Abstract
Brain function and its effect on motor performance in Duchenne muscular dystrophy (DMD) is an emerging concept. The present study explored how cumulative dystrophin isoform loss, age, and a corticosteroid treatment affect DMD motor outcomes. A total of 133 genetically confirmed DMD patients from Sri Lanka were divided into two groups based on whether their shorter dystrophin isoforms (Dp140, Dp116, and Dp71) were affected: Group 1, containing patients with Dp140, Dp116, and Dp71 affected (n = 98), and Group 2, containing unaffected patients (n = 35). A subset of 52 patients (Group 1, n = 38; Group 2, n = 14) was followed for up to three follow-ups performed in an average of 28-month intervals. The effect of the cumulative loss of shorter dystrophin isoforms on the natural history of DMD was analyzed. A total of 74/133 (56%) patients encountered developmental delays, with 66/74 (89%) being in Group 1 and 8/74 (11%) being in Group 2 (p < 0.001). Motor developmental delays were predominant. The hip and knee muscular strength, according to the Medical Research Council (MRC) scale and the North Star Ambulatory Assessment (NSAA) activities, “standing on one leg R”, “standing on one leg L”, and “walk”, declined rapidly in Group 1 (p < 0.001 In the follow-up analysis, Group 1 patients became wheelchair-bound at a younger age than those of Group 2 (p = 0.004). DMD motor dysfunction is linked to DMD mutations that affect shorter dystrophin isoforms. When stratifying individuals for clinical trials, considering the DMD mutation site and its impact on a shorter dystrophin isoform is crucial.
1. Introduction
With an incidence rate of 4.80 per 100,000 [1], Duchenne muscular dystrophy (DMD, OMIM #310200), stands as the most prevalent form of muscular dystrophy in the pediatric population [2]. Thus far, a plethora of mutations in the DMD gene leading to the production of a truncated, nonfunctional dystrophin protein have been identified as the etiology of this X-linked recessive disorder [3].
The DMD gene is known to produce a minimum of seven major isoforms or variants of the dystrophin protein (Dp) through the utilization of seven established promoters [4]. From the upstream promoter/first exon sequences, three full-length isoforms are derived: Dp427m for muscle, Dp427c/b for cerebral, and Dp427p for Purkinje. Intriguingly, Dp427m and Dp427c are ubiquitously distributed across the cortex and basal ganglia in the human brain, with the hippocampus and amygdala exhibiting the highest expression levels and the cerebellum displaying the lowest. In addition, it is noteworthy that there exist four shorter isoforms, namely Dp260, Dp140, Dp116, and Dp71, with Dp140 and Dp71 being the most predominantly expressed in the brain. Conversely, the Dp260 and Dp116 isoforms are not expressed in the brain, with the former being exclusive to the retina and the latter exclusive to peripheral nerves [5,6,7] (Figure 1).
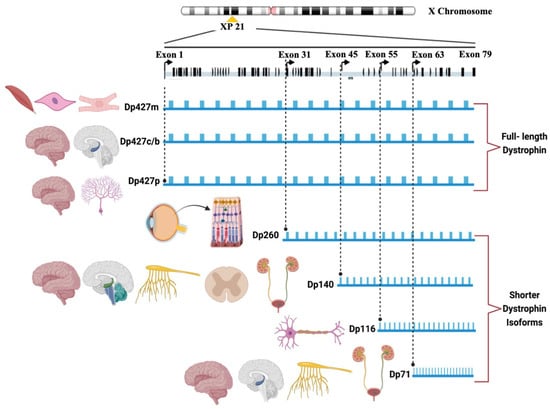
Figure 1.
The DMD isoforms and their expression patterns.
The significance of Dp140 and Dp71 in the involvement of the central nervous system (CNS) in DMD has been extensively studied [4,8,9,10,11,12] and was reviewed by Doorenweerd N et al., 2020 [13]. However, the relationship between brain function and motor performance in individuals with DMD is an emerging concept. In this context, Chesshyre et al., 2022, and Coratti et al., 2022, [12,14] reported that DMD boys lacking both the Dp140 and Dp71 isoforms experienced a notable decrease in their motor function as assessed by the North Star Ambulatory Assessment (NSAA), highlighting the cumulative effect of brain dystrophin isoform loss on motor performance. Further, the reported variations in motor function may be attributed to the direct influence of CNS involvement in motor executive function [12,14].
The objective of this study was to analyze the impact of multiple variables, including the cumulative loss of shorter dystrophin isoforms (Dp140, Dp71, and Dp116), age, and corticosteroid treatment, on the variability of motor outcomes in a group of 138 genetically confirmed DMD patients from a developing country, Sri Lanka, a geographically defined population in South Asia. To the best of our knowledge, the present study is the first of its kind from a South Asian perspective.
2. Materials and Methods
2.1. Patient Recruitment
Patient recruitment was conducted through neurology clinics in various government hospitals across Sri Lanka’s western, northwestern, north-central, central, southern, and northern provinces, as well as through pro bono mobile clinics and home visits.
A total of 138 male patients (age range (mean): 1.5–18 Yrs (8 Yrs)) genetically confirmed through multiplex ligation-dependent probe amplification (MLPA) (SALSA MLPA Kit P034/P035 DMD/Becker, MRC Holland, Amsterdam, the Netherlands) to have deletions/duplications in the DMD gene were enrolled in the study. The molecular diagnostic procedure was established by utilizing the primary molecular diagnostic recommendations, as outlined by Abbs et al. in 2010 [15], as well as the revised edition by Fratter et al. in 2020, in alignment with the European Molecular Quality Genetics Network’s (EMQN’s) optimal practice guidelines for genetic testing in dystrophinopathies [16].
