Endovascular Revascularisation versus Open Surgery with Prosthetic Bypass for Femoro-Popliteal Lesions in Patients with Peripheral Arterial Disease
Abstract
:1. Introduction
2. Materials and Methods
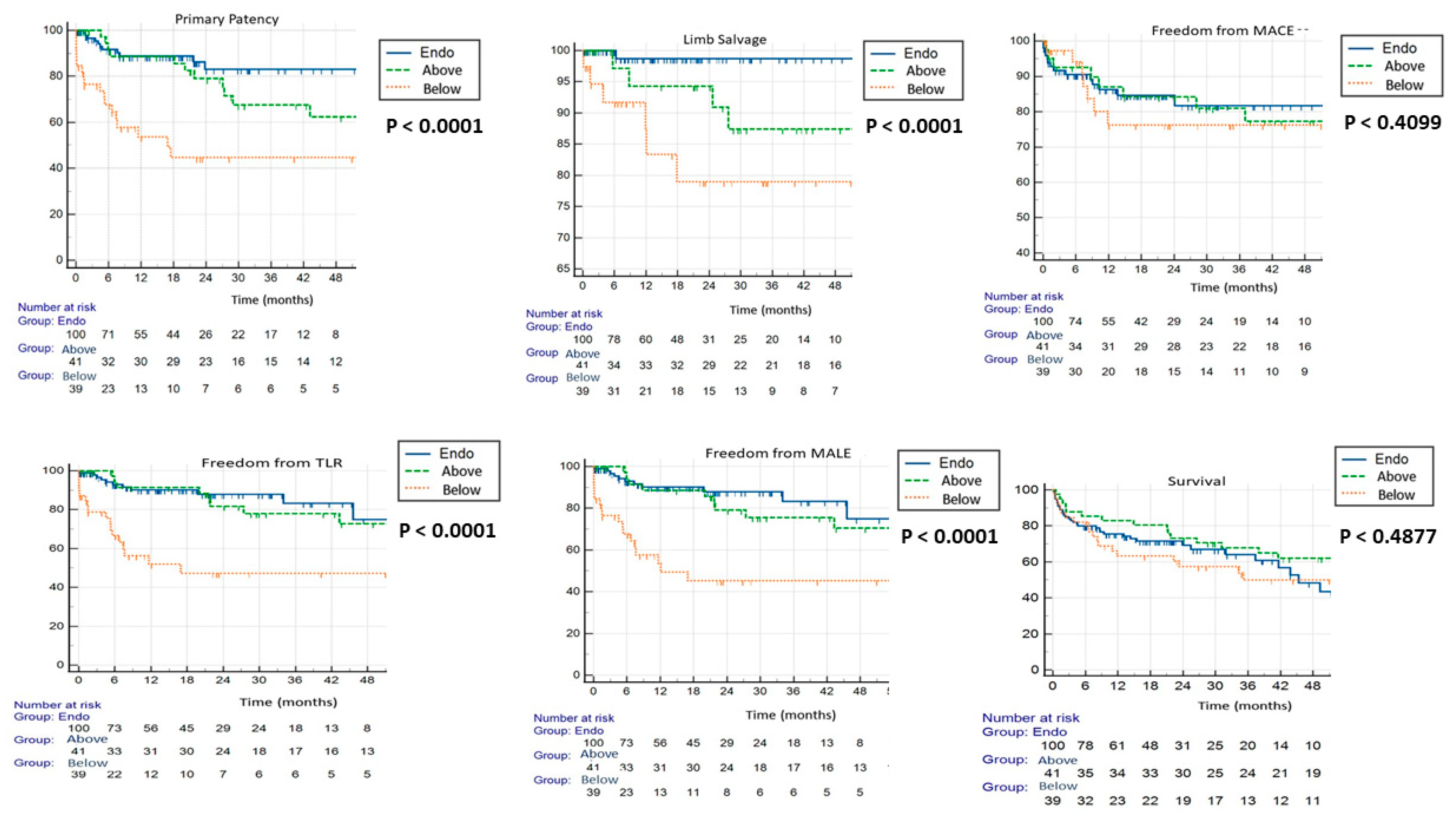
3. Results
4. Discussion
Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 2007, 33, S1–S75. [Google Scholar] [CrossRef] [PubMed]
- Almasri, J.; Adusumalli, J.; Asi, N.; Lakis, S.; Alsawas, M.; Prokop, L.J.; Bradbury, A.; Kolh, P.; Conte, M.S.; Murad, M.H. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 126S–136S. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.; Wilmink, T.; Lee, A.J.; Bell, J.; Prescott, R.; Gillespie, I.; Stansby, G.; Fowkes, F.R. Bypass versus angioplasty to treat severe limb ischemia: Factors that affect treatment preferences of UK surgeons and interventional radiologists. J. Vasc. Surg. 2004, 39, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef]
- Poletti, L.F.; Matsuura, J.H.; Dattilo, J.B.; Posner, M.P.; Lee, H.M.; Scouvart, M.; Sobel, M. Should vein be saved for future operations? A 15-year review of infrainguinal bypasses and the subsequent need for autogenous vein. Ann. Vasc. Surg. 1998, 12, 143–147. [Google Scholar] [CrossRef]
- Dirven, M.; Scharn, D.; Blankensteijn, J.; van der Vliet, J. Preservation for future use of the autologous saphenous vein during femoro-popliteal bypass surgery is inexpedient. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 420–423. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538, Erratum in J. Vasc. Surg. 2001, 33, 805. [Google Scholar] [CrossRef]
- Zenunaj, G.; Traina, L.; Serra, R.; Camagni, A.; Mucignat, M.; Gasbarro, V. The impact of a prior revascularization procedure on the outcome of a future lower limb bypass for chronic threatened limb ischemia. Ital. J. Vasc. Endovasc. Surg. 2021, 28, 65–70. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Zenunaj, G.; Traina, L.; Acciarri, P.; Mucignat, M.; Scian, S.; Alesiani, F.; Serra, R.; Gasbarro, V. Superficial Femoral Artery Access for Infrainguinal Antegrade Endovascular Interventions in the Hostile Groin: A Prospective Randomized Study. Ann. Vasc. Surg. 2022, 86, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Troisi, N.; De Blasis, G.; Salvini, M.; Michelagnoli, S.; Setacci, C.; LIMBSAVE Registry Collaborative Group. Preliminary six-month outcomes of LIMBSAVE (treatment of critical Limb IscheMia with infragenicular Bypass adopting in situ SAphenous VEin technique) registry. Vascular 2021, 29, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.J.; Beard, J.D.; Cleveland, T.; Bell, J.; Bradbury, A.W.; Forbes, J.F.; Fowkes, F.G.R.; Gillepsie, I.; Ruckley, C.V.; Raab, G.; et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005, 366, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- van Reijen, N.S.; Ponchant, K.; Ubbink, D.T.; Koelemay, M.J. Editor’s Choice—The Prognostic Value of the WIfI Classification in Patients with Chronic Limb Threatening Ischaemia: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 362–371. [Google Scholar] [CrossRef]
- Schillinger, M.; Sabeti, S.; Loewe, C.; Dick, P.; Amighi, J.; Mlekusch, W.; Schlager, O.; Cejna, M.; Lammer, J.; Minar, E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N. Engl. J. Med. 2006, 354, 1879–1888. [Google Scholar] [CrossRef]
- Dick, P.; Wallner, H.; Sabeti, S.; Loewe, C.; Mlekusch, W.; Lammer, J.; Koppensteiner, R.; Minar, E.; Schillinger, M. Balloon angioplasty versus stenting with nitinol stents in intermediate length superficial femoral artery lesions. Catheter. Cardiovasc. Interv. 2009, 74, 1090–1095. [Google Scholar] [CrossRef]
- Laird, J.R.; Katzen, B.T.; Scheinert, D.; Lammer, J.; Carpenter, J.; Buchbinder, M.; Dave, R.; Ansel, G.; Lansky, A.; Cristea, E.; et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: Twelve-month results from the RESILIENT randomized trial. Circ. Cardiovasc. Interv. 2010, 3, 267–276. [Google Scholar] [CrossRef]
- Bausback, Y.; Wittig, T.; Schmidt, A.; Zeller, T.; Bosiers, M.; Peeters, P.; Brucks, S.; Lottes, A.E.; Scheinert, D.; Steiner, S. Drug-Eluting Stent Versus Drug-Coated Balloon Revascularization in Patients with Femoropopliteal Arterial Disease. J. Am. Coll. Cardiol. 2019, 73, 667–679. [Google Scholar] [CrossRef]
- Okuno, S.; Iida, O.; Shiraki, T.; Fujita, M.; Masuda, M.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Takahara, M.; et al. Impact of Calcification on Clinical Outcomes after Endovascular Therapy for Superficial Femoral Artery Disease: Assessment Using the Peripheral Artery Calcification Scoring System. J. Endovasc. Ther. 2016, 23, 731–737. [Google Scholar] [CrossRef]
- Fanelli, F.; Cannavale, A.; Gazzetti, M.; Lucatelli, P.; Wlderk, A.; Cirelli, C.; D’adamo, A.; Salvatori, F.M. Calcium burden assessment and impact on drug-eluting balloons in peripheral arterial disease. Cardiovasc. Interv. Radiol. 2014, 37, 898–907. [Google Scholar] [CrossRef]
- Lorenzoni, R.; Ferraresi, R.; Manzi, M.; Roffi, M. Guidewires for lower extremity artery angioplasty: A review. EuroIntervention 2015, 11, 799–807. [Google Scholar] [CrossRef]
