Enhanced Recovery after Pelvic Organ Prolapse Surgery
Abstract
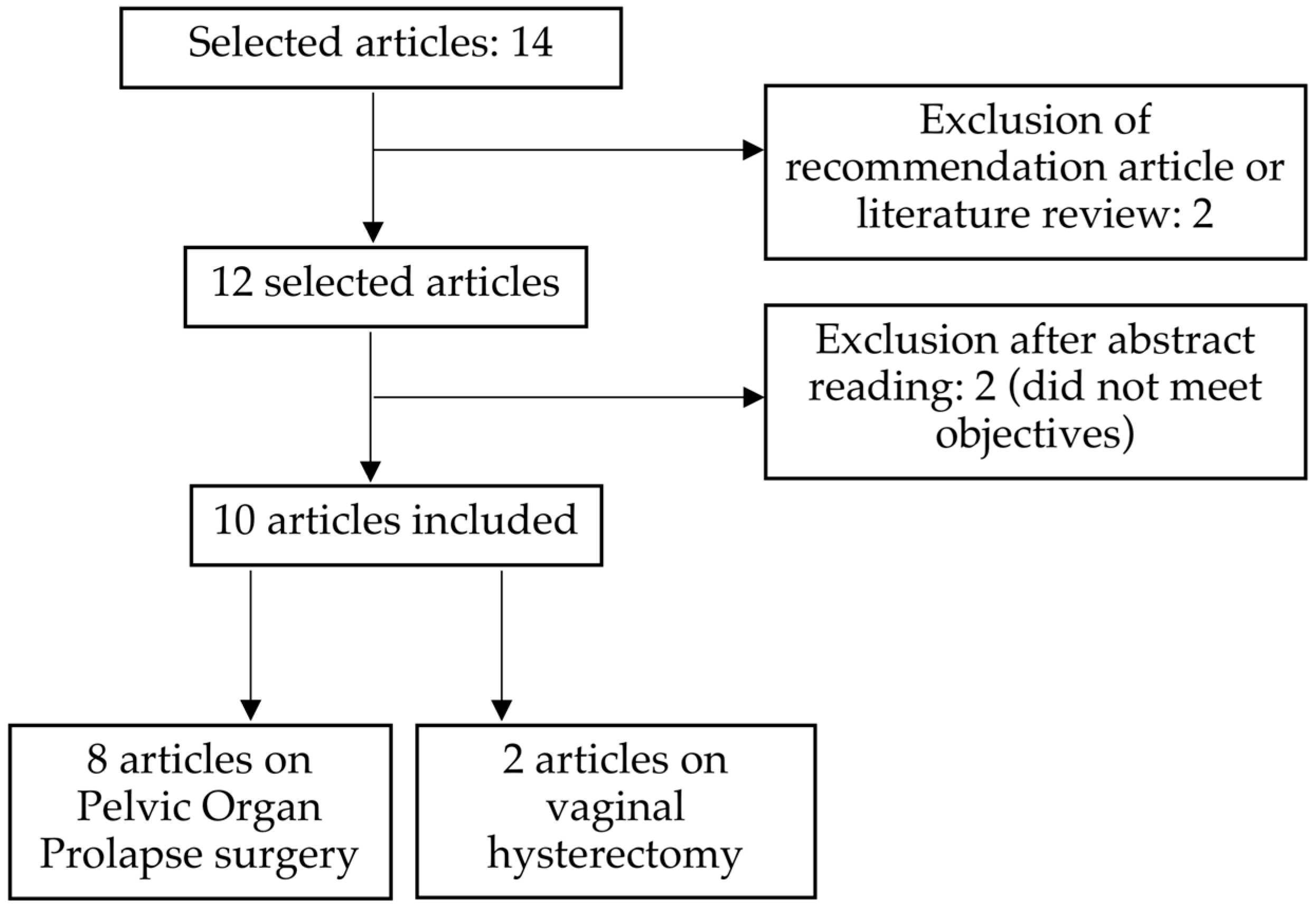
:1. Introduction
2. Materials and Methods
3. Results
| Type of Study | Time Period | Number of Patients | |
|---|---|---|---|
| Carter-Brooks [7] USA 2018 | Observational Retrospective and prospective Single-center | January 2016– June 2016 | 137 not ERAS 121 ERAS |
| Evans [8] USA 2021 | Observational Retrospective Single-center | January 2017– June 2019 | 83 not ERAS 83 ERAS |
| Gong [11] China 2020 | Observational Retrospective Single-center | August 2015– November 2017 | 85 not ERAS 92 ERAS |
| Abrao Trad [12] USA 2020 | Observational Retrospective Single-center | April 2009– November 2017 | 128 not ERAS 83 ERAS |
| Trowbridge [13] USA 2018 | Observational Retrospective and prospective Single-center |
January 2014– February 2016 | 76 not ERAS 118 ERAS |
| Evans [14] USA 2020 | Observational Retrospective Single-center | December 2018–February 2019 | 14 ERAS (7 outputs at day 0 and 7 outputs at day 1) |
| Pan [15] India 2021 | Observational Retrospective and prospective Single-center | December 2013–February 2020 | 169 not ERAS 89 ERAS |
| Carter-Brooks [16] USA 2021 | Observational Retrospective Single-center | January 2016– October 2017 | 210 not ERAS 172 ERAS (3 groups: <61 years, 61 to 75 years, >75 years) |
| Relph [9] England 2014 | Observational Retrospective and prospective Single-center | November 2010–February 2012 | 45 not ERAS 45 ERAS |
| Yoong [10] England 2014 | Observational Single-center | November 2009–March 2012 | 50 not ERAS 50 ERAS |
| Type of Surgery | Aim of the Study | |
|---|---|---|
| Carter- Brooks [7] | Laparoscopic or robot-assisted prolapse surgery: 43.4% Vaginal prolapse surgery: 22.5% Colpocleisis: 23.6% ±Hysterectomy: 57.4% | To evaluate whether the implementation of ERAS resulted in a reduction in hospital length of stay |
| Evans [8] | Laparoscopic sacrocolpopexy (44%) and robotic-assisted sacrocolpopexy (56%) with:
