The Complexities of Diagnosis with Co-Existing Gaucher Disease and Hemato-Oncology—A Case Report and Review of the Literature
Abstract
1. Introduction
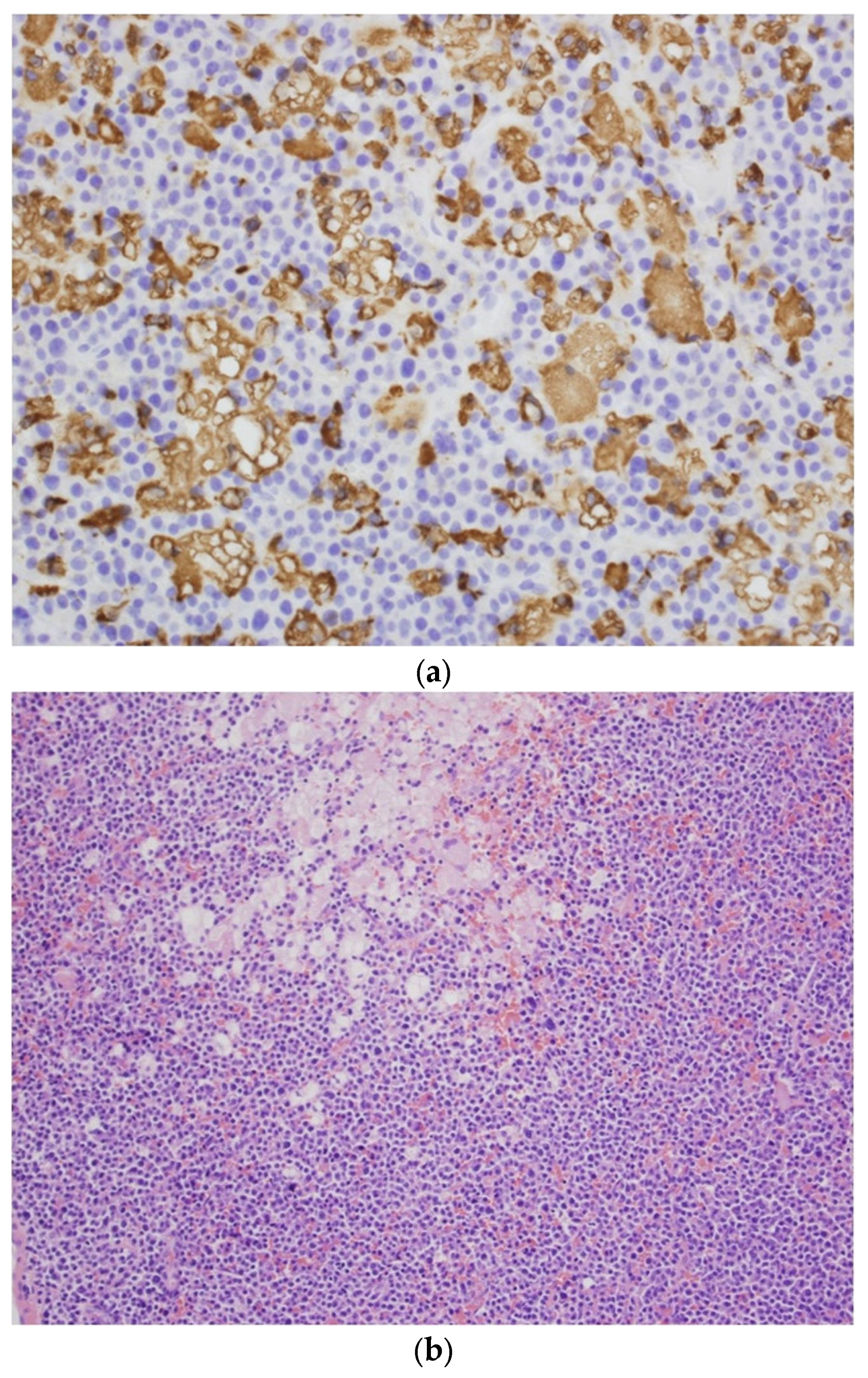
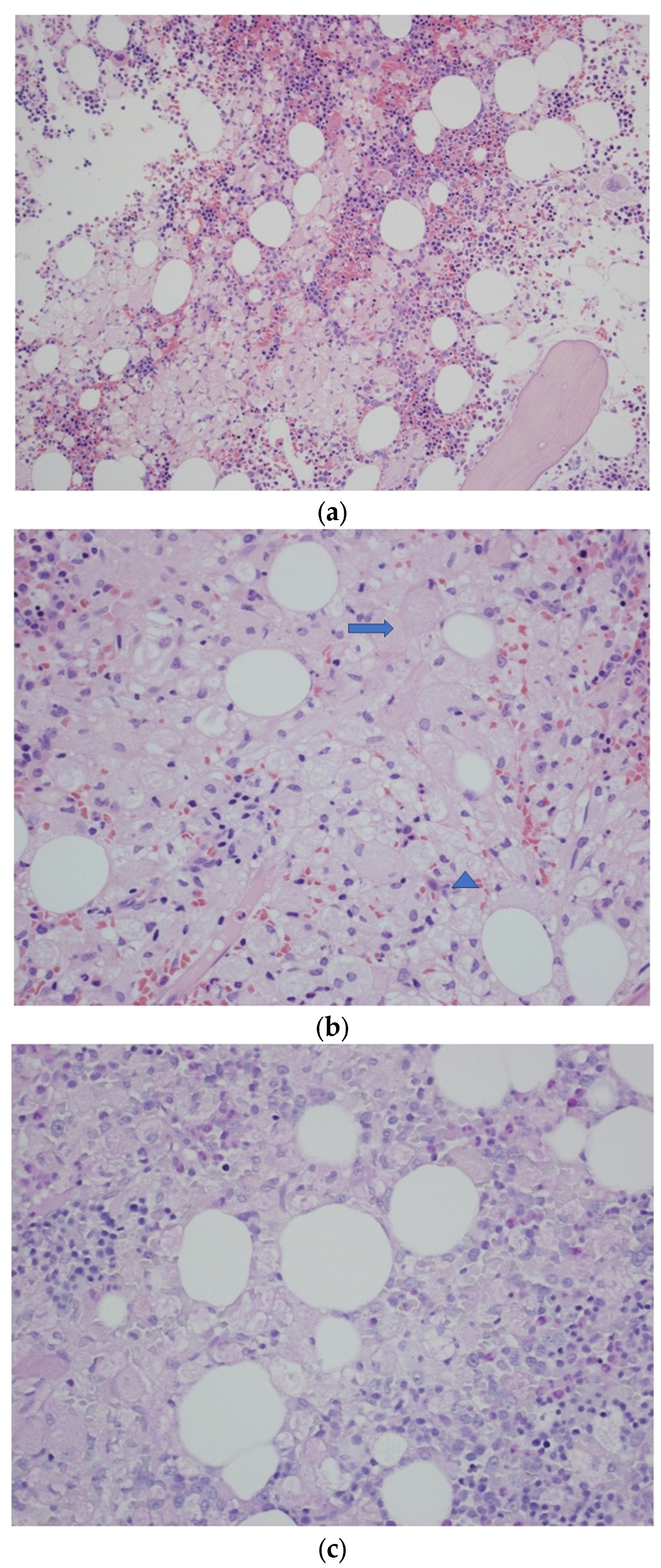
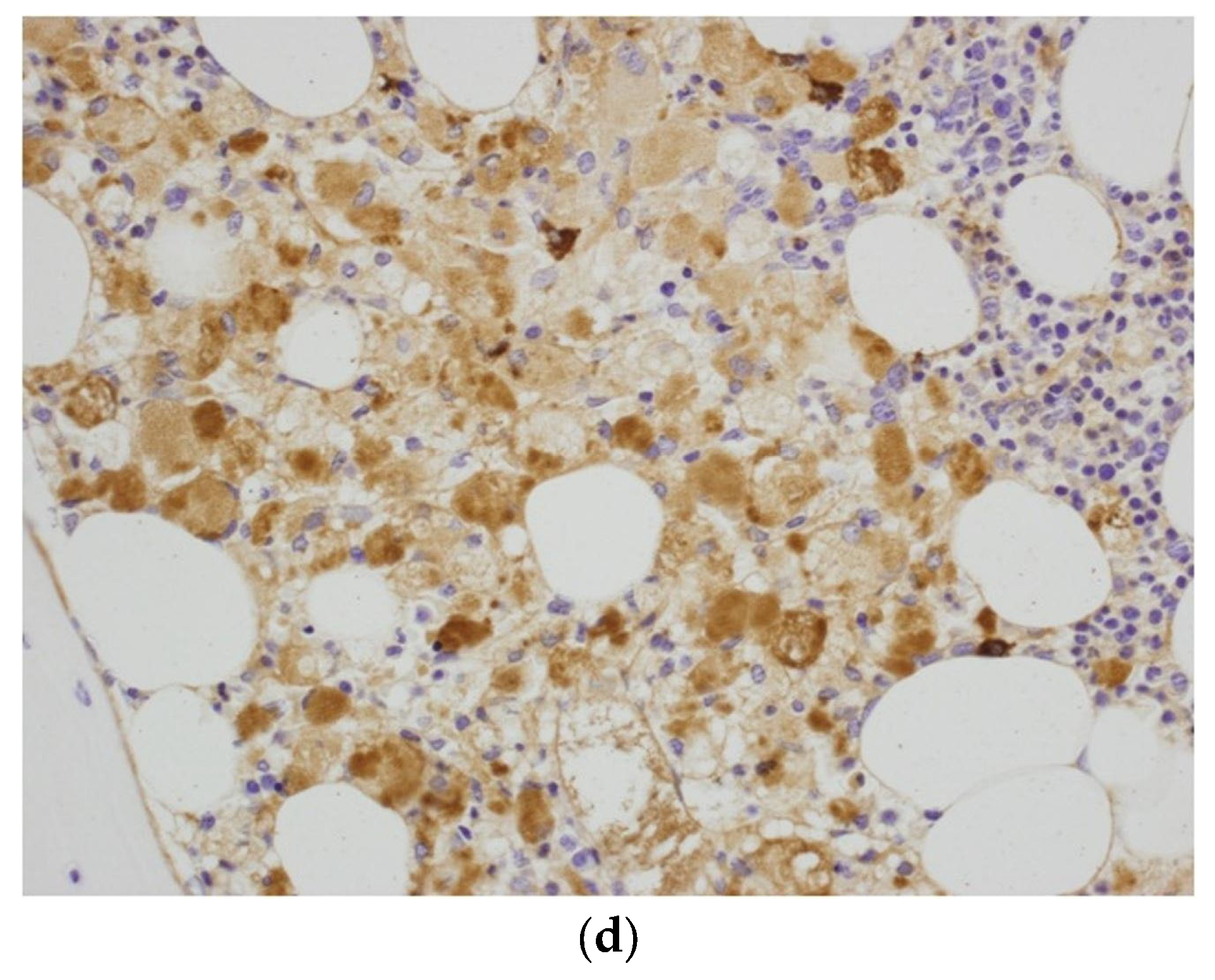
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabowski, G.A.; Kolodny, E.H.; Weinreb, N.J.; Rosenbloom, B.E.; Prakash-Cheng, A.; Kaplan, P.; Charrow, J.; Pastores, G.M.; Mistry, P.K. Gaucher disease: Phenotypic and genetic variation. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; The McGraw-Hill Companies Hill, Inc.: New York, NY, USA, 2019; Available online: https://ommbid.mhmedical.com/content.aspx?bookid=2709§ionid=225546386 (accessed on 8 May 2023).
- Revel-Vilk, S.; Szer, J.; Zimran, A. Hematological manifestations and complications of Gaucher disease. Expert Rev. Hematol. 2021, 14, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Biegstraaten, M.; van Schaik, I.N.; Aerts, J.M.; Hollak, C.E. ‘Non-neuronopathic’ Gaucher disease reconsidered. Prevalence of neurological manifestations in a Dutch cohort of type I Gaucher disease patients and a systematic review of the literature. J. Inherit. Metab. Dis. 2008, 31, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Shchelochkov, O.A.; Plon, S.E.; Scaglia, F.; Lee, B. Insights into the pathogenesis and treatment of cancer from inborn errors of metabolism. Am. J. Hum. Genet. 2011, 88, 402–421. [Google Scholar] [CrossRef] [PubMed]
- Radin, N.S. The development of aggressive cancer: A possible role for sphingolipids. Cancer Investig. 2002, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Balreira, A.; Lacerda, L.; Miranda, C.S.; Arosa, F.A. Evidence for a link between sphingolipid metabolism and expression of CD1d and MHC-class II: Monocytes from Gaucher disease patients as a model. Br. J. Haematol. 2005, 129, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.A.; Antommaria, A.H.M.; Kolodny, E.H.; Mistry, P.K. Gaucher disease: Basic and translational science needs for more complete therapy and management. Mol. Genet. Metab. 2021, 132, 59–75. [Google Scholar] [CrossRef]