The sociodemographic characteristics and clinical data of the patients were documented using a standard questionnaire and clinical batteries that included the NSAA, Vignos scale, Brooke scale, and Medical Research Council (MRC) scale for muscle strength. It is important to note that the clinical assessments, which included evaluating muscle strength using the MRC scale, were conducted by a medical practitioner by the pro bono research team under the guidance and observation of the principal investigator and the respective consultant neurologist or pediatric neurologist who was treating the patient. Information on developmental milestones was directly recorded from the child health development record (CHDR) of the Family Health Bureau, Ministry of Health Nutrition and Indigenous Medicine, Sri Lanka, during the baseline evaluation of the patient. Follow-ups were conducted for 52 patients, for which data from an extended 2nd follow-up were available for 20 patients, and follow-up data for up to the 3rd follow-up were available for 3 patients. The follow-ups were conducted at the following time intervals: the first follow-up occurred 24 months after the initial assessment, the second follow-up took place 48 months after the initial assessment, and the third follow-up was conducted 84 months after the initial assessment.
Every participant provided written informed consent where applicable. The assent of a proxy was obtained for patients unable to provide their own consent. This study adheres to the ethical standards of Sri Lankan institutional review boards that follow the Declaration of Helsinki (ethical approval Nos. 449/09 and 38/19 from the Ethics Review Committee, Faculty of Medical Sciences, University of Sri Jayewardenepura, and ethical approval No. LRH/D/06/2007 from the Lady Ridgeway Hospital for Children, Colombo 08, 0800, Sri Lanka).
2.2. Patient Categorization
Based on the mutation location and the published literature [4,5,10], missing dystrophin isoforms (Dp427m, Dp427c, Dp427p, Dp260, Dp140, Dp116, and Dp71) were predicted. The patients were categorized into the following two groups by considering whether single or multiple, shorter dystrophin isoforms expressed in the CNS (Dp140 and Dp71) and the peripheral nervous system (PNS) (Dp116) were affected or not. Group 1 comprised patients with single or multiple, shorter dystrophin isoforms expressed in the CNS and PNS that were affected (n = 98), and Group 2 comprised patients with shorter dystrophin isoforms expressed in the CNS and PNS that were not affected (n = 35).
2.3. Statistical Analysis
ANOVA tests and multivariate analysis were performed to analyze the effect of shorter dystrophin isoforms expressed in the CNS and PNS on the performance of patients in the motor function scales and their subsets. After conducting the exploratory data analysis, the impact of the averaged power variables (e.g., average ankle power) and the scores for NSAA subcategories across different age groups were examined while also considering the group effect. Regression models were fitted to analyze the relationship between the average power variable and age, as well as the NSAA subcategory scores and age, for Group 1 and Group 2, respectively. Scatterplots were used to visually compare the results. A Weibull regression analysis was performed using the follow-up data to identify the effect of shorter dystrophin isoforms expressed in the CNS and PNS on the age at which patients became wheelchair-bound, with the ages of the as-yet unaffected patients treated as censored observations. A statistical analysis was performed using R statistical software, version 4.2. Microsoft Power BI software was used for data visualization. Outlier analysis was performed statistically (Supplementary Figure S1), and 5 patients were identified as outliers and removed from further statistical analyses, leaving 133 patients for the final analysis (Table 1).

Table 1.
Summary of demographic factors for each patient group at baseline.
3. Results
3.1. Genotype and Demographic Characteristics of the Patient Cohort
A total of 133 male patients (age range (mean): 1.5–16 Yrs (8 Yrs)) genetically confirmed through MLPA to have deletions/duplications in the DMD gene were enrolled in the study. We observed the clustering of deletion mutations in exons 45–55 and regions 6–15 of the DMD gene and the clustering of duplications in exons 6–10 (Figure 2a). Almost all the patients had a defective Dp427m, Dp427c, and Dp427p, while mutations affecting the Dp116 and Dp71 isoforms were rare (Figure 2b).
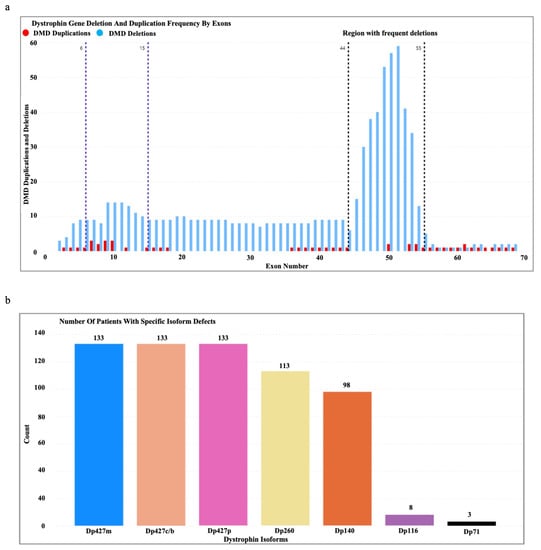
Figure 2.
(a) Dystrophin gene deletion and duplication patterns in the patient cohort. (b) Number of patients with specific isoform defects (Dp427m, Dp427c, Dp427p, Dp260, Dp140, Dp116, and Dp71) identified based on the DMD gene mutation location.
The patients were categorized into the following two groups based on whether the shorter dystrophin isoforms expressed in the CNS (Dp140 and Dp71) and PNS (Dp116) were affected or not: Group 1 comprised patients with single or multiple, shorter dystrophin isoforms expressed in the CNS and PNS that were affected (n = 98), and Group 2 comprised patients with shorter dystrophin isoforms expressed in the CNS and PNS that were not affected (n = 35). Table 1 is a summary of the demographic characteristics of the patient cohort.
The distribution of patient ages and the ages of onset in each group exhibited a left skew, and no statistically significant differences in age (p = 0.4) or the age of onset (p = 0.6) between the groups were discovered. Interestingly, there was a significant difference (p = 0.02) in the age at which individuals in the two groups became wheelchair-bound. Specifically, patients in Group 1 (the median age upon becoming wheelchair-bound—9 years) lost their ability to walk at a younger age compared to those in Group 2 (the median age upon becoming wheelchair-bound—11 years) (Table 1).
When examining the relationship between age and creatine phosphokinase (CPK) values in patients, it was observed that there was a declining trend in the CPK values as age increased. However, there was no statistically significant difference observed in the decreasing trend of CPK values with age among the different groups (age of 0–5.5 Yrs, Group 1 vs. Group 2: p = 0.825; age of 5.5–9.75 Yrs, Group 1 vs. Group 2: p = 0.810; age of 9.75+ Yrs, Group 1 vs. Group 2: p = 0.981), as presented in Table 1.
