuroscience: A new synthesis of methodological issues. Neurosci. Biobehav. Rev. 2011, 35, 516–536. [Google Scholar] [CrossRef]
- Jannati, A.; Oberman, L.M.; Rotenberg, A.; Pascual-Leone, A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 2023, 48, 191–208. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Lisanby, S.H.; Sackeim, H.A. Transcranial Magnetic Stimulation: Applications in Neuropsychiatry. Arch. Gen. Psychiatry 1999, 56, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Keck, M.E. Transcranial magnetic stimulation as a therapeutic tool in psychiatry: What do we know about the neurobiological mechanisms? J. Psychiatr. Res. 2001, 35, 193–215. [Google Scholar] [CrossRef]
- George, M.S.; Nahas, Z.; Kozel, F.A.; Li, X.; Denslow, S.; Yamanaka, K.; Mishory, A.; Foust, M.J.; Bohning, D.E. Mechanisms and State of the Art of Transcranial Magnetic Stimulation. J. ECT 2002, 18, 170–181. [Google Scholar] [CrossRef]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2017, 10, 51–58. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.H.; Trapp, N.T.; Hacker, C.D.; Laumann, T.O.; Kandala, S.; Hong, X.; Trillo, L.; Shahim, P.; Leuthardt, E.C.; Carter, A.R.; et al. Repetitive Transcranial Magnetic Stimulation with Resting-State Network Targeting for Treatment-Resistant Depression in Traumatic Brain Injury: A Randomized, Controlled, Double-Blinded Pilot Study. J. Neurotrauma 2019, 36, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, M.K. Effect of Low Frequency Repetitive Transcranial Magnetic Stimulation on Depression and Cognition of Patients with Traumatic Brain Injury: A Randomized Controlled Trial. Med. Sci. Monit. 2018, 24, 8789–8794. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Metzger-Smith, V.; He, Y.; Cordero, J.; Ehlert, B.; Song, D.; Lin, L.; Shahrokh, G.; Tsai, A.; Vaninetti, M.; et al. Left Dorsolateral Prefrontal Cortex rTMS in Alleviating MTBI Related Headaches and Depressive Symptoms. Neuromodulation 2018, 21, 390–401. [Google Scholar] [CrossRef]
- Franke, L.M.; Gitchel, G.T.; Perera, R.A.; Hadimani, R.L.; Holloway, K.L.; Walker, W.C. Randomized trial of rTMS in traumatic brain injury: Improved subjective neurobehavioral symptoms and increases in EEG delta activity. Brain Inj. 2022, 36, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Hoy, K.E.; McQueen, S.; Elliot, D.; Herring, S.E.; Maller, J.J.; Fitzgerald, P.B. A Pilot Investigation of Repetitive Transcranial Magnetic Stimulation for Post-Traumatic Brain Injury Depression: Safety, Tolerability, and Efficacy. J. Neurotrauma 2019, 36, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Galetto, V.; Dimitri, D.; Geda, E.; Perotti, F.; Zettin, M.; Geminiani, G.C. Concomitant Use of Transcranial Direct Current Stimulation and Computer-Assisted Training for the Rehabilitation of Attention in Traumatic Brain Injured Patients: Behavioral and Neuroimaging Results. Front. Behav. Neurosci. 2016, 10, 57. [Google Scholar] [CrossRef]
- Beedham, W.; Belli, A.; Ingaralingam, S.; Haque, S.; Upthegrove, R. The management of depression following traumatic brain injury: A systematic review with meta-analysis. Brain Inj. 2020, 34, 1287–1304. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Chen, Y.C.; Wang, J.Y.; Chung, K.H.; Lai, C.H. Effect of repetitive transcranial magnetic stimulation on depression and cognition in individuals with traumatic brain injury: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16940. [Google Scholar] [CrossRef]
- Gertler, P.; Tate, R.L.; Cameron, I.D. Non-pharmacological interventions for depression in adults and children with traumatic brain injury. Cochrane Database Syst. Rev. 2015, 2015, CD009871. [Google Scholar] [CrossRef]
- Annegers, J.F.; Coan, S.P. The risks of epilepsy after traumatic brain injury. Seizure 2000, 9, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Wang, S.S.; Zhang, M.M.; Wang, J.W.; Hu, J.B.; Huang, M.L.; Wei, N.; Zhou, W.H.; Qi, H.L.; Xu, W.J.; et al. Repetitive transcranial magnetic stimulation-induced seizure of a patient with adolescent-onset depression: A case report and literature review. J. Int. Med. Res. 2011, 39, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Rushby, J.A.; De Blasio, F.M.; Logan, J.A.; Wearne, T.; Kornfeld, E.; Wilson, E.J.; Loo, C.; Martin, D.; McDonald, S. tDCS effects on task-related activation and working memory performance in traumatic brain injury: A within group randomized controlled trial. Neuropsychol. Rehabil. 2021, 31, 814–836. [Google Scholar] [CrossRef]
