MDS-Related Anemia Is Associated with Impaired Quality of Life but Improvement Is Not Always Achieved by Increased Hemoglobin Level
Abstract
:1. Introduction
2. Methods
2.1. Study Design & Patients
2.2. QoL
2.3. Analysis
2.4. Statistics
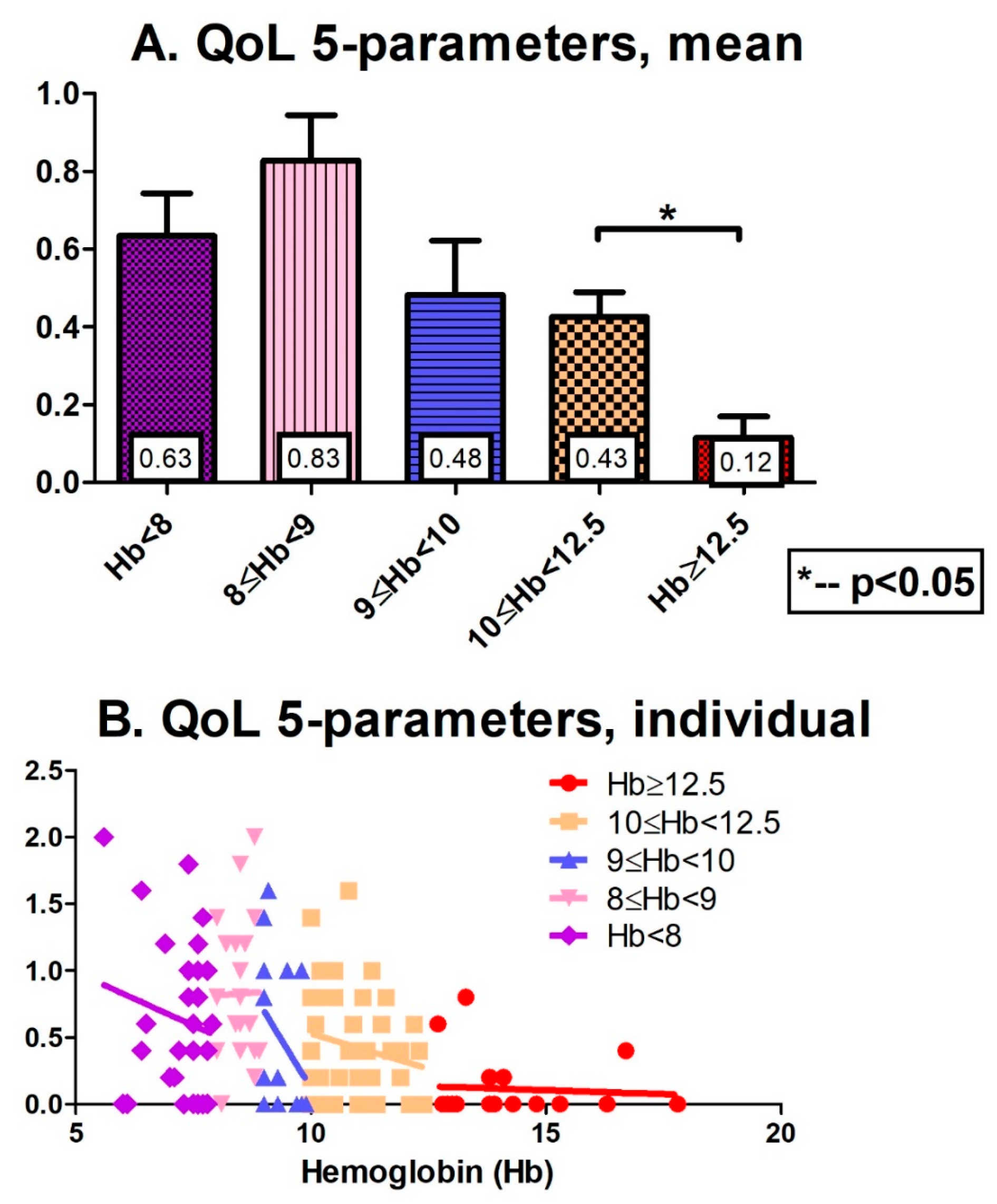
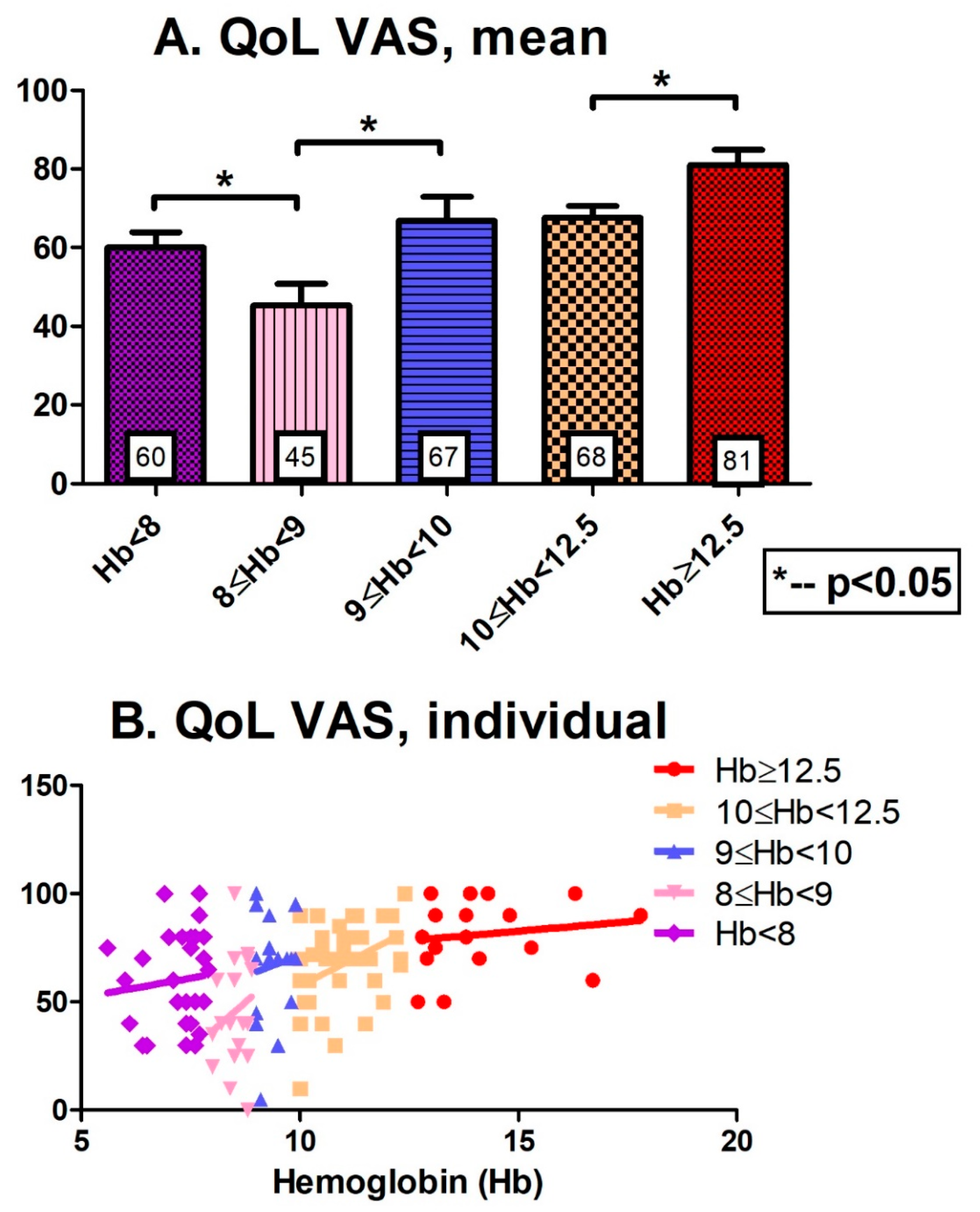
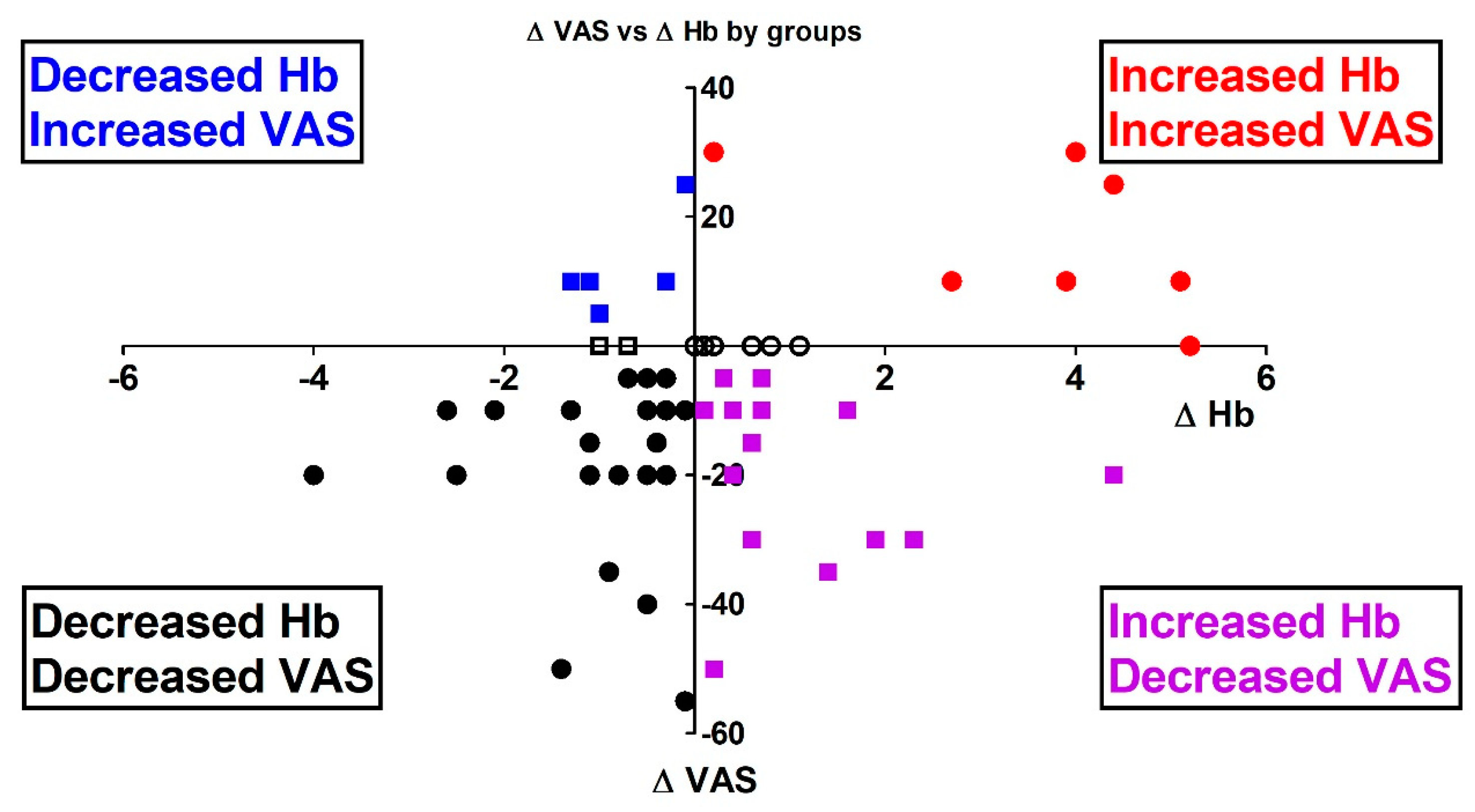
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UpToDate. Clinical Manifestations, Diagnosis, and Classification of Myelodysplastic Syndromes (MDS); UpToDate Inc.: Waltham, MA, USA, 2023; Available online: https://www.uptodate.com (accessed on 5 June 2023).