- Mulholland, D.; Thulasidasan, N.; Patel, A.; Katsanos, K.; Diamantopoulos, A. The Outback Re-Entry Device. A Pictorial Review. Vasc. Endovasc. Surg. 2021, 55, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.M.; Afari, M.E.; Garcia, L.A. Atherectomy in Peripheral Artery Disease: A Review. J. Invasive Cardiol. 2017, 29, 135–144. [Google Scholar] [PubMed]
- Wardle, B.G.; Ambler, G.K.; Radwan, R.W.; Hinchliffe, R.J.; Twine, C.P. Atherectomy for peripheral arterial disease. Cochrane Database Syst. Rev. 2020, 9, CD006680. [Google Scholar] [CrossRef]
- Zenunaj, G.; Traina, L.; Acciarri, P.; Cosacco, A.M.; Alesiani, F.; Baldazzi, G.; Gasbarro, V. Primary Drug-Coated Balloon Versus Drug-Eluting Stent for Native Atherosclerotic Femoropopliteal Lesions: A Systematic Review and Meta-Analysis. Ann. Vasc. Surg. 2023, 92, 294–303. [Google Scholar] [CrossRef]
- Tepe, G.; Schneider, P.A.; Brodmann, M.; Krishnan, P.; Micari, A.; Metzger, C.; Scheinert, D.; Zeller, T.; Cohen, D.J.; Snead, D.B.; et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015, 131, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Jaff, M.R.; Rosenfield, K.; Scheinert, D.; Rocha-Singh, K.; Benenati, J.; Nehler, M.; White, C.J. Drug-coated balloons to improve femoropopliteal artery patency: Rationale and design of the LEVANT 2 trial. Am. Heart J. 2015, 169, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.W.; Moakes, C.A.; Popplewell, M.; Meecham, L.; Bate, G.R.; Kelly, L.; Chetter, I.; Diamantopoulos, A.; Ganeshan, A.; Hall, J.; et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): An open-label, randomised, multicentre, phase 3 trial. Lancet 2023, 401, 1798–1809. [Google Scholar] [CrossRef]
- Kluckner, M.; Gratl, A.; Wipper, S.H.; Hitzl, W.; Nierlich, P.; Aspalter, M.; Linni, K.; Enzmann, F.K. Comparison of Prosthetic and Vein Bypass with Nitinol Stents in Long Femoropopliteal Lesions. Ann. Vasc. Surg. 2022, 78, 272–280. [Google Scholar] [CrossRef]
- Chaudhuri, A.; York, A.; Dey, R. Percutaneous vascular closure using an anchored collagen plug provides effective haemostasis following both antegrade and retrograde femoral arterial punctures. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 220–225. [Google Scholar] [CrossRef]
- Noori, V.J.; Eldrup-Jørgensen, J. A systematic review of vascular closure devices for femoral artery puncture sites. J. Vasc. Surg. 2018, 68, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Zenunaj, G.; Traina, L.; Acciarri, P.; Spataro, C.; Gasbarro, V. Revascularisation through the obturator foramen of lower limbs with a compromised ipsilateral groin due to infection. Ann. R. Coll. Surg. Engl. 2020, 102, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Doshi, R.; Rao, G.; Shlofmitz, E.; Donnelly, J.; Meraj, P. Comparison of In-Hospital Outcomes After Percutaneous Revascularization for Peripheral Arterial Disease in Patients with a Body Mass Index of >30 kg/m2 versus ≤30 kg/m2 (from the National Inpatient Sample). Am. J. Cardiol. 