Frequent Follow-Up of Delisted Liver Transplant Candidates Is Necessary: An Observational Study about Characteristics and Outcomes of Delisted Liver Transplant Candidates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Listing and Delisting Criteria/Policy
2.3. Variables and Statistical Analysis
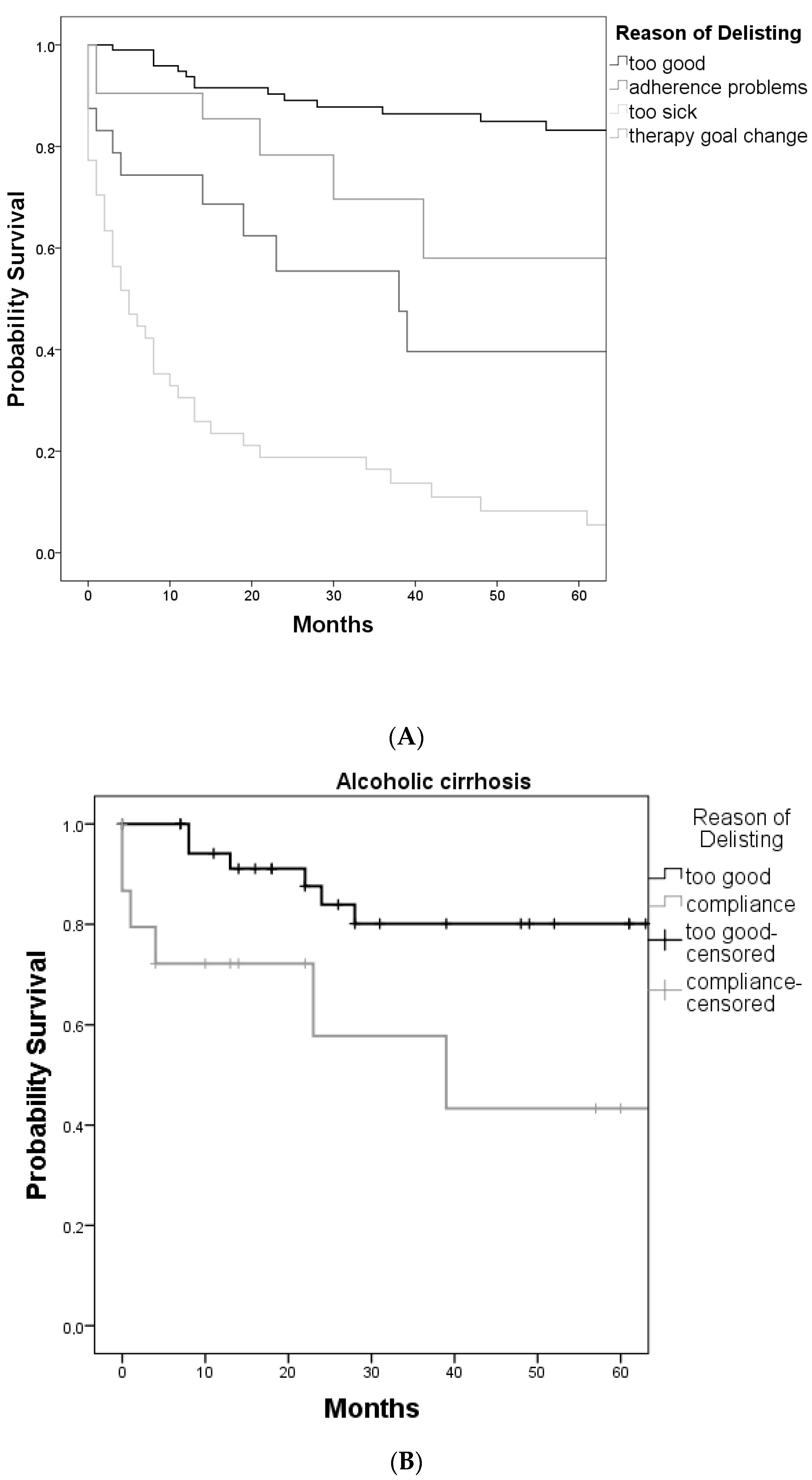
3. Results
3.1. Study Population and Patients Characteristics
3.2. Primary Indications and Reasons for Delisting
3.3. Outcome after Delisting
3.4. Relisting after Delisting
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samuel, D.; Coilly, A. Management of patients with liver diseases on the waiting list for transplantation: A major impact to the success of liver transplantation. BMC Med. 2018, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; van Rosmalen, M.; Pirenne, J.; Samuel, U. Adult Liver Allocation in Eurotransplant. Transplantation 2017, 101, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.D.; Barbas, A.S.; Doyle, A.C.; Tazari, M.; Sapisochin, G.; Cattral, M.S.; Ghanekar, A.; McGilvray, I.D.; Selzner, M.; Greig, P.D.; et al. Characteristics of liver transplant candidates delisted following recompensation and predictors of such delisting in alcohol-related liver disease: A case-control study. Transpl. Int. 2017, 30, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Bababekov, Y.J.; Hung, Y.C.; Chang, D.C.; Rickert, C.G.; Adler, J.T.; Bethea, E.; Pomfret, E.A.; Pomposelli, J.J.; Yeh, H. Do Social Determinants Define “Too Sick” to Transplant in Patients with End-stage Liver Disease? Transplantation 2020, 104, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Cullaro, G.; Sarkar, M.; Lai, J.C. Sex-based disparities in delisting for being “too sick” for liver transplantation. Am. J. Transplant. 2018, 18, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Karunungan, K.L.; Sanaiha, Y.; Hernandez, R.A.; Wilhalme, H.; Rudasill, S.; Hadaya, J.; DiNorcia, J.; Benharash, P. Impact of Payer Status on Delisting Among Liver Transplant Candidates in the United States. Liver Transplant. 2020, 27, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Leanza, J.; Massie, A.B.; Garonzik-Wang, J.M.; Haugen, C.E.; Gentry, S.E.; Ottmann, S.E.; Segev, D.L. MELD as a metric for survival benefit of liver transplantation. Am. J. Transplant. 2018, 18, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Poonja, Z.; Brisebois, A.; van Zanten, S.V.; Tandon, P.; Meeberg, G.; Karvellas, C.J. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin. Gastroenterol. Hepatol. 2014, 12, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Belli, L.S.; Berenguer, M.; Cortesi, P.A.; Strazzabosco, M.; Rockenschaub, S.-R.; Martini, S.; Morelli, C.; Donato, F.; Volpes, R.; Pageaux, G.