The dosage of the corticosteroid (prednisolone) did not exhibit a significant difference (p = 0.19) between the patients in Group 1 and Group 2. It is important to mention that, during the baseline evaluation and follow-up periods, none of the patients were receiving corticosteroids. This is because the average age of the patients in both groups at the time of the baseline evaluation was 8 years, and corticosteroids were administered prior to the age of 6 years, as outlined in Table 1.
3.2. Effect of Shorter Dystrophin Isoforms Expressed in the CNS and PNS on the Performance in Motor Function Scales and Their Subsets
The study observed a significant difference (p < 0.001) in the deteriorating trend of the subcategories for the MRC scale and the NSAA concerning age between Group 1 and Group 2. This difference was evident in all subcategories among the patients in both Group 1 and Group 2, as shown in Table 2. Interestingly, an accelerated decline in hip muscle power (p < 0.001) and knee muscle power (p < 0.001) was observed concerning the MRC scale for muscle strength (Figure 3a). Despite the decline in muscle power associated with aging, there was no discernible accelerated decline observed in the upper-extremity muscles (Table 2). Moreover, the NSAA revealed that certain activities, namely “standing on one leg R” (p < 0.001), “standing on one leg L” (p < 0.001), and “walk” (p < 0.001), exhibited an accelerated decline in performance with age in Group 1 compared to Group 2 (Figure 3b and Table 2).

Table 2.
The deteriorating trend of the subcategories of the Medical Research Council (MRC) scale for muscle strength and the North Star Ambulatory Assessment (NSAA) with age in Group 1 (G1) and Group 2 (G2) at p < 0.001.
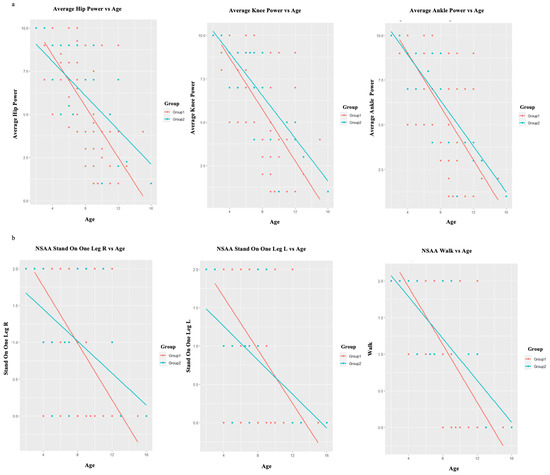
Figure 3.
(a) The accelerated decline in hip muscle power and knee muscle power as measured by the MRC scale for muscle strength, with regard to age. (b) The accelerated decline in the performance of the activities of NSAA: “standing on one leg R”, “standing on one leg L”, and “walk” with regard to age. Group 1—red color, Group 2—blue color.
3.3. Follow-Up Evaluations of the Patients
The results of the Weibull regression survival analysis for the follow-up evaluations of the patients revealed a statistically significant difference (p = 0.004) for the age at which individuals became wheelchair-bound. Specifically, patients in Group 1 exhibited a tendency to become wheelchair-bound at a younger age with the progression of the disease compared to those in Group 2, as illustrated in Figure 4.
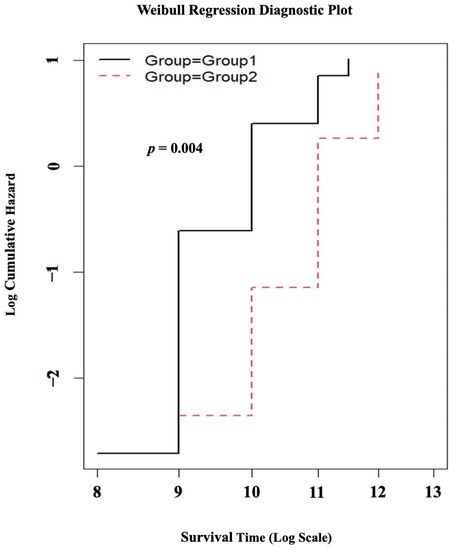
Figure 4.
The results of the Weibull regression survival analysis. The black line represents Group 1, and the red dotted line represents Group 2.
4. Discussion
The DMD gene variation hotspots of our cohort (Figure 2a) represent a distal variation hotspot spanning from exon 45 to 56, which is consistent with previous studies on DMD in South Asian populations [17,18,19,20]. While the investigation of the intricate correlation between shorter dystrophin isoforms and motor performance in DMD is a relatively new idea, previous research has examined the impact of brain dystrophin isoforms on the attainment of early developmental milestones [21,22,23,24,25]. These investigations have identified a delayed attainment of motor developmental milestones and language milestones in individuals with DMD. Nevertheless, it is important to acknowledge that there could potentially be delays in the attainment of milestones related to social, emotional, and behavioral development, as well as fine motor development [25].
Consistent with Dommelen et al., 2020, and as depicted in Table 1, a motor developmental delay was identified in our cohort as the most common type of developmental delay, while the least commonly observed issue was a delay in social–emotional or behavioral development. Importantly, there was a significant difference between Group 1 and Group 2 observed for the categories of developmental delays: motor developmental delays (p < 0.001), language delays (p < 0.001), and delays in vision and fine motor development (p = 0.038). These results suggest that the delay observed may be attributed to the involvement of dystrophin isoforms in the brain, specifically Dp140 and Dp71 isoforms, that have implications for brain functionality.
There has been an increasing emphasis in recent years on identifying early indicators of impairment in young boys affected with DMD [22,26,27,28,29]. In this context, Dommelen et al., 2020 [25], reported a correlation between developmental milestones and heightened susceptibility to DMD, where an inability to walk proficiently at 24 months was found to elevate the risk of DMD from 1 in 5000 to approximately 1 in 100 male infants. Despite the fact that 56% (74/133) of the patients in our cohort exhibited a delay in developmental milestones, only 35% (26/74) of these patients had their abnormality recognized by their parents at the onset, leading them to seek medical attention at an early age (Supplementary Table S1). If healthcare professionals and parents possessed adequate knowledge regarding the potential risk of DMD in male children exhibiting delayed developmental milestones, it is plausible that the age at which DMD is diagnosed could be decreased in 65% (48/74) of the remaining patients who had a delay in developmental milestones. Therefore, it is contended that commencing investigations on DMD with the identification of developmental delays could potentially lead to an earlier diagnosis.