2017, 120, 1648–1652. [Google Scholar] [CrossRef]
- Dakour-Aridi, H.; Arora, M.; Nejim, B.; Locham, S.; Malas, M.B. Association between Drug Use and In-hospital Outcomes after Infrainguinal Bypass for Peripheral Arterial Occlusive Disease. Ann. Vasc. Surg. 2019, 58, 122–133.e4. [Google Scholar] [CrossRef] [PubMed]

| Demographics Co-Morbidities | Open Group (n = 76) | Endovascular Group (n = 98) | p-Value |
|---|---|---|---|
| Mean age, years | 74 8 SD | 79 9 SD | - |
| Age 80 years | 20 (26.3%) | 40 (40.8%) | 0.045 |
| Age 90 years | 0 (0%) | 6 (6.1%) | 0.028 |
| Men | 49 (64%) | 59 (60%) | 0.5648 |
| Women | 27 (36%) | 39 (40%) | 0.5648 |
| Current smokers | 22 (29%) | 18 (18%) | 0.0999 |
| Systemic hypertension | 72 (95%) | 90 (92%) | 0.4539 |
| Dyslipidaemia | 44 (58%) | 62 (63%) | 0.4714 |
| Diabetes | 34 (45%) | 47 (48%) | 0.6725 |
| Chronic renal disease | 19 (25%) | 27 (28%) | 0.7050 |
| CRD dialysis | 8 (11%) | 9 (9%) | 0.7673 |
| Coronary artery disease | 35 (46%) | 66 (67%) | 0.0047 |
| COPD | 13 (17%) | 15 (15%) | 0.7487 |
| Stroke * | 7 (9%) | 22 (22%) | 0.0201 |
| Run-Off | Open Group (n = 80 Limbs) | Endovascular Group (n = 105 Limbs) | p Value |
|---|---|---|---|
| Number of BTK vessels | Number of limbs | Number of limbs | |
| 0 | 0 | 11 | 0.002 |
| 1 | 30 | 40 | 0.462 |
| 2 | 22 | 44 | 0.042 |
| 3 | 28 | 10 | 0.001 |
| Open Group | Number of Limbs (%) (n = 80) |
|---|---|
| Above the knee | 41 (51.2%) |
| Below the knee | 39 (48.7%) |
| Graft material | |
| Heparin-bonded ePTFE | 80 (100%) |
| Graft diameter | |
| 6 mm | 38 (47.5%) |
| 7 mm | 36 (45%) |
| 8 mm | 6 (7.5%) |
| Endovascular group | Number of limbs (%) (n = 105) |
| Vascular access | |
| Cut down | 53 (50.4%) |
| Percutaneous | 52 (49.5%) |
| Ipsilateral | 12 (11.4%) |
| Contralateral | 40 (38%) |
| Haemostasis | |
| Percutaneous closure device (AngioSeal Vip 6 Fr) | 52 (49.5%) |
| Surgical suture | 53 (50.4%) |
| Target lesion | |
| SFA | 71 (67.6%) |
| SFA and popliteal artery | 34 (32.3%) |
| Endovascular devices | |
| POBA (Evercross) * | 105 (100%) |
| DCB (Lutonix) # | 4 (3.8%) |
| pBMS (Zilver Flex) & | 24 (22.8%) |
| pDES (Zilver PTX) & | 5 (48.7%) |
| Anaesthesiologic Management | Open Group (n = 80) | Endovascular Group (n = 105) | p-Value |