- Pandey, M.K.; Grabowski, G.A. Immunological cells and functions in Gaucher disease. Crit. Rev. Oncog. 2013, 18, 197–220. [Google Scholar] [CrossRef]
- Cabrera-Reyes, F.; Parra-Ruiz, C.; Yuseff, M.I.; Zanlungo, S. Alterations in Lysosome Homeostasis in Lipid-Related Disorders: Impact on Metabolic Tissues and Immune Cells. Front. Cell Dev. Biol. 2021, 9, 790568. [Google Scholar] [CrossRef]
- Milnik, A.; Roggenbuck, D.; Conrad, K.; Bartels, C. Acute inflammatory neuropathy with monoclonal anti-GM2 IgM antibodies, IgM-κ paraprotein and additional autoimmune processes in association with a diffuse large B-cell non-Hodgkin’s lymphoma. BMJ. Case Rep. 2013, 2013, bcr1120115087. [Google Scholar] [CrossRef]
- Weinreb, N.J.; Camelo, J.S., Jr.; Charrow, J.; McClain, M.R.; Mistry, P.; Belmatoug, N. International Collaborative Gaucher Group (ICGG) Gaucher Registry (NCT00358943) investigators. Gaucher disease type 1 patients from the ICGG Gaucher Registry sustain initial clinical improvements during twenty years of imiglucerase treatment. Mol. Genet. Metab. 2021, 132, 100–111. [Google Scholar] [CrossRef]
- Ayto, R.; Hughes, D.A. Gaucher disease and myeloma. Crit. Rev. Oncog. 2013, 18, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Monge, J.; Chadburn, A.; Gergis, U. Synchronous multiple myeloma and Gaucher disease. Hematol. Oncol. Stem Cell Ther. 2020, 13, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Zeindl-Eberhart, E.; Müller, E.C.; Müller-Höcker, J.; Jungblut, P.R.; Emmerich, B.; Löhrs, U. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: Molecular analysis of a disorder with rapid clinical course and review of the literature. Blood 2002, 100, 1817–1827. [Google Scholar] [CrossRef]