Diagnostic delays in DMD remain frequent in traditionally disadvantaged groups, including patients from developing countries and those with a lower socioeconomic status [30,31]. Therefore, a potential approach could be suggested that involves identifying young male children with delayed developmental milestones and examining them for suggestive clinical symptoms of muscular dystrophy, followed by performing a CPK test. This would be a cost-effective approach to practice, even in a primary care setting in countries with limited resources, to achieve the early clinical detection of DMD. This approach could be further expanded when coupled with a cost-effective molecular diagnostic approach such as multiplex PCR followed by MLPA, which would facilitate the early confirmation of DMD.
One of the limitations observed in prior studies examining developmental delays in large cohorts of individuals with DMD [23,32] is the absence of developmental milestones documented by healthcare professionals and parental reporting in a retrospective context, which may be influenced by recall bias. To address this issue, Dommelen et al., 2020, examined the developmental achievements of infants with DMD during their initial months of life, with the medical professionals involved in the study being unaware of the participants’ diagnoses [25]. In our study, we sought to mitigate the concerns surrounding retrospective parental reporting and recall bias by implementing a method where the information about developmental milestones was directly recorded from the CHDR, as described in the methodology. The CHDR serves as a pivotal document within the National Growth Monitoring and Promotion Programme for children under the age of five in Sri Lanka.
Individuals with DMD experience a decline in their ability to walk independently, with a loss of independent ambulation occurring around the age of 13 years [33]. In our patient cohort, the median age at which individuals in Group 1 experienced wheelchair dependency was 9 years, whereas for Group 2, it was 11 years (p = 0.02) (Table 1). Furthermore, the survival analysis for the follow-up evaluation of the patients revealed that, with the progression of the disease, the patients in Group 1 were more prone to becoming wheelchair-bound at a younger age compared to those in Group 2 (p = 0.004), as depicted in Table 3 and Figure 4. The reported data on the age of wheelchair dependency in DMD patients of different populations are as follows: in India, the age is 10.4 years [18] and 13 years [34]; in Iran, the age is 10.9 years [35]; in Saudi Arabia, the age is 10.1 years [36]; in the Netherlands, the age is 9.7 years [37]; and in the UK, according to the North Star database and other related studies, the age is 13 years [10,38]. The variability observed in the age at which individuals become dependent on wheelchairs in different populations can potentially be attributed to regional disparities in patient management, healthcare resources, the socioeconomic background of the patients, genetic factors, and the influence of disease modifier genes specific to certain populations [30,39,40,41,42].

Table 3.
Yearly follow-up data up to three follow-ups.
Additionally, it was postulated that mutations occurring in the DMD gene, which are expected to disrupt brain dystrophin isoforms, could potentially impact motor outcomes among individuals diagnosed with DMD [12,14]. Moreover, Cyrulnik et al., 2007, put forth the argument that DMD can be characterized as a “cerebellar disorder” due to the expression of the Dp140 and Dp71 brain dystrophin isoforms [5,23]. They proposed that the absence of dystrophin during brain development could potentially impair the effectiveness of the cerebro–cerebellar pathways. Interestingly, there are reports indicating that cerebro–cerebellar loops establish connections between the lateral regions of the cerebellar hemisphere and the cerebral cortex. These loops have conventionally been associated with the coordination of movement [43,44,45].
Taking into consideration the aforementioned, our study observed a significant difference (p < 0.001) in the decline pattern of the subcategories for the MRC scale and the NSAA in relation to age when comparing Group 1 and Group 2 (Table 2). It is noteworthy that a rapid deterioration in hip muscle power and knee muscle power was observed based on the MRC scale for muscle strength (Figure 3a). In a comparable manner, the NSAA demonstrated that certain activities, specifically “standing on one leg R”, “standing on one leg L”, and “walk”, displayed a heightened deterioration in performance with increasing age in relation to Group 1 to Group 2 (Figure 3b). It is important to highlight that, in contrast to the MRC scale, the NSAA is documented to possess a greater complexity and necessitate elevated levels of motor coordination and planning [14]. Hence, considering the role of Dp140 and Dp71 in brain function, it can be contended that our observations regarding the rapid decline of motor abilities in individuals with DMD may be associated with the impact of the CNS on motor executive functions [11,46].
It is plausible to hypothesize that the observed variability in the functional outcomes could be attributed to the variability in the expression of Dp71 in skeletal muscle. A recent study identified the expression of Dp71 in skeletal muscle [47], although it is widely reported that Dp71 is primarily expressed in the brain [48]. Furthermore, numerous studies have indicated the involvement of Dp71 in the proliferation of myoblast cells [49,50] and the activation of satellite cells [51]. These findings suggest that the lower expression of Dp71 in the muscles of individuals with DMD is a secondary event that is closely associated with the muscle degeneration and regeneration that are characteristic of DMD [14].
Furthermore, it is important to highlight that the administration of corticosteroids has the potential to exert an influence on the progression of DMD, as suggested by Zambon et al., 2022 [52]. In the present study, there was no statistically significant difference (p = 0.19) observed for the dosage of the corticosteroid (prednisolone) administered to patients belonging to Group 1 or Group 2, as presented in Table 1. It is imperative to note that, throughout the baseline assessment and subsequent follow-up periods, none of the patients were on corticosteroids. This is because the mean age of individuals in both cohorts during the initial assessment was 8 years, and the administration of corticosteroids occurred before the age of 6 years, as indicated in Table 1. Despite the fact that it is common practice to begin corticosteroids for DMD around the age of 4–5 years [53], an expert survey on the clinical practice of steroid usage in DMD performed in Asia and Oceania revealed that most clinicians in Asia and Oceania considered 4–7 years old to be an appropriate age to begin steroids for DMD [54]. This is consistent with the 5.6-year mean age of corticosteroid initiation in our patients (Table 1). Despite the fact that the most recent guidelines strongly advise continuing corticosteroids throughout life [55], the mean durations of corticosteroids in our group were 4.9 months and 4.4 months for individuals in Group 1 and Group 2, respectively. This was primarily because the parents of DMD children stopped using corticosteroids due to the adverse effects.