| To assess whether ERAS was associated with a higher rate of day 0 discharge after laparoscopic (±robotic assisted) sacrocolpopexy and to describe the safety and feasibility of day 0 discharge after this type of procedure |
| Gong [11] | Pelvic organ prolapse surgery with vaginal mesh | To study the impact of ERAS on intra-operative outcomes after pelvic organ prolapse surgery with vaginal mesh |
| Abrao Trad [12] | Sacrocolpopexy | To evaluate the impact of ERAS on drug use, length of hospital stay, costs, and morbidity |
| Trowbridge [13] | Pelvic organ prolapse surgery:
| To evaluate outcomes after an ERAS program for minimally invasive or vaginal pelvic surgery |
| Evans [14] | Sacrocolpopexy by laparoscopy or robot-assisted laparoscopy ±concomitant surgery | To describe patient experiences with an enhanced recovery protocol after minimally invasive sacrocolpopexy |
| Pan [15] | Sacrocolpopexy by laparoscopy | To determine whether an ERAS program improved the perception of post-operative recovery after laparoscopic sacrocolpopexy |
| Carter- Brooks [16] | Laparoscopic sacrocolpopexy: 42.7%. Vaginal apex suspension (sacrospinous ligament or utero- sacral ligament fixation): 30.3% Colpocleisis: 27% ±Hysterectomy: 56.3% | To evaluate discharges at day 0, opioid administration, pain scores, and differences in complications in three age categories (<61 years, 61 to 75 years, >75 years), before and after implementation of ERAS. |
| Relph [9] | Vaginal hysterectomy | To explore the cost savings after implementation of an ERAS program for vaginal hysterectomy |
| Yoong [10] | Vaginal hysterectomy | To evaluate the impact of ERAS on patients after vaginal hysterectomy for benign indications. |
| Age (Years) | BMI (kg/m2) | ASA Score | Stage of Prolapse | History of Laparotomy | |
|---|---|---|---|---|---|
| Carter-Brooks [7] | 65 | 28.2 | NA | 0: 4.3% 1: 0.4% 2: 20.2% 3: 65.1% 4: 10.1% | NA |
| Evans [8] | 64.3 | 26.9 | 1: 5.5% 2: 73% 3: 21% 4: 0.5% | 1: 3.5% 2: 35.5% 3: 50% 4: 11% | 26% |
| Gong [11] | 67 | 28.9 | NA | 2: 4.5% 3: 72.9% 4: 22.6% | NA |
| Abrao Trad [12] | NA | NA | NA | NA | NA |