- Ahorsu, D.K.; Adjaottor, E.S.; Lam, B.Y.H. Intervention Effect of Non-Invasive Brain Stimulation on Cognitive Functions among People with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Brain Sci. 2021, 11, 840. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Stilling, J.; Paxman, E.; Mercier, L.; Gan, L.S.; Wang, M.; Amoozegar, F.; Dukelow, S.P.; Monchi, O.; Debert, C. Treatment of Persistent Post-Traumatic Headache and Post-Concussion Symptoms Using Repetitive Transcranial Magnetic Stimulation: A Pilot, Double-Blind, Randomized Controlled Trial. J. Neurotrauma 2020, 37, 312–323. [Google Scholar] [CrossRef]
- Rao, V.; Bechtold, K.; McCann, U.; Roy, D.; Peters, M.; Vaishnavi, S.; Yousem, D.; Mori, S.; Yan, H.; Leoutsakos, J.; et al. Low-Frequency Right Repetitive Transcranial Magnetic Stimulation for the Treatment of Depression After Traumatic Brain Injury: A Randomized Sham-Controlled Pilot Study. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, Z.; Suleiman, A.; Rutherford, G.; Ranjbar Pouya, O.; Dastgheib, Z.; Zhang, W.; Salter, J.; Wang, X.; Mansouri, B.; Lithgow, B. A Pilot Randomised Double-Blind Study of the Tolerability and efficacy of repetitive Transcranial Magnetic Stimulation on Persistent Post-Concussion Syndrome. Sci. Rep. 2019, 9, 5498. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Available online: https://training.cochrane.org/handbook/current/chapter-10 (accessed on 16 January 2022).
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Terry, P.C.; Lane, A.M.; Lane, H.J.; Keohane, L. Development and validation of a mood measure for adolescents. J. Sports Sci. 1999, 17, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Beck, A.T. A systematic investigation of depression. Compr. Psychiatry 1961, 2, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, L.; Li, J.; Liu, Y.; Chen, Y. Comparative efficacy and acceptability of neuromodulation procedures in the treatment of treatment-resistant depression: A network meta-analysis of randomized controlled trials. J. Affect. Disord. 2021, 287, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cai, Z.; Liu, F.; Zhang, Z.; Ni, G. Repetitive Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation as Treatment of Poststroke Depression: A Systematic Review and Meta-Analysis. Neurologist 2022, 27, 177–182. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Chaimani, A.; Moffa, A.H.; Razza, L.B.; Gattaz, W.F.; Daskalakis, Z.J.; Carvalho, A.F. Repetitive Transcranial Magnetic Stimulation for the Acute Treatment of Major Depressive Episodes: A Systematic Review with Network Meta-analysis. JAMA Psychiatry 2017, 74, 143–152. [Google Scholar] [CrossRef]
- Shen, X.; Liu, M.; Cheng, Y.; Jia, C.; Pan, X.; Gou, Q.; Liu, X.; Cao, H.; Zhang, L. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: A systematic review and meta-analysis of randomized controlled clinical trials. J. Affect. Disord. 2017, 211, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J. A systematic review of factors contributing to outcomes in patients with traumatic brain injury. J. Clin. Nurs. 2011, 20, 1518–1532. [Google Scholar] [CrossRef]
- Lan, M.J.; Chhetry, B.T.; Liston, C.; Mann, J.J.; Dubin, M. Transcranial Magnetic Stimulation of Left Dorsolateral Prefrontal Cortex Induces Brain Morphological Changes in Regions Associated with a Treatment Resistant Major Depressive Episode: An Exploratory Analysis. Brain Stimul. 2016, 9, 577–583. [Google Scholar] [CrossRef]
- Kito, S.; Hasegawa, T.; Koga, Y. Cerebral blood flow ratio of the dorsolateral prefrontal cortex to the ventromedial prefrontal cortex as a potential predictor of treatment response to transcranial magnetic stimulation in depression. Brain Stimul. 2012, 5, 547–553. [Google Scholar] [CrossRef]
- Tik, M.; Hoffmann, A.; Sladky, R.; Tomova, L.; Hummer, A.; Navarro de Lara, L.; Bukowski, H.; Pripfl, J.; Biswal, B.; Lamm, C.; et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage 2017, 162, 289–296. [Google Scholar] [CrossRef]