Our research will shed light on the growing emphasis on identifying early indicators of impairment in DMD, advising clinicians to identify young male children with delayed developmental milestones, examine them for suggestive clinical symptoms of muscular dystrophy, and then administer a CPK test to achieve the early clinical detection of DMD, even in a primary care setting. Furthermore, it is not standard practice in modern clinical trial settings to group patients based on the number of dystrophin isoforms affected. Our findings on the effect of the cumulative loss of brain dystrophin isoforms on the motor function outcome suggest that not stratifying clinical trial participants based on the cumulative loss of brain dystrophin isoforms may have a matrix effect on the outcome measurements, concealing the treatment effects. Moreover, we were able to identify 82/138 (60%) patients as amenable to the available exon-skipping therapies using MLPA diagnostics. Interestingly, the majority of our patients were eligible for exon 51 skipping (30/82), followed by exon 53 skipping (19/82) and exon 45 skipping (19/82). This was comprehensively discussed by the same authors in Wijekoon et al.’s 2023 study; thus, this is not discussed in this paper [56].
We would like to acknowledge the following limitations of this study. The evaluation of muscle strength using the MRC scale is reported to be evaluator-dependent. As mentioned in the methodology, the assessments were conducted by a medical practitioner to minimize this effect; however, we acknowledge the potential effect of evaluator dependency. Furthermore, because there are a limited number of patients with DMD mutations that specifically affect the Dp116 and Dp71 isoforms (Figure 2b), we had to combine patients with mutations in shorter dystrophin isoforms (Dp140, Dp116, and Dp71) into a single group in order to maintain statistical power. One limitation we acknowledge is that this approach limited our ability to distinguish the effects of each shorter dystrophin isoform on motor function. The analysis of the impact of dystrophin mutations on cardiac and respiratory phenotypes was hindered by the limited availability of data, which precluded the execution of a comprehensive statistical analysis. In addition, the annual follow-up evaluations were restricted to a cohort of 52 patients as a result of limitations imposed by the COVID-19 pandemic in Sri Lanka, which hindered clinic visits and home visits by the research team for a period of approximately two years.
5. Conclusions
In conclusion, we identified that mutations occurring in the DMD gene, which impact the expression of the shorter dystrophin isoforms Dp 140, Dp71, and Dp116, are correlated with the impairment of motor functions in individuals with DMD. Furthermore, it was evident that the cumulative loss of shorter dystrophin isoforms (Dp 140, Dp71, and Dp116) will have an effect on the natural history of DMD, where patients with mutations in the DMD gene affecting shorter dystrophin expression are more prone to having an accelerated decline in hip muscle power, knee muscle power, and NSAA activities involving the lower limbs. Moreover, it is advised for clinicians to identify young male children with delayed developmental milestones, examine them for suggestive clinical symptoms of muscular dystrophy, and then administer a CPK test to achieve early clinical detection of DMD, even in a primary care setting. Finally, it is suggested to consider the DMD mutation site and its effect on the cumulative loss of brain dystrophin isoforms when stratifying patients for clinical trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12175637/s1, Supplementary Figure S1: Outlier removal of the data set. Supplementary Table S1: Details of the abnormality identified by the parents at the onset, which prompted them to seek medical attention.
Author Contributions
N.W. and L.G.: Conceptualization, methodology, validation, formal analysis, data curation, writing—original draft preparation, writing—review and editing, and visualization. D.A.: formal analysis, methodology, and writing—review and editing. P.R., Y.H. and C.M.: resources. H.W.M.S., A.D. and E.P.H.: supervision and resources. K.R.D.d.S.: Supervision, project administration, funding acquisition, conceptualization, formal analysis, investigation, resources, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The corresponding author in Sri Lanka received funding from the Muscular Dystrophy Association, Washington DC, USA (grant number FMS/7090/2010), the World Health Organization (WHO) (grant number 2010/81594-0), the World Class University Grant Project (University of Sri Jayewardenepura, Sri Lanka, grant numbers WCUP/Ph.D./19/2013 and WCUP/Ph.D./19B 2013), the Ministry of Primary Industries, Sri Lanka (grant number SP/CIN/2016/02), the University of Sri Jayewardenepura (grant numbers ASP/06/RE/2010/07, ASP/06/RE/2012/18, ASP/06/RE/2013/28), General Sir John Kotelawala Defence University, Sri Lanka (grant numbers KDU/RG/2021/CARE/005 and KDU/RG/2021/CARE/006), and the Interdisciplinary Center for Innovation in Biotechnology and Neuroscience, University of Sri Jayewardenepura (ICIBN/USJ). The equipment was donated by the National Institutes of Health (Bethesda, MD, USA) through IBRO-APRC and by the Chinese Neuroscience Society. Moreover, the corresponding author has received funding from IBRO-APRC and the International Society for Neurochemistry (ISN) for international training scholarships for postgraduates and to conduct neuroscience workshops in Sri Lanka.