-P.; et al. Delisting of liver transplant candidates with chronic hepatitis C after viral eradication: A European study. J. Hepatol. 2016, 65, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.B.; Ehmer, U.; Umgelter, A.; Bucher, J.N.; Koch, D.T.; Börner, N.; Nieß, H.; Denk, G.; De Toni, E.N.; Seidensticker, M.; et al. Liver Transplantation versus Watchful-Waiting in Hepatocellular Carcinoma Patients with Complete Response to Bridging-Therapy. Transpl. Int. 2020, 34, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Schoenberg, M.B.; Anger, H.J.W.; Bucher, J.N.; Denk, G.; De Toni, E.N.; Seidensticker, M.; Andrassy, J.; Angele, M.K.; Werner, J.; Guba, M.O. Liver Transplantation for Extended Criteria Hepatocellular Carcinoma Using Stable Response to Locoregional Therapy and Alpha-Fetoprotein as Selection Criteria. Visc. Med. 2020, 36, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C. Defining the threshold for too sick for transplant. Curr. Opin. Organ Transplant. 2016, 21, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Linecker, M.; Krones, T.; Berg, T.; Niemann, C.U.; Steadman, R.H.; Dutkowski, P.; Clavien, P.-A.; Busuttil, R.W.; Truog, R.D.; Petrowsky, H. Potentially inappropriate liver transplantation in the era of the “sickest first” policy—A search for the upper limits. J. Hepatol. 2018, 68, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.J.; Flores, A.; Saracino, G.; Boutté, J.; McKenna, G.; Testa, G.; Bahirwani, R.; Wall, A.; Kim, W.R.; Klintmalm, G.; et al. Center Variation in Intention-to-Treat Survival Among Patients Listed for Liver Transplant. Liver Transplant. 2020, 26, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
| Overall (n = 196) | “Too Good” (n = 106) | “Too Sick” (n = 44) | Adherence Problems (n = 24) | Therapy Goal Change (n = 22) | p-Value | |
|---|---|---|---|---|---|---|
| Age at listing (years) * | 52 (14.25) | 52 (16.5) | 54 (13.25) | 48 (13) | 54 (13.5) | 0.32 |
| Age at delisting (years) * | 55 (16) | 55 (17) | 55 (13.25) | 50.5 (14) | 57 (14) | 0.62 |
| Gender (m/f) | 123/73 | 63/43 | 35/9 | 12/12 | 13/9 | 0.055 |
| BMI at listing (range) | 24 (14–43) | 24 (14–39) | 24 (19–43) | 24 (15–37) | 24 (18–35) | 0.74 |
| Blood group (%) | 0.49 | |||||
| O | 66 (33.7) | 35 (33) | 16 (36.4) | 7 (29.2) | 8 (36.4) | |
| A | 98 (50) | 52 (49.1) | 20 (45.5) | 17 (70.8) | 9 (40.9) | |
| B | 21 (10.7) | 12 (11.3) | 5 (11.4) | 0 (0) | 4 (18.2) | |
| AB | 11 (5.6) | 7 (6.6) | 3 (6.8) | 0 (0) | 1 (4.5) | |
| Insurance status (n/%) | 0.84 | |||||
| private | 30 (15.3) | 18 (16.9) | 5 (11.4) | 4 (16.7) | 3 (13.6) | |
| public | 166 (84.7) | 88 (83.1) | 39 (88.6) | 20 (83.3) | 19 (86.4) | |
| Waiting time (months) * | 21 (41) | 28 (46) | 7 (18) | 18 (25) | 24 (38) | |
| labMELD * | ||||||