- Niu, X.; Bai, L.; Sun, Y.; Wang, S.; Cao, J.; Sun, C.; Wang, Z.; Xu, H.; Gan, S.; Fan, G.; et al. Disruption of periaqueductal grey-default mode network functional connectivity predicts persistent post-traumatic headache in mild traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 2019, 90, 326–332. [Google Scholar] [CrossRef]
- Hocke, L.M.; Duszynski, C.C.; Debert, C.T.; Dleikan, D.; Dunn, J.F. Reduced Functional Connectivity in Adults with Persistent Post-Concussion Symptoms: A Functional Near-Infrared Spectroscopy Study. J. Neurotrauma 2018, 35, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Huntley, J.H.; Rezvani Habibabadi, R.; Vaishnavi, S.; Khoshpouri, P.; Kraut, M.A.; Yousem, D.M. Transcranial Magnetic Stimulation and its Imaging Features in Patients with Depression, Post-traumatic Stress Disorder, and Traumatic Brain Injury. Acad. Radiol. 2023, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]


| Study (First Author, Year) | Patient Population | N | Gender (%Male) | Mean Age (Years) | Depression Measure | Baseline Mean Depression Scores | Depression Outcome | NIBS | Brain Target | Frequency | Intensity (% RMT) | Sessions | Total Dose (Pulses) | Time Since Injury |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Franke et al., 2022 [25] | Mild-to-moderate TBI | 26 | 85.7 | 45.57 (10.01) | PHQ-9 | 10.07 (5.33) | immediate two-week | rTMS | Right DLPFC | 10 Hz | 80 | 5 | 6000 | 12.04 (6.80) years |
| Stilling et al., 2020 [36] | Mild TBI | 20 | 10.0 | 36.0 (11.4) | PHQ-9 | 11.90 (6.74) | one-month | rTMS | Left DLPFC | 10 Hz | 70 | 10 | 6000 | 32.5 (13.9) months |
| Rushby et al., 2020 [33] | Severe TBI | 30 | 70.0 | 50.0 (1.1) | POMS Depression HAMD | 2.2 (2.8) | immediate | tDCS | left inferior parietal cortex (corresponding to the P3 location) and the cathode was placed over the right inferior parietal cortex (P4 location) | 2 mA of tDCS for 20 min | Not applicable | 1 | NA | 13.9 (12.1) years |
| Siddiqi et al., 2019 [22] | Moderate TBI | 15 | 73.3 | 45.8 (15.1) | MADRS | 32.2 | immediate | rTMS | Bilateral sequential DLPFC (left → right) | Right: 1 Hz Left: 10 Hz | 120 | 20 | 10,000 | 8.3 (9.5) years |
| Rao et al., 2019 [37] | Mild to moderate TBI | 30 | 53.3 | 40.0 (14.4) | HAMD | 23.5 (4.4) | immediate one-month two-month three-month | rTMS | Right DLPFC | 1 Hz | 110 | 20 | 24,000 | 3 months to >10 years |
| Moussavi et al., 2019 [38] | Mild TBI | 18 | 50.0 | 49.9 (12.5) | MADRS | 14.6 (8.8) | immediate one-month two-month | rTMS | Left DLPFC | 20 Hz | 100 | 13 | 9750 | 1.7 (1.3) years |
| Hoy et al., 2019 [26] | Mild to severe TBI | 21 | 47.6 | 46.3 (11.7) | MADRS IDS-CR IDS-SR | 34.0 (8.0) | immediate | rTMS | Bilateral sequential DLPFC (right → left) | Right: 1 Hz Left: 10 Hz | 110 | 20 | 48,000 | 18.2 (12.2) years |
| Lee et al., 2018 [23] | Mild to moderate TBI | 13 | 69.2 | 42.0 (11.2) | MADRS | 23.8 (4.3) | immediate | rTMS | Right DLPFC | 1 Hz | 100 | 10 | 20,000 | NA |
| Leung et al., 2018 [24] | Mild TBI | 29 | 79.3 | 34.1 (7.9) | HAMD | 23.9 (7.5) | immediate | rTMS | Left DLPFC | 10 Hz | 80 | 4 | 4000 | 97.1 (71.1) months |
| Sacco et al., 2016 [27] | Severe TBI | 32 | 50.0 | 18 to 66 | BDI | NA | one month | tDCS | F3 or F4 anodal (anode on the lesioned hemisphere and cathode on the other hemisphere); bi-montage F3/F4 anodal in case of equal hemispheric lesion distribution | 10 min of anodal tDCS, 1 mA | Not applicable | 10 | NA | 3.16 to 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Chou, P.-H.; Chuang, H.-Y.; Yao, C.-Y.; Chen, W.-J.; Tsai, H.-C. Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 6030. https://doi.org/10.3390/jcm12186030
Chang C-H, Chou P-H, Chuang H-Y, Yao C-Y, Chen W-J, Tsai H-C. Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. Journal of Clinical Medicine. 2023; 12(18):6030. https://doi.org/10.3390/jcm12186030
Chicago/Turabian StyleChang, Chun-Hung, Po-Han Chou, Hao-Yu Chuang, Chi-Yu Yao, Wei-Jen Chen, and Hsin-Chi Tsai. 2023. "Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials" Journal of Clinical Medicine 12, no. 18: 6030. https://doi.org/10.3390/jcm12186030
APA StyleChang, C.-H., Chou, P.-H., Chuang, H.-Y., Yao, C.-Y., Chen, W.-J., & Tsai, H.-C. (2023). Efficacy of Non-Invasive Brain Stimulation for Treating Depression in Patients with Traumatic Brain Injury: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. Journal of Clinical Medicine, 12(18), 6030. https://doi.org/10.3390/jcm12186030