|---|---|---|---|
| General | 71 (87.5%) | 41 (39%) | <0.00001 |
| Local | 0 (0%) | 37 (35.2%) | <0.00001 |
| Local + systemic sedation | 0 (0%) | 17 (16.1%) | 0.0001 |
| Epidural analgesia | 9 (2.5%) | 5 (4.7%) | 0.434 |
| Adjunctive procedures | |||
| CFA endarterectomy | 17 (21.2%) | 1 (0.9%) | <0.001 |
| CFA and PFA endarterectomy | 4 (5%) | 0 (0%) | 0.02 |
| CFA angioplasty | 0 (0%) | 22 (20.9%) | <0.001 |
| Popliteal endarterectomy | 8 (10%) | 0 (0%) | 0.001 |
| Minor amputations | 14 (17.5%) | 13 (12.3%) | 0.328 |
| Technical success | 0 (0%) | 5 (4.7%) | 0.047 |
| Overall intervention duration, minutes * | 273 96 | 146 89 | <0.0001 |
| Duration of the intervention, minutes * | Open group (n = 80) | Endovascular percutaneous group (n = 52) | |
| 273 96 | 78 37 | <0.00001 | |
| Haemoglobin loss ≥ 3 g/dL | 13 (16.25%) | 6 (5.7%) | 0.019 |
| Overall vascular access # | 9 (11.2%) | 3 (2.8%) | 0.021 |
| Dehiscence | 6 | 0 | |
| Infection | 4 | 0 | |
| Lymphorrhoea | 6 | 0 | |
| Haematoma | 1 | 3 | |
| Post-intervention therapy | |||
| DAPT 3 months | 52 (65%) | 80 (76.1%) | - |
| Anti-coagulant | 13 (16.2%) | 0 (0%) | - |
| Anti-coagulant plus anti-platelet | 15 (18.7%) | 25 (23.8%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zenunaj, G.; Acciarri, P.; Baldazzi, G.; Cosacco, A.M.; Gasbarro, V.; Traina, L. Endovascular Revascularisation versus Open Surgery with Prosthetic Bypass for Femoro-Popliteal Lesions in Patients with Peripheral Arterial Disease. J. Clin. Med. 2023, 12, 5978. https://doi.org/10.3390/jcm12185978
Zenunaj G, Acciarri P, Baldazzi G, Cosacco AM, Gasbarro V, Traina L. Endovascular Revascularisation versus Open Surgery with Prosthetic Bypass for Femoro-Popliteal Lesions in Patients with Peripheral Arterial Disease. Journal of Clinical Medicine. 2023; 12(18):5978. https://doi.org/10.3390/jcm12185978
Chicago/Turabian StyleZenunaj, Gladiol, Pierfilippo Acciarri, Giulia Baldazzi, Alessio Mario Cosacco, Vincenzo Gasbarro, and Luca Traina. 2023. "Endovascular Revascularisation versus Open Surgery with Prosthetic Bypass for Femoro-Popliteal Lesions in Patients with Peripheral Arterial Disease" Journal of Clinical Medicine 12, no. 18: 5978. https://doi.org/10.3390/jcm12185978
APA StyleZenunaj, G., Acciarri, P., Baldazzi, G., Cosacco, A. M., Gasbarro, V., & Traina, L. (2023). Endovascular Revascularisation versus Open Surgery with Prosthetic Bypass for Femoro-Popliteal Lesions in Patients with Peripheral Arterial Disease. Journal of Clinical Medicine, 12(18), 5978. https://doi.org/10.3390/jcm12185978