- Regazzoli, A.; Pozzi, A.; Rossi, G. Pseudo-Gaucher plasma cells in the bone marrow of a patient with monoclonal gammopathy of undetermined significance. Haematologica 1997, 82, 727. [Google Scholar]
- Hughes, D.; Cappellini, M.D.; Berger, M.; Van Droogenbroeck, J.; de Fost, M.; Janic, D.; Marinakis, T.; Rosenbaum, H.; Villarubia, J.; Zhukovskaya, E.; et al. Recommendations for the management of the haematological and onco-haematological aspects of Gaucher disease. Br. J. Haematol. 2007, 138, 676–686. [Google Scholar] [CrossRef]
- Dinur, T.; Istaiti, M.; Frydman, D.; Becker-Cohen, M.; Szer, J.; Zimran, A.; Revel-Vilk, S. Patient reported outcome measures in a large cohort of patients with type 1 Gaucher disease. Orphanet. J. Rare Dis. 2020, 15, 284. [Google Scholar] [CrossRef]
- Rosenbloom, B.E.; Weinreb, N.J.; Zimran, A.; Kacena, K.A.; Charrow, J.; Ward, E. Gaucher disease and cancer incidence: A study from the Gaucher Registry. Blood 2005, 105, 4569–4572. [Google Scholar] [CrossRef]
- Rosenbloom, B.E.; Cappellini, M.D.; Weinreb, N.J.; Dragosky, M.; Revel-Vilk, S.; Batista, J.L.; Sekulic, D.; Mistry, P.K. Cancer risk and gammopathies in 2123 adults with Gaucher disease type 1 in the International Gaucher Group Gaucher Registry. Am. J. Hematol. 2022, 97, 1337–1347. [Google Scholar] [CrossRef]
- Hegab, A.E.; Ozaki, M.; Kagawa, S.; Hamamoto, J.; Yasuda, H.; Naoki, K.; Soejima, K.; Yin, Y.; Kinoshita, T.; Yaguchi, T.; et al. Tumor associated macrophages support the growth of FGF9-induced lung adenocarcinoma by multiple mechanisms. Lung Cancer 2018, 119, 25–35. [Google Scholar] [CrossRef]