| Trowbridge [13] | 58.1 | 28.3 | 1: 9% 2: 75% 3: 16% | NA | NA |
| Evans [14] | 65.5 | 26.8 | 2: 71.4% 3: 28.6% | 2: 42.9% 3: 57.1% | NA |
| Pan [15] | 62.9 | 28.1 | NA | NA | NA |
| Carter-Brooks [16] | 66 | 27.8 | NA | 2: 16.8% 3: 70.7% 4: 12.5% | NA |
| Relph [9] | 53 | 27.85 | NA | NA | 26.6% |
| Yoong [10] | 50 | NA | 1 to 3: 96% | NA | 24% |
3.1. Contributions of ERAS in the Intra-Operative
3.1.1. Operating Time
3.1.2. Intra-Operative Complications
3.1.3. Intra-Operative Blood Loss
3.1.4. Intra-Operative Intravenous Intakes
3.1.5. Intra-Operative Morphine Rate
3.2. Benefits of ERAS in Post-Operative Care
3.2.1. Length of Stay
3.2.2. Post-Operative Opioids
3.2.3. Pain
3.2.4. Mobilization, Walking, and Recovery
3.3. Post-Operative Complications
3.3.1. Readmission
3.3.2. Post-Operative Complications within 30 Days and at One Year after Surgery
3.4. Hospital Cost
3.5. Patient Satisfaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- High Authority of Health (Haute Autorité de Santé). Enhanced Recovery After Surgery (ERAS) Programs: Current Status and Prospects (Programmes de Récupération Améliorée Après Chirurgie (RAAC): Etat des Lieux et Perspectives). 2016. Available online: https://www.has-sante.fr/upload/docs/application/forcedownload/2016-09/rapport_orientation_raac.pdf (accessed on 2 December 2020).
- Lee, L.; Tran, T.; Mayo, N.E.; Carli, F.; Feldman, L.S. What does it really mean to “recover” from an operation? Surgery 2014, 155, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; Macfie, J.; Liberman, A.S.; Soop, M.; et al. Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN). Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef]
- High Authority of Health (Haute Autorité de Santé). Enhanced Recovery After Surgery (ERAS) Programs. Summary of Orientation Report (Programmes de Récupération Améliorée après Chirurgie (RAAC). Synthèse du Rapport D’orientation). 2016. Available online: https://www.has-sante.fr/upload/docs/application/forcedownload/2016-09/synthese_raac_2016-09-01_15-49-32_230.pdf (accessed on 2 December 2020).