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef]
- Mittelman, M. The myelodysplastic syndromes. Isr. J. Med. Sci. 1990, 26, 468–478. [Google Scholar]
- Sekeres, M.A.; Taylor, J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef]
- Malcovati, L.; Hellstrom-Lindberg, E.; Bowen, D.; Adès, L.; Cermak, J.; del Cañizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964. [Google Scholar] [CrossRef] [PubMed]
- Cella, D. Factors influencing quality of life in cancer patients: Anemia and fatigue. Semin. Oncol. 1998, 25, 43–46. [Google Scholar] [PubMed]
- Oliva, E.N.; Platzbecker, U.; Fenaux, P.; Garcia-Manero, G.; LeBlanc, T.W.; Patel, B.J.; Kubasch, A.S.; Sekeres, M.A. Targeting health-related quality of life in patients with myelodysplastic syndromes—Current knowledge and lessons to be learned. Blood Rev. 2021, 50, 100851. [Google Scholar] [CrossRef]
- Oliva, E.N.; Dimitrov, B.D.; Benedetto, F.; D’Angelo, A.; Nobile, F. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk. Res. 2005, 29, 1217–1219. [Google Scholar] [CrossRef]
- Rosko, A.E.; Cordoba, R.; Abel, G.; Artz, A.; Loh, K.P.; Klepin, H.D. Advances in Management for Older Adults with Hematologic Malignancies. J. Clin. Oncol. 2021, 39, 2102–2114. [Google Scholar] [CrossRef]
- Steensma, D.P.; Heptinstall, K.V.; Johnson, V.M.; Novotny, P.J.; Sloan, J.A.; Camoriano, J.K.; Niblack, J.; Bennett, J.M.; Mesa, R.A. Common troublesome symptoms and their impact on quality of life in patients with myelodysplastic syndromes (MDS): Results of a large internet-based survey. Leuk. Res. 2008, 32, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Koinig, K.; Cottone, F.; Bowen, D.; Mittelman, M.; Sommer, K.; Langemeijer, S.; Culligan, D.; Filanovsky, K.; Storck, M.; et al. Raising the standards of patient-centered outcomes research in myelodysplastic syndromes: Clinical utility and validation of the subscales of the QUALMS from the MDS-RIGHT project. Cancer Med. 2023, 12, 7529–7539. [Google Scholar] [CrossRef]
- Stauder, R.; Yu, G.; Koinig, K.A.; Bagguley, T.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Cermak, J.; Mittelman, M.; Hellström-Lindberg, E.; et al. Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: A European LeukemiaNet study. Leukemia 2018, 32, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- De Swart, L.; Smith, A.; Johnston, T.W.; Haase, D.; Droste, J.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Hellström-Lindberg, E.; Cermák, J.; et al. Validation of the revised international prognostic scoring system (IPSS-R) in patients with lower-risk myelodysplastic syndromes: A report from the prospective European LeukaemiaNet MDS (EUMDS) registry. Br. J. Haematol. 2015, 170, 372–383. [Google Scholar] [PubMed]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Efficace, F.; Cottone, F.; Oswald, L.B.; Cella, D.; Patriarca, A.; Niscola, P.; Breccia, M.; Platzbecker, U.; Palumbo, G.A.; Caocci, G.; et al. The IPSS-R more accurately captures fatigue severity of newly diagnosed patients with myelodysplastic syndromes compared with the IPSS index. Leukemia 2020, 34, 2451–2459. [Google Scholar] [CrossRef]