Institutional Review Board Statement
Every participant provided written informed consent, where applicable. The assent of a proxy was obtained for patients unable to provide their own. This study adheres to the ethical standards of Sri Lankan institutional review boards that follow the Helsinki Declaration (ethical approval nos. 449/09 and 38/19 from the Ethics Review Committee, the Faculty of Medical Sciences, University of Sri Jayewardenepura, and ethical approval no. LRH/D/06/2007, Lady Ridgeway Hospital for Children, Sri Lanka.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We sincerely thank the patients and their family members for taking part in the study. Special thanks to the members of the pro bono research team of ICIBN of the University of Sri Jayewardenepura, which included Ruwani Wijayakoon, Vindika Suriyakumara, Chamara Jayasinghe, Beneeta Hettiarachchi, Roshani Karunarathne, Kasunka Gamage, Jayantha Udurawana, Navami Samaranayake, Gayatri Wijeweera, Kasinathar Lokajini, Pulasthi Dissanayaka, Yoonus Imran, M.K.D.K. Attanayake, and Shamali Wasala, and provided support. Moreover, we highly appreciate the technical support provided by Isuru Lokuge, a freelance analyst in performing statistical analysis. The authors kindly acknowledge the support received from the School of Pharmacy and Pharmaceutical Sciences, Binghamton University, Binghamton, 13902, NY, USA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salari, N.; Fatahi, B.; Valipour, E.; Kazeminia, M.; Fatahian, R.; Kiaei, A.; Shohaimi, S.; Mohammadi, M. Global prevalence of Duchenne and Becker muscular dystrophy: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 96. [Google Scholar] [CrossRef]
- Wang, R.T.; Barthelemy, F.; Martin, A.S.; Douine, E.D.; Eskin, A.; Lucas, A.; Lavigne, J.; Peay, H.; Khanlou, N.; Sweeney, L. DMD genotype correlations from the Duchenne Registry: Endogenous exon skipping is a factor in prolonged ambulation for individuals with a defined mutation subtype. Hum. Mutat. 2018, 39, 1193–1202. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Van Deutekom, J.C.; Fokkema, I.F.; Van Ommen, G.J.B.; Den Dunnen, J.T. Entries in the Leiden Duchenne muscular dystrophy mutation database: An overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2006, 34, 135–144. [Google Scholar] [CrossRef]
- Taylor, P.J.; Betts, G.A.; Maroulis, S.; Gilissen, C.; Pedersen, R.L.; Mowat, D.R.; Johnston, H.M.; Buckley, M.F. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS ONE 2010, 5, e8803. [Google Scholar] [CrossRef]
- Doorenweerd, N.; Mahfouz, A.; van Putten, M.; Kaliyaperumal, R.; t’Hoen, P.A.; Hendriksen, J.G.; Aartsma-Rus, A.M.; Verschuuren, J.J.; Niks, E.H.; Reinders, M.J. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci. Rep. 2017, 7, 12575. [Google Scholar] [CrossRef]
- Falzarano, M.; Rachele, R.; Mietto, M.; Fortunato, F.; Selvatici, R.; Spitali, P.; Montanaro, F.; Muntoni, F.; Ferlini, A. DMD-BRAIN: EP. 133 Expression and localization of dystrophin isoforms transcripts in human adult control brain areas. Neuromuscul. Disord. 2021, 31, S89. [Google Scholar] [CrossRef]
- Maresh, K.; Papageorgiou, A.; Ridout, D.; Harrison, N.A.; Mandy, W.; Skuse, D.; Muntoni, F. Startle responses in Duchenne muscular dystrophy: A novel biomarker of brain dystrophin deficiency. Brain 2023, 146, 252–265. [Google Scholar] [CrossRef]
- Wijekoon, N.; Gonawala, L.; Ratnayake, P.; Dissanayaka, P.; Gunarathne, I.; Amaratunga, D.; Liyanage, R.; Senanayaka, S.; Wijesekara, S.; Gunasekara, H.H. Integrated genomic, proteomic and cognitive assessment in Duchenne Muscular Dystrophy suggest astrocyte centric pathology. Heliyon 2023, 9, e18530. [Google Scholar] [CrossRef]
- Moizard, M.P.; Billard, C.; Toutain, A.; Berret, F.; Marmin, N.; Moraine, C. Are Dp71 and Dp140 brain dystrophin isoforms related to cognitive impairment in Duchenne muscular dystrophy? Am. J. Med. Genet. 1998, 80, 32–41. [Google Scholar] [CrossRef]
- Ricotti, V.; Mandy, W.P.; Scoto, M.; Pane, M.; Deconinck, N.; Messina, S.; Mercuri, E.; Skuse, D.H.; Muntoni, F. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 2016, 58, 77–84. [Google Scholar] [CrossRef]
- Felisari, G.; Boneschi, F.M.; Bardoni, A.; Sironi, M.; Comi, G.; Robotti, M.; Turconi, A.; Lai, M.; Corrao, G.; Bresolin, N. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000, 55, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Lenkowicz, J.; Norcia, G.; Lucibello, S.; Ferraroli, E.; d’Amico, A.; Bello, L.; Pegoraro, E.; Messina, S.; Ricci, F. Age, corticosteroid treatment and site of mutations affect motor functional changes in young boys with Duchenne Muscular Dystrophy. PLoS ONE 2022, 17, e0271681. [Google Scholar] [CrossRef]
- Doorenweerd, N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy-a narrative review. Neuromuscul. Disord. 2020, 30, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Chesshyre, M.; Ridout, D.; Hashimoto, Y.; Ookubo, Y.; Torelli, S.; Maresh, K.; Ricotti, V.; Abbott, L.; Gupta, V.A.; Main, M. Investigating the role of dystrophin isoform deficiency in motor function in Duchenne muscular dystrophy. J. Cachexia Sarcopenia Muscle 2022, 13, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Abbs, S.; Tuffery-Giraud, S.; Bakker, E.; Ferlini, A.; Sejersen, T.; Mueller, C.R. Best practice guidelines on molecular diagnostics in Duchenne/Becker muscular dystrophies. Neuromuscul. Disord. 2010, 20, 422–427. [Google Scholar] [CrossRef]