- Altman, A.D.; Robert, M.; Armbrust, R.; Fawcett, W.J.; Nihira, M.; Jones, C.N.; Tamussino, K.; Sehouli, J.; Dowdy, S.C.; Nelson, G. Guidelines for vulvar and vaginal surgery: Enhanced Recovery After Surgery Society recommendations. Am. J. Obstet. Gynecol. 2020, 223, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Latthe, P.; Panza, J.; Marquini, G.V.; Jankowski, C.J.; Heisler, C.; Achtari, C.; Reagan, K.; Hickman, L.C.; Haddad, J. AUGS-IUGA Joint Clinical Consensus Statement on Enhanced Recovery After Urogynecologic Surgery: Developed by the Joint Writing Group of the International Urogynecological Association and the American Urogynecologic Society. Individual writing group members are noted in the Acknowledgements section. Urogynecology 2022, 28, 716–734. [Google Scholar] [CrossRef] [PubMed]
- Carter-Brooks, C.M.; Du, A.L.; Ruppert, K.M.; Romanova, A.L.; Zyczynski, H.M. Implementation of a urogynecology-specific enhanced recovery after surgery (ERAS) pathway. Am. J. Obstet. Gynecol. 2018, 219, 495.e1–495.e10. [Google Scholar] [CrossRef]
- Evans, S.; McCarter, M.; Abimbola, O.; Myers, E.M. Enhanced Recovery and Same-Day Discharge After Minimally Invasive Sacrocolpopexy. Female Pelvic. Med. Reconstr. Surg. 2021, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Relph, S.; Bell, A.; Sivashanmugarajan, V.; Munro, K.; Chigwidden, K.; Lloyd, S.; Fakokunde, A.; Yoong, W. Cost effectiveness of enhanced recovery after surgery programme for vaginal hysterectomy: A comparison of pre and post-implementation expenditures. Int. J. Health Plan. Manag. 2014, 29, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yoong, W.; Sivashanmugarajan, V.; Relph, S.; Bell, A.; Fajemirokun, E.; Davies, T.; Munro, K.; Chigwidden, K.; Evan, F.; Lodhi, W. Enhanced Recovery After Surgery (ERAS) Team for Gynaecology and Anaesthesia. Can enhanced recovery pathways improve outcomes of vaginal hysterectomy? Cohort control study. J. Minim. Invasive Gynecol. 2014, 21, 83–89. [Google Scholar] [CrossRef]
- Gong, R.; Hu, Q.; Liu, D.; Zu, J.; Wu, Y.; Xia, Z. Enhanced recovery after surgery versus traditional care in total pelvic floor reconstruction surgery with transvaginal mesh. Int. J. Gynaecol. Obstet. 2020, 148, 107–112. [Google Scholar] [CrossRef]
- Abrao Trad, A.T.; Tamhane, P.; Weaver, A.; Baker, M.V.; Visscher, S.; Borah, D.; Kalogera, E.; Klingele, C.; Gebhart, J.; Trabuco, E. 93: The impact of an urogynecologic-specific enhanced recovery after surgery (ERAS) pathway implementation in open prolapse surgery. Am. J. Obstet. Gynecol. 2020, 222, S829. [Google Scholar] [CrossRef]
- Trowbridge, E.R.; Evans, S.L.; Sarosiek, B.M.; Modesitt, S.C.; Redick, D.L.; Tiouririne, M.; Thiele, R.H.; Hedrick, T.L.; Hullfish, K.L. Enhanced recovery program for minimally invasive and vaginal urogynecologic surgery. Int. Urogynecol. J. 2019, 30, 313–321. [Google Scholar] [CrossRef]
- Evans, S.; Snook, L.; Yates, T.; Bundy, H.; Abimbola, O.; Myers, E.M. Patient experience with enhanced recovery and early discharge after minimally invasive sacrocolpopexy: A qualitative study. Int. Urogynecol. J. 2021, 32, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Hale, D.; Heit, M. Enhanced Recovery Protocol Enhances Postdischarge Recovery After Laparoscopic Sacrocolpopexy. Female Pelvic. Med. Reconstr. Surg. 2021, 27, 667–671. [Google Scholar] [CrossRef]