- Oliva, E.N.; Platzbecker, U.; Garcia-Manero, G.; Mufti, G.J.; Santini, V.; Sekeres, M.A.; Komrokji, R.S.; Shetty, J.K.; Tang, D.; Guo, S.; et al. Health-Related Quality of Life Outcomes in Patients with Myelodysplastic Syndromes with Ring Sideroblasts Treated with Luspatercept in the MEDALIST Phase 3 Trial. J. Clin. Med. 2021, 11, 27. [Google Scholar] [CrossRef]
- Stojkov, I.; Conrads-Frank, A.; Rochau, U.; Koinig, K.A.; Arvandi, M.; Puntscher, S.; van Marrewijk, C.J.; Fenaux, P.; Symeonidis, A.; Chermat, F.; et al. Core set of patient-reported outcomes for myelodysplastic syndromes: An EUMDS Delphi study involving patients and hematologists. Blood Adv. 2022, 6, 1–12. [Google Scholar] [CrossRef]
- Rochau, U.; Stojkov, I.; Conrads-Frank, A.; Borba, H.H.; Koinig, K.A.; Arvandi, M.; van Marrewijk, C.; Garelius, H.; Germing, U.; Symeonidis, A.; et al. Development of a core outcome set for myelodysplastic syndromes—A Delphi study from the EUMDS Registry Group. Br. J. Haematol. 2020, 191, 405–417. [Google Scholar] [CrossRef]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at older age: Etiologies, clinical implications, and management. Blood 2018, 131, 505–514. [Google Scholar]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef]
- Wouters, H.; van der Klauw, M.M.; de Witte, T.; Stauder, R.; Swinkels, D.W.; Wolffenbuttel, B.H.; Huls, G. Association of anemia with health-related quality of life and survival: A large population-based cohort study. Haematologica 2019, 104, 468–476. [Google Scholar] [CrossRef]
- Regnault, A.; Pompilus, F.; Ciesluk, A.; Mazerolle, F.; Bejar, R.; Fram, R.J.; Faller, D.V.; Marquis, P.; Bell, J.A. Measuring patient-reported physical functioning and fatigue in myelodysplastic syndromes using a modular approach based on EORTC QLQ-C30. J. Patient Rep. Outcomes 2021, 5, 60. [Google Scholar] [CrossRef]
- Gamper, E.M.; Cottone, F.; Sommer, K.; Norman, R.; King, M.; Breccia, M.; Caocci, G.; Patriarca, A.; Palumbo, G.A.; Stauder, R.; et al. The EORTC QLU-C10D was more efficient in detecting clinical known group differences in myelodysplastic syndromes than the EQ-5D-3L. J. Clin. Epidemiol. 2021, 137, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Abel, G.A.; Klaassen, R.; Lee, S.J.; Young, N.L.; Cannella, L.; Steensma, D.P.; Efficace, F. Patient-reported outcomes for the myelodysplastic syndromes: A new MDS-specific measure of quality of life. Blood 2014, 123, 451–452. [Google Scholar] [CrossRef]
- Abel, G.A.; Efficace, F.; Buckstein, R.J.; Tinsley, S.; Jurcic, J.G.; Martins, Y.; Steensma, D.P.; Watts, C.D.; Raza, A.; Lee, S.J.; et al. Prospective international validation of the Quality of Life in Myelodysplasia Scale (QUALMS). Haematologica 2016, 101, 781–788. [Google Scholar] [CrossRef]
- Rabin, R.; de Charro, F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001, 33, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A. EQ-5D: Moving from Three Levels to Five. Value Health 2018, 21, 57–58. [Google Scholar] [CrossRef]
- Osaki, K.; Morishita, S.; Takami, S.; Sakai, Y.; Kamimura, A.; Shindo, A.; Kawata, E. Quality of life of patients with hematological malignancies and factors affecting health state utility values. Support. Care Cancer 2022, 30, 5319–5327. [Google Scholar] [CrossRef]