| at listing | 13 (7) | 14 (9) | 12 (6) | 16 (3) | 12 (8) | 0.26 |
| at delisting | 11 (7) | 10 (5) | 14 (7) | 17 (9) | 12 (7) | 0.80 |
| Etiology of LD (%) | ||||||
| ALF | 14 (7.1) | 11 (10.4) | 2 (4.5) | 0 | 1 (4.5) | |
| ALD w/o HCC | 58 (29.6) | 37 (34.9) | 4 (9.1) | 15 (62.5) | 2 (9.1) | |
| HBV w/o HCC | 6 (3.1) | 4 (3.8) | 0 | 0 | 2 (9.1) | |
| HBV/HDV w/o HCC | 2 (1) | 1 (0.9) | 1 (2.3) | 0 | 0 | |
| HCV w/o HCC | 16 (8.2) | 7 (6.6) | 6 (13.6) | 2 (8.3) | 1 (4.5) | |
| HCC in ALD | 18 (9.2) | 5 (4.7) | 10 (22.7) | 1 (4.2) | 2 (9.1) | |
| HCC in HBV | 6 (3.1) | 1 (0.9) | 5 (11.4) | 0 | 0 | |
| HCC in HCV | 11 (5.6) | 2 (1.9) | 6 (13.6) | 2 (8.3) | 1 (4.5) | |
| HCC unknown | 10 (5.1) | 4 (3.8) | 2 (4.5) | 0 | 4 (18.2) | |
| AIH w/o HCC | 5 (2.6) | 4 (3.8) | 0 | 0 | 1 (4.5) | |
| Cholestatic LD | 17 (8.7) | 11 (10.4) | 2 (4.5) | 2 (8.3) | 2 (9.1) | |
| Cryptogenic LC | 8 (4.1) | 3 (2.8) | 2 (4.5) | 1 (4.2) | 2 (9.1) | |
| Metabolic/genetic | 18 (9.2) | 11 (10.4) | 4 (9.1) | 1 (4.2) | 2 (9.1) | |
| Other | 7(3.5) | 5 (4.7) | 0 | 0 | 2 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payani, E.; Koliogiannis, D.; Schoenberg, M.B.; Koch, D.; Eser-Valeri, D.; Denk, G.; Rehm, M.; Schäfer, S.; Ehmer, U.; Kremer, A.E.; et al. Frequent Follow-Up of Delisted Liver Transplant Candidates Is Necessary: An Observational Study about Characteristics and Outcomes of Delisted Liver Transplant Candidates. J. Clin. Med. 2023, 12, 5880. https://doi.org/10.3390/jcm12185880
Payani E, Koliogiannis D, Schoenberg MB, Koch D, Eser-Valeri D, Denk G, Rehm M, Schäfer S, Ehmer U, Kremer AE, et al. Frequent Follow-Up of Delisted Liver Transplant Candidates Is Necessary: An Observational Study about Characteristics and Outcomes of Delisted Liver Transplant Candidates. Journal of Clinical Medicine. 2023; 12(18):5880. https://doi.org/10.3390/jcm12185880
Chicago/Turabian StylePayani, Elnaz, Dionysios Koliogiannis, Markus Bo Schoenberg, Dominik Koch, Daniela Eser-Valeri, Gerald Denk, Markus Rehm, Simon Schäfer, Ursula Ehmer, Andreas E. Kremer, and et al. 2023. "Frequent Follow-Up of Delisted Liver Transplant Candidates Is Necessary: An Observational Study about Characteristics and Outcomes of Delisted Liver Transplant Candidates" Journal of Clinical Medicine 12, no. 18: 5880. https://doi.org/10.3390/jcm12185880
APA StylePayani, E., Koliogiannis, D., Schoenberg, M. B., Koch, D., Eser-Valeri, D., Denk, G., Rehm, M., Schäfer, S., Ehmer, U., Kremer, A. E., Meiser, B., Werner, J., Guba, M., & Börner, N. (2023). Frequent Follow-Up of Delisted Liver Transplant Candidates Is Necessary: An Observational Study about Characteristics and Outcomes of Delisted Liver Transplant Candidates. Journal of Clinical Medicine, 12(18), 5880. https://doi.org/10.3390/jcm12185880