- Carter-Brooks, C.M.; Romanova, A.L.; DeRenzo, J.S.; Shepherd, J.P.; Zyczynski, H.M. Age and Perioperative Outcomes After Implementation of an Enhanced Recovery After Surgery Pathway in Women Undergoing Major Prolapse Repair Surgery. Female Pelvic. Med. Reconstr. Surg. 2021, 27, e392–e398. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Lim, S.; Broadwater, G.; Cobb, L.; Valea, F.; Marosky Thacker, J.; Habib, A.; Havrilesky, L. Reduction in opioid use and postoperative pain scores after elective laparotomy with implementation of enhanced recovery after surgery protocol on a gynecologic oncology service. Int. J. Gynecol. Cancer 2019, 29, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, J.E.; Scott, M.E.; Alimi, Y.; Yen, T.T.; Hobson, D.; Machado, K.K.; Tanner EJ 3rd Fader, A.N.; Temkin, S.M.; Wethington, S.; Levinson, K.; et al. Narcotics reduction, quality and safety in gynecologic oncology surgery in the first year of enhanced recovery after surgery protocol implementation. Gynecol. Oncol. 2018, 149, 554–559. [Google Scholar] [CrossRef]
- Kalogera, E.; Bakkum-Gamez, J.N.; Jankowski, C.J.; Trabuco, E.; Lovely, J.K.; Dhanorker, S.; Grubbs, P.L.; Weaver, A.L.; Haas, L.R.; Borah, B.J.; et al. Enhanced recovery in gynecologic surgery. Obstet. Gynecol. 2013, 122 Pt 1, 319–328. [Google Scholar] [CrossRef]
- Meyer, L.A.; Lasala, J.; Iniesta, M.D.; Nick, A.M.; Munsell, M.F.; Shi, Q.; Wang, X.S.; Cain, K.E.; Lu, K.H.; Ramirez, P.T. Effect of an Enhanced Recovery After Surgery Program on Opioid Use and Patient-Reported Outcomes. Obstet. Gynecol. 2018, 132, 281–290. [Google Scholar] [CrossRef]
- Peters, A.; Siripong, N.; Wang, L.; Donnellan, N.M. Enhanced recovery after surgery outcomes in minimally invasive nonhysterectomy gynecologic procedures. Am. J. Obstet. Gynecol. 2020, 223, 234.e1–234.e8. [Google Scholar] [CrossRef]
- Ferrari, F.; Forte, S.; Sbalzer, N.; Zizioli, V.; Mauri, M.; Maggi, C.; Sartori, E.; Odicino, F. Validation of an enhanced recovery after surgery protocol in gynecologic surgery: An Italian randomized study. Am. J. Obstet. Gynecol. 2020, 223, 543.e1–543.e14. [Google Scholar] [CrossRef]
- Chapman, J.S.; Roddy, E.; Ueda, S.; Brooks, R.; Chen, L.L.; Chen, L.M. Enhanced Recovery Pathways for Improving Outcomes after Minimally Invasive Gynecologic Oncology Surgery. Obstet. Gynecol. 2016, 128, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wijk, L.; Franzen, K.; Ljungqvist, O.; Nilsson, K. Implementing a structured Enhanced Recovery After Surgery (ERAS) protocol reduces length of stay after abdominal hysterectomy. Acta Obstet. Gynecol. Scand. 2014, 93, 749–756. [Google Scholar] [CrossRef]
- Bisch, S.P.; Wells, T.; Gramlich, L.; Faris, P.; Wang, X.; Tran, D.T.; Thanh, N.X.; Glaze, S.; Chu, P.; Ghatage, P.; et al. Enhanced Recovery After Surgery (ERAS) in gynecologic oncology: System-wide implementation and audit leads to improved value and patient outcomes. Gynecol. Oncol. 2018, 151, 117–123. [Google Scholar] [CrossRef]
- Iniesta, M.D.; Lasala, J.; Mena, G.; Rodriguez-Restrepo, A.; Salvo, G.; Pitcher, B.; Washington, L.D.; Harris, M.; Meyer, L.A.; Ramirez, P.T. Impact of compliance with an enhanced recovery after surgery pathway on patient outcomes in open gynecologic surgery. Int. J. Gynecol. Cancer 2019, 29, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Modesitt, S.C.; Sarosiek, B.M.; Trowbridge, E.R.; Redick, D.L.; Shah, P.M.; Thiele, R.H.; Tiouririne, M.; Hedrick, T.L. Enhanced Recovery Implementation in Major Gynecologic Surgeries: Effect of Care Standardization. Obstet. Gynecol. 