- Giesinger, J.M.; La Nasa, G.; Sparano, F.; Angermeyer, M.; Morelli, E.; Mulas, O.; Efficace, F.; Caocci, G. Health-Related Quality of Life Assessment in Patients with Myelodysplastic Syndromes: Evidence from Randomized Clinical Trials. Clin. Pract. Epidemiol. Ment. Health 2021, 17, 307–314. [Google Scholar] [CrossRef]
- Heyrman, B. Quality of Life in Low-Risk MDS: An Undervalued Endpoint. J. Clin. Med. 2022, 11, 5699. [Google Scholar] [CrossRef]
- Saad, S.T.; Vassallo, J.; Arruda, V.A.; Lorand-Metze, I. The role of bone marrow study in diagnosis and prognosis of myelodysplastic syndrome. Pathologica 1994, 86, 47–51. [Google Scholar]
- Mozessohn, L.; Li, Q.; Liu, N.; Leber, B.; Khalaf, D.; Sabloff, M.; Christou, G.; Yee, K.; Chodirker, L.; Parmentier, A.; et al. Impact of Frailty on Health Care Resource Utilization and Costs of Care in Myelodysplastic Syndromes. JCO Oncol. Pract. 2023, 19, e559–e569. [Google Scholar] [CrossRef]
- Wouters, H.; van Zeventer, I.A.; van der Klauw, M.M.; Wolffenbuttel, B.H.R.; Huls, G. Association Between Peripheral Blood Cell Count Abnormalities and Health-Related Quality of Life in the General Population. Hemasphere 2021, 5, e503. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.; Conrads-Frank, A.; Koinig, K.A.; Smith, A.; Yu, G.; de Witte, T.; Wolffenbuttel, B.H.R.; Huls, G.; Siebert, U.; Stauder, R.; et al. The anemia-independent impact of myelodysplastic syndromes on health-related quality of life. Ann. Hematol. 2021, 100, 2921–2932. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Buckstein, R.; Chodirker, L.; Yee, K.W.L.; Geddes, M.; Leitch, H.A.; Christou, G.; Banerji, V.; Leber, B.; Khalaf, D.; St-Hilaire, E.; et al. The burden of red blood cell transfusions in patients with lower-risk myelodysplastic syndromes and ring sideroblasts: An analysis of the prospective MDS-CAN registry. Leuk. Lymphoma 2023, 64, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Tanasijevic, A.M.; Revette, A.; Klepin, H.D.; Zeidan, A.; Townsley, D.; DiNardo, C.D.; Sebert, M.; DeZern, A.E.; Stone, R.M.; Magnavita, E.S.; et al. Consensus minimum hemoglobin level above which patients with myelodysplastic syndromes can safely forgo transfusions. Leuk. Lymphoma 2020, 61, 2900–2904. [Google Scholar] [CrossRef]
- Stanworth, S.J.; Killick, S.; McQuilten, Z.K.; Karakantza, M.; Weinkove, R.; Smethurst, H.; Pankhurst, L.A.; Hodge, R.L.; Hopkins, V.; Thomas, H.L.; et al. Red cell transfusion in outpatients with myelodysplastic syndromes: A feasibility and exploratory randomised trial. Br. J. Haematol. 2020, 189, 279–290. [Google Scholar] [CrossRef]
- Buckstein, R.J.; Prica, A.; Leber, B.; Heddle, N.; Yee, K.W.; Stanworth, S.J.; Bowen, D.; Chodirker, L.; Gallagher, J.; Parmentier, A.; et al. RBC-Enhance: A Randomized Pilot Feasibility Trial of Red Cell Transfusion Thresholds in Myelodysplastic Syndromes. Blood 2020, 136, 3–4. [Google Scholar] [CrossRef]
- Vijenthira, A.; Starkman, R.; Lin, Y.; Stanworth, S.J.; Bowen, D.; Harrison, L.; Wintrich, S.; Callum, J.; Buckstein, R. Multi-national survey of transfusion experiences and preferences of patients with myelodysplastic syndrome. Transfusion 2022, 62, 1355–1364. [Google Scholar] [CrossRef]
- Abel, G.A.; Buckstein, R. Integrating Frailty, Comorbidity, and Quality of Life in the Management of Myelodysplastic Syndromes. Am. Soc. Clin. Oncol. Educ. Book. 2016, 35, e337–e344. [Google Scholar] [CrossRef] [PubMed]