- Cortés, M.; Sanchez-Moral, L.; de Barrios, O.; Fernández-Aceñero, M.J.; Martínez-Campanario, M.C.; Esteve-Codina, A.; Darling, D.S.; Győrffy, B.; Lawrence, T.; Dean, D.C.; et al. Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J. 2017, 36, 3336–3355. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.; Limgala, R.P.; Changsila, E.; Kamath, R.; Ioanou, C.; Goker-Alpan, O. Gaucheromas: When macrophages promote tumor formation and dissemination. Blood Cells Mol. Dis. 2018, 68, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.K.; Taddei, T.; vom Dahl, S.; Rosenbloom, B.E. Gaucher disease and malignancy: A model for cancer pathogenesis in an inborn error of metabolism. Crit. Rev. Oncog. 2013, 18, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wątek, M.; Piktel, E.; Wollny, T.; Durnaś, B.; Fiedoruk, K.; Lech-Marańda, E.; Bucki, R. Defective Sphingolipids Metabolism and Tumor Associated Macrophages as the Possible Links between Gaucher Disease and Blood Cancer Development. Int. J. Mol. Sci. 2019, 20, 843. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Koduru, S.; Wong, E.; Strowig, T.; Sundaram, R.; Zhang, L.; Strout, M.P.; Flavell, R.A.; Schatz, D.G.; Dhodapkar, K.M.; Dhodapkar, M.V. Dendritic cell-mediated activation-induced cytidine deaminase (AID)-dependent induction of genomic instability in human myeloma. Blood 2012, 119, 2302–2309. [Google Scholar] [CrossRef]
- Dubot, P.; Astudillo, L.; Therville, N.; Sabourdy, F.; Stirnemann, J.; Levade, T.; Andrieu-Abadie, N. Are Glucosylceramide-Related Sphingolipids Involved in the Increased Risk for Cancer in Gaucher Disease Patients? Review and Hypotheses. Cancers 2020, 12, 475. [Google Scholar] [CrossRef]
- Aflaki, E.; Moaven, N.; Borger, D.K.; Lopez, G.; Westbroek, W.; Chae, J.J.; Marugan, J.; Patnaik, S.; Maniwang, E.; Gonzalez, A.N.; et al. Lysosomal storage and impaired autophagy lead to inflammasome activation in Gaucher macrophages. Aging Cell 2016, 15, 77–88. [Google Scholar] [CrossRef]
- Pastores, G.M.; Hughes, D.A. Lysosomal Storage Disorders and Malignancy. Diseases 2017, 5, 8. [Google Scholar] [CrossRef]