2016, 128, 457–466. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.J.; van Es, L.E.; Maessen, J.M.; Dejong, C.H.; Kruitwagen, R.F.; Slangen, B.F. Diffusion of Enhanced Recovery principles in gynecologic oncology surgery: Is active implementation still necessary? Gynecol. Oncol. 2014, 134, 570–575. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.J.; Ament, S.M.; Maessen, J.M.; Dejong, C.H.; Kleijnen, J.M.; Slangen, B.F. Enhanced recovery pathways in abdominal gynecologic surgery: A systematic review and meta-analysis. Acta Obstet. Gynecol. Scand. 2016, 95, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yi, J.; Cornella, J.; Butterfield, R.; Buras, M.; Wasson, M. Same-Day Discharge after Vaginal Hysterectomy with Pelvic Floor Reconstruction: Pilot Study. J. Minim. Invasive Gynecol. 2020, 27, 498–503.e1. [Google Scholar] [CrossRef]
- Lloyd, J.C.; Guzman-Negron, J.; Goldman, H.B. Feasibility of same day discharge after robotic assisted pelvic floor reconstruction. Can. J. Urol. 2018, 25, 9307–9312. [Google Scholar]
- Hickman, L.C.; Paraiso, M.F.R.; Goldman, H.B.; Propst, K.; Ferrando, C.A. Same-Day Discharge after Minimally Invasive Sacrocolpopexy Is Feasible, Safe, and Associated with High Patient Satisfaction. Female Pelvic. Med. Reconstr. Surg. 2021, 27, e614–e619. [Google Scholar] [CrossRef]
- Kisby, C.K.; Polin, M.R.; Visco, A.G.; Siddiqui, N.Y. Same-Day Discharge after Robotic-Assisted Sacrocolpopexy. Female Pelvic. Med. Reconstr. Surg. 2019, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Illiano, E.; Trama, F.; Crocetto, F.; Califano, G.; Aveta, A.; Motta, G.; Pastore, A.L.; Brancorsini, S.; Fabi, C.; Costantini, E. Prolapse Surgery: What Kind of Antibiotic Prophylaxis Is Necessary? Urol. Int. 2021, 105, 771–776. [Google Scholar] [CrossRef]
- Adamina, M.; Kehlet, H.; Tomlinson, G.A.; Senagore, A.J.; Delaney, C.P. Enhanced recovery pathways optimize health outcomes and resource utilization: A meta-analysis of randomized controlled trials in colorectal surgery. Surgery 2011, 149, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Ore, A.S.; Shear, M.A.; Liu, F.W.; Dalrymple, J.L.; Awtrey, C.S.; Garrett, L.; Stack-Dunnbier, H.; Hacker, M.R.; Esselen, K.M. Adoption of enhanced recovery after laparotomy in gynecologic oncology. Int. J. Gynecol. Cancer 2020, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
| Operating Time in Minutes | Blood Loss in Milliliters | Length of Hospital Stay in Hours | Readmission Rate after Initial Hospitalization | Total Hospital Costs by Currency of Origin and Euro Equivalent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bE | wE | S | bE | wE | S | bE | wE | S | bE | wE | S | bE | wE | S | |
| Carter-Brooks [7] | 156 | 156 | 0.96 * | 64 | 78 | 0.35 * | 25.9 | 12.2 | <0.01 * | 1.5% | 6.7% | 0.03 * | - | - | - |
| Evans [8] | 160 | 161 | 0.48 * | 50 | 50 | 2 * | 28 | 26 | 0.01 * | - | - | - | - | - | - |
| Gong [11] | 66 | 58 | 0.16 * | 90 | 92 | 0.26 * | 121.4 | 70.3 | <0.01 * | - | - | - | CNY 46,838.65 EUR 6511 | CNY 42,793.57 EUR 5948 | CNY 4045.08 EUR 562 (p < 0.01) |
| Abrao Trad [12] | - | - | - | - | - | - | - | - | - | 6.3% | 6.3% | NS | - | - | USD 869 EUR 769 |
| Trowbridge [13] | 165 | 156 | 0.19 * | 102 | 97 | 0.72 * | 29.9 | 27.9 | 0.04 * | 3.9% | 3.4% | 0.84 * | USD 7908 EUR 6997.30 | USD 8072 EUR 7142.42 | USD 164 EUR 145.11 (p = 0.79) |