- Buckstein, R.J. Integrating patient-centered factors in the risk assessment of MDS. Hematol. Am. Soc. Hematol. Educ. Program. 2019, 2019, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, M.T.; Huisingh-Scheetz, M.; Meltzer, D. Sex differences in fatigue and symptoms of anemia in relation to hemoglobin level in hospitalized patients. Ann. Hematol. 2022, 101, 1873–1875. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, I.; Conrads-Frank, A.; Rochau, U.; Arvandi, M.; Koinig, K.A.; Schomaker, M.; Mittelman, M.; Fenaux, P.; Bowen, D.; Sanz, G.F.; et al. Determinants of low health-related quality of life in patients with myelodysplastic syndromes: EUMDS registry study. Blood Adv. 2023, 7, 2772–2783. [Google Scholar] [CrossRef]
- Abel, G.A.; Klepin, H.D.; Magnavita, E.S.; Jaung, T.; Lu, W.; Shallis, R.M.; Hantel, A.; Bahl, N.E.; Dellinger-Johnson, R.; Winer, E.S.; et al. Peri-transfusion quality-of-life assessment for patients with myelodysplastic syndromes. Transfusion 2021, 61, 2830–2836. [Google Scholar] [CrossRef]

| All Patients | No Anemia/Normal | Mild Anemia | Moderate Anemia | Severe Anemia | Very Severe Anemia | |
|---|---|---|---|---|---|---|
| n | 127 | 19 | 41 | 17 | 21 | 29 |
| F/M | 44/81 (35%/65%) | 7/12 (37%/63%) | 17/24 (41%/59%) | 7/10 (41%/59%) | 3/18 (15%/85%) | 12/17 (41%/59%) |
| Age mean (range) | 74.9 (36–91) | 67.6 (36–87) | 77.3 (62–89) | 75.1 (57–88) | 75.1 (40–91) | 75.8 (57–90) |
| Hb, Mean (g/dL) | 10.0 | 14.1 | 11.0 | 9.4 | 8.5 | 7.2 |
| Mean MCV (Fl) | 95.2 | 90.9 | 95.6 | 98.0 | 95.0 | 96.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haring, Y.; Goldschmidt, N.; Taha, S.; Stemer, G.; Filanovsky, K.; Hellman, I.; Okasha, D.; Krayem, B.; Levi, I.; Rosenbaum, H.; et al. MDS-Related Anemia Is Associated with Impaired Quality of Life but Improvement Is Not Always Achieved by Increased Hemoglobin Level. J. Clin. Med. 2023, 12, 5865. https://doi.org/10.3390/jcm12185865
Haring Y, Goldschmidt N, Taha S, Stemer G, Filanovsky K, Hellman I, Okasha D, Krayem B, Levi I, Rosenbaum H, et al. MDS-Related Anemia Is Associated with Impaired Quality of Life but Improvement Is Not Always Achieved by Increased Hemoglobin Level. Journal of Clinical Medicine. 2023; 12(18):5865. https://doi.org/10.3390/jcm12185865
Chicago/Turabian StyleHaring, Yael, Noa Goldschmidt, Shaimaa Taha, Galia Stemer, Kalman Filanovsky, Ilana Hellman, Doaa Okasha, Baher Krayem, Itai Levi, Hanna Rosenbaum, and et al. 2023. "MDS-Related Anemia Is Associated with Impaired Quality of Life but Improvement Is Not Always Achieved by Increased Hemoglobin Level" Journal of Clinical Medicine 12, no. 18: 5865. https://doi.org/10.3390/jcm12185865
APA StyleHaring, Y., Goldschmidt, N., Taha, S., Stemer, G., Filanovsky, K., Hellman, I., Okasha, D., Krayem, B., Levi, I., Rosenbaum, H., Koren-Michowitz, M., Yagna, S., Nemets, A., Gino-Moor, S., Saban, R., Cohen, J., Halperin, E., Wolach, O., Dally, N., ... Mittelman, M., on behalf of the Israel MDS Working Group. (2023). MDS-Related Anemia Is Associated with Impaired Quality of Life but Improvement Is Not Always Achieved by Increased Hemoglobin Level. Journal of Clinical Medicine, 12(18), 5865. https://doi.org/10.3390/jcm12185865