- Fratter, C.; Dalgleish, R.; Allen, S.K.; Santos, R.; Abbs, S.; Tuffery-Giraud, S.; Ferlini, A. EMQN best practice guidelines for genetic testing in dystrophinopathies. Eur. J. Hum. Genet. 2020, 28, 1141–1159. [Google Scholar] [CrossRef]
- Tyagi, R.; Kumar, S.; Dalal, A.; Mohammed, F.; Mohanty, M.; Kaur, P.; Anand, A. Repurposing pathogenic variants of DMD gene and its isoforms for DMD exon skipping intervention. Curr. Genom. 2019, 20, 519–530. [Google Scholar] [CrossRef]
- Vengalil, S.; Preethish-Kumar, V.; Polavarapu, K.; Mahadevappa, M.; Sekar, D.; Purushottam, M.; Thomas, P.T.; Nashi, S.; Nalini, A. Duchenne muscular dystrophy and Becker muscular dystrophy confirmed by multiplex ligation-dependent probe amplification: Genotype-phenotype correlation in a large cohort. J. Clin. Neurol. 2017, 13, 91–97. [Google Scholar] [CrossRef]
- Polavarapu, K.; Preethish-Kumar, V.; Sekar, D.; Vengalil, S.; Nashi, S.; Mahajan, N.P.; Thomas, P.T.; Sadasivan, A.; Warrier, M.; Gupta, A. Mutation pattern in 606 Duchenne muscular dystrophy children with a comparison between familial and non-familial forms: A study in an Indian large single-center cohort. J. Neurol. 2019, 266, 2177–2185. [Google Scholar] [CrossRef]
- Ansar, Z.; Nasir, A.; Moatter, T.; Khan, S.; Kirmani, S.; Ibrahim, S.; Imam, K.; Ather, A.; Samreen, A.; Hasan, Z. MLPA analyses reveal a spectrum of dystrophin gene deletions/duplications in Pakistani patients suspected of having Duchenne/Becker muscular dystrophy: A retrospective study. Genet. Test. Mol. Biomark. 2019, 23, 468–472. [Google Scholar] [CrossRef]
- Mirski, K.T.; Crawford, T.O. Motor and cognitive delay in Duchenne muscular dystrophy: Implication for early diagnosis. J. Pediatr. 2014, 165, 1008–1010. [Google Scholar] [CrossRef]
- Connolly, A.; Florence, J.; Cradock, M.; Malkus, E.; Schierbecker, J.; Siener, C.; Wulf, C.; Anand, P.; Lowes, L.; Alfano, L. Motor and Cognitive Assessment of Infants and Young Boys with Duchenne Muscular Dystrophy; Results from the Muscular Dystrophy Association DMD Clinical Research Center Network (P04. 084). Neuromuscul. Disord. 2012, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Cyrulnik, S.E.; Fee, R.J.; Darryl, C.; Goldstein, E.; Hinton, V.J. Delayed developmental language milestones in children with Duchenne’s muscular dystrophy. J. Pediatr. 2007, 150, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.P.; Clarke, A.J.; Bradley, D.M. Developmental progress in Duchenne muscular dystrophy: Lessons for earlier detection. Eur. J. Paediatr. Neurol. 2004, 8, 145–153. [Google Scholar] [CrossRef]
- van Dommelen, P.; van Dijk, O.; de Wilde, J.A.; Verkerk, P.H. Early developmental milestones in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2020, 62, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.F.; Alfano, L.N.; Iammarino, M.A.; Connolly, A.M.; Moore-Clingenpeel, M.; Powers, B.R.; Tsao, C.-Y.; Waldrop, M.A.; Flanigan, K.M.; Mendell, J.R. Natural history of steroid-treated young boys with Duchenne muscular dystrophy using the NSAA, 100m, and timed functional tests. Pediatr. Neurol. 2020, 113, 15–20. [Google Scholar] [CrossRef]
- Coratti, G.; Brogna, C.; Norcia, G.; Ricotti, V.; Abbott, L.; D’Amico, A.; Berardinelli, A.; Vita, G.L.; Lucibello, S.; Messina, S. Longitudinal natural history in young boys with Duchenne muscular dystrophy. Neuromuscul. Disord. 2019, 29, 857–862. [Google Scholar] [CrossRef]
- Connolly, A.M.; Florence, J.M.; Cradock, M.M.; Eagle, M.; Flanigan, K.M.; McDonald, C.M.; Karachunski, P.I.; Darras, B.T.; Bushby, K.; Malkus, E.C. One year outcome of boys with Duchenne muscular dystrophy using the Bayley-III scales of infant and toddler development. Pediatr. Neurol. 2014, 50, 557–563. [Google Scholar] [CrossRef]
- Pane, M.; Scalise, R.; Berardinelli, A.; D’Angelo, G.; Ricotti, V.; Alfieri, P.; Moroni, I.; Hartley, L.; Pera, M.C.; Baranello, G. Early neurodevelopmental assessment in Duchenne muscular dystrophy. Neuromuscul. Disord. 2013, 23, 451–455. [Google Scholar] [CrossRef]
- Samaranayake, N.; Dissanayaka, P.; Gunarathna, I.; Gonawala, L.; Wijekoon, N.; Rathnayake, P.; Sirisena, D.; Gunasekara, H.; Dissanayake, A.; Senanayake, S. What we fail to see in neuro-genetic diseases: A bird’s eye view from the developing world. Ann. Neurosci. 2020, 27, 91–97. [Google Scholar] [CrossRef]
- Counterman, K.J.; Furlong, P.; Wang, R.T.; Martin, A.S. Delays in diagnosis of Duchenne muscular dystrophy: An evaluation of genotypic and sociodemographic factors. Muscle Nerve 2020, 61, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ciafaloni, E.; Fox, D.J.; Pandya, S.; Westfield, C.P.; Puzhankara, S.; Romitti, P.A.; Mathews, K.D.; Miller, T.M.; Matthews, D.J.; Miller, L.A. Delayed diagnosis in duchenne muscular dystrophy: Data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J. Pediatr. 2009, 155, 380–385. [Google Scholar] [CrossRef]
- Darras, B.; Korf, B.; Urion, D. Dystrophinopathies. In Gene Reviews; University of Washington: Seattle, WA, USA, 2000. [Google Scholar]
- Rao, M.V.; Sindhav, G.M.; Mehta, J.J. Duchenne/Becker muscular dystrophy: A report on clinical, biochemical, and genetic study in Gujarat population, India. Ann. Indian Acad. Neurol. 2014, 17, 303–307. [Google Scholar] [CrossRef]