| Evans [14] | 194 | 178 | 0.34 * | 50 | 50 | 0.60 * | 27.1 | 12.1 | <0.01 * | - | - | - | - | - | - |
| Pan [15] | 253 | 249 | 8–15 † | 124 | 173 | 31.2–70.4 † | 44.2 | 33 | 6.4–16.0 † | - | - | - | - | - | - |
| Carter-Brooks [16] | - | - | - | - | - | - | 25.5 | 10.5 | - | - | - | - | - | - | - |
| Relph [9] | - | - | - | - | - | - | 42.9 | 23.5 | - | 0% | 0% | NS | GBP 1302 EUR 1548 | GBP 1137 EUR 1352 | GBP 165 EUR 196 |
| Yoong [10] | 90 | 90 | NS | 200 | 175 | NS | 45.5 | 22 | <0.01 * | 0% | 4% | 0.29 * | GBP 1149 EUR 1366 | GBP 1042 EUR 1239 | GBP 106 EUR 94 |
| Complications Taken into Account | Results | |
|---|---|---|
| Within 30 Days of Surgery | ||
| Carter- Brooks [7] | Intra-operative complications Hospital complications, including hypoxia, chest pain, arrhythmia, hyponatremia, uncontrolled pain, oliguria, nausea/ileus, scar complications Discharge complications, including urinary disorders, scar complications, angina, arrhythmia, nausea/ileus, hematoma, dizziness and ureteral obstruction Unscheduled post-hospitalization consultation Emergency room visit Readmission, including myocardial infarction, chest pain, arrhythmia, hyponatremia, scar complications, nausea/ileus, and ureteral obstruction Surgical re-intervention Urinary tract infection | 31.4% before ERAS 35.5% with ERAS p = 0.48 |
| Evans [8] | Post-operative complications | 5% before and 11% with ERAS, p = 0.15 |
| Emergency room visits | 4% before and with ERAS, p = 0.99 | |
| Unscheduled consultations | 12% before and 7% with ERAS, p = 0.29 | |
| Phone calls, median per patient | 1 before and with ERAS, p = 0.08 | |
| Abrao Trad [12] | NA | 6.6% before and with ERAS |
| Trowbridge[13] | Surgical site infection, urinary tract infection, blood transfusion, unplanned surgical re-intervention, pneumonia, pulmonary embolism, unplanned intubation, acute renal failure, cardiac arrest, septic shock, sepsis, death within 30 days, readmission | 6.58% before ERAS 8.47% with ERAS p = 0.79 |
| Carter- Brooks [16] | Any aberration in the normal recovery | 7.6% before and 12.8% with ERAS, p = 0.121 |
| Yoong [10] | Emergency room visits | 0% before and 12.0% with ERAS, p = 0.05 |
| One year after surgery | ||
| Gong [11] | Anterior mesh exposure | 9% before and 6% with ERAS, p = 0.48 |
| Posterior mesh exposure | 6.4% before and 3.7% with ERAS, p = 0.96 | |
| Pain or discomfort during sexual intercourse | 34% before and 35% with ERAS, p = 0.115 (vs. 30% and 31% before surgery (p = 0.19)) | |
| Chronic pelvic pain | 5.1% before and 7.3% with ERAS, p = 0.57 | |
| De novo urinary incontinence | 0% before and with ERAS | |
| Recurrence of prolapse | 0% before and with ERAS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tresch, C.; Lallemant, M.; Ramanah, R. Enhanced Recovery after Pelvic Organ Prolapse Surgery. J. Clin. Med. 2023, 12, 5911. https://doi.org/10.3390/jcm12185911
Tresch C, Lallemant M, Ramanah R. Enhanced Recovery after Pelvic Organ Prolapse Surgery. Journal of Clinical Medicine. 2023; 12(18):5911. https://doi.org/10.3390/jcm12185911
Chicago/Turabian StyleTresch, Caroline, Marine Lallemant, and Rajeev Ramanah. 2023. "Enhanced Recovery after Pelvic Organ Prolapse Surgery" Journal of Clinical Medicine 12, no. 18: 5911. https://doi.org/10.3390/jcm12185911
APA StyleTresch, C., Lallemant, M., & Ramanah, R. (2023). Enhanced Recovery after Pelvic Organ Prolapse Surgery. Journal of Clinical Medicine, 12(18), 5911. https://doi.org/10.3390/jcm12185911