- Zamani, G.; Hosseinpour, S.; Ashrafi, M.R.; Mohammadi, M.; Badv, R.S.; Tavasoli, A.R.; Akbari, M.G.; Bereshneh, A.H.; Malamiri, R.A.; Heidari, M. Characteristics of disease progression and genetic correlation in ambulatory Iranian boys with Duchenne muscular dystrophy. BMC Neurol. 2022, 22, 162. [Google Scholar] [CrossRef] [PubMed]
- AlSaman, A.S.; Al Ghamdi, F.; Bamaga, A.K.; AlShaikh, N.; Al Muqbil, M.; Muthaffar, O.; Bashiri, F.A.; Ali, B.; Mulayim, A.; Heider, E. Patient demographics and characteristics from an ambispective, observational study of patients with duchenne muscular dystrophy in Saudi Arabia. Front. Pediatr. 2022, 10, 1020059. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergen, J.; Ginjaar, H.; Van Essen, A.; Pangalila, R.; De Groot, I.; Wijkstra, P.; Zijnen, M.; Cobben, N.; Kampelmacher, M.; Wokke, B. Forty-five years of Duchenne muscular dystrophy in the Netherlands. J. Neuromuscul. Dis. 2014, 1, 99–109. [Google Scholar] [CrossRef]
- Muntoni, F.; Domingos, J.; Manzur, A.Y.; Mayhew, A.; Guglieri, M.; Network, U.N.; Sajeev, G.; Signorovitch, J.; Ward, S.J. Categorising trajectories and individual item changes of the North Star Ambulatory Assessment in patients with Duchenne muscular dystrophy. PLoS ONE 2019, 14, e0221097. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.I.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, D.; Wu, L.; Li, M.; Song, L.; Wei, C.; Lu, C.; Zhang, X.; Hong, S.; Ma, M. Genotype characterization and delayed loss of ambulation by glucocorticoids in a large cohort of patients with Duchenne muscular dystrophy. Orphanet J. Rare Dis. 2021, 16, 188. [Google Scholar] [CrossRef]
- Bello, L.; Morgenroth, L.P.; Gordish-Dressman, H.; Hoffman, E.P.; McDonald, C.M.; Cirak, S. DMD genotypes and loss of ambulation in the CINRG Duchenne Natural History Study. Neurology 2016, 87, 401–409. [Google Scholar] [CrossRef]
- Jumah, M.A.; Muhaizea, M.A.; Rumayyan, A.A.; Saman, A.A.; Shehri, A.A.; Cupler, E.; Jan, M.; Madani, A.A.; Fathalla, W.; Kashyape, P. Current management of Duchenne muscular dystrophy in the Middle East: Expert report. Neurodegener. Dis. Manag. 2019, 9, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.A.; Miall, R.C.; Ivry, R.B. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn. Sci. 2017, 21, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.S.; Tsai, P. The role of the pediatric cerebellum in motor functions, cognition, and behavior: A clinical perspective. Neuroimaging Clin. 2016, 26, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000, 71, 44–56. [Google Scholar] [CrossRef]
- Daoud, F.; Angeard, N.; Demerre, B.; Martie, I.; Benyaou, R.; Leturcq, F.; Cossee, M.; Deburgrave, N.; Saillour, Y.; Tuffery, S. Analysis of Dp71 contribution in the severity of mental retardation through comparison of Duchenne and Becker patients differing by mutation consequences on Dp71 expression. Hum. Mol. Genet. 2009, 18, 3779–3794. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Niba, E.T.E.; Rani, A.Q.M.; Onishi, Y.; Koizumi, M.; Awano, H.; Matsumoto, M.; Nagai, M.; Yoshida, S.; Sakakibara, S. Detection of dystrophin Dp71 in human skeletal muscle using an automated capillary western assay system. Int. J. Mol. Sci. 2018, 19, 1546. [Google Scholar] [CrossRef]
- Naidoo, M.; Anthony, K. Dystrophin Dp71 and the neuropathophysiology of Duchenne muscular dystrophy. Mol. Neurobiol. 2020, 57, 1748–1767. [Google Scholar] [CrossRef]
- Gosselin, M.R.; Mournetas, V.; Borczyk, M.; Verma, S.; Occhipinti, A.; Róg, J.; Bozycki, L.; Korostynski, M.; Robson, S.C.; Angione, C. Loss of full-length dystrophin expression results in major cell-autonomous abnormalities in proliferating myoblasts. eLife 2022, 11, e75521. [Google Scholar] [CrossRef]
- Farea, M.; Rani, A.Q.M.; Maeta, K.; Nishio, H.; Matsuo, M. Dystrophin Dp71ab is monoclonally expressed in human satellite cells and enhances proliferation of myoblast cells. Sci. Rep. 2020, 10, 17123. [Google Scholar] [CrossRef]
- Farea, M.; Maeta, K.; Nishio, H.; Matsuo, M. Human Dystrophin Dp71ab Enhances the Proliferation of Myoblasts Across Species But Not Human Nonmyoblast Cells. Front. Cell Dev. Biol. 2022, 10, 877612. [Google Scholar] [CrossRef]
- Zambon, A.A.; Ayyar Gupta, V.; Ridout, D.; Manzur, A.Y.; Baranello, G.; Trucco, F.; Muntoni, F.; Network, U.N.C.; Tirupath, S.; Douglas, M. Peak functional ability and age at loss of ambulation in Duchenne muscular dystrophy. Dev. Med. Child Neurol. 2022, 64, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Biggar, W.; Politano, L.; Harris, V.; Passamano, L.; Vajsar, J.; Alman, B.; Palladino, A.; Comi, L.; Nigro, G. Deflazacort in Duchenne muscular dystrophy: A comparison of two different protocols. Neuromuscul. Disord. 2004, 14, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Nakamura, H.; Yonemoto, N.; Komaki, H.; Rosales, R.L.; Kornberg, A.J.; Bretag, A.H.; Dejthevaporn, C.; Goh, K.J.; Jong, Y.-J. Clinical practice with steroid therapy for Duchenne muscular dystrophy: An expert survey in Asia and Oceania. Brain Dev. 2020, 42, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, N.; Gonawala, L.; Ratnayake, P.; Sirisena, D.; Gunasekara, H.; Dissanayake, A.; Senanayake, S.; Keshavaraj, A.; Hathout, Y.; Steinbusch, H.W. Gene Therapy for selected Neuromuscular and Trinucleotide Repeat Disorders-An Insight to Subsume South Asia for Multicenter Clinical Trials. IBRO Neurosci. Rep. 2023, 14, 146–153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).