- Allen, M.J.; Myer, B.J.; Khokher, A.M.; Rushton, N.; Cox, T.M. Pro-inflammatory cytokines and the pathogenesis of Gaucher’s disease: Increased release of interleukin-6 and interleukin-10. QJM 1997, 90, 19–25. [Google Scholar] [CrossRef]
- Boven, L.A.; van Meurs, M.; Boot, R.G.; Mehta, A.; Boon, L.; Aerts, J.M.; Laman, J.D. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am. J. Clin. Pathol. 2004, 122, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Arikan-Ayyildiz, Z.; Yüce, A.; Emre, S.; Baysoy, G.; Saltik-Temizel, I.N.; Gürakan, F. Outcome of enzyme replacement therapy in Turkish patients with Gaucher disease: Does late intervention affect the response? Turk. J. Pediatr. 2011, 53, 499–507. [Google Scholar] [PubMed]
- Saadi, T.; Rosenbaum, H.; Veitsman, E.; Baruch, Y. Gaucher’s disease type I: A disease masked by the presence of abnormal laboratory tests common to primary liver disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1019–1021. [Google Scholar] [CrossRef]
- Mehta, A.; Belmatoug, N.; Bembi, B.; Deegan, P.; Elstein, D.; Göker-Alpan, Ö.; Lukina, E.; Mengel, E.; Nakamura, K.; Pastores, G.M.; et al. Exploring the patient journey to diagnosis of Gaucher disease from the perspective of 212 patients with Gaucher disease and 16 Gaucher expert physicians. Mol. Genet. Metab. 2017, 122, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.K.; Sadan, S.; Yang, R.; Yee, J.; Yang, M. Consequences of diagnostic delays in type 1 Gaucher disease: The need for greater awareness among hematologists-oncologists and an opportunity for early diagnosis and intervention. Am. J. Hematol. 2007, 82, 697–701. [Google Scholar] [CrossRef]
- Hughes, D.; Mikosch, P.; Belmatoug, N.; Carubbi, F.; Cox, T.; Goker-Alpan, O.; Kindmark, A.; Mistry, P.; Poll, L.; Weinreb, N.; et al. Gaucher disease in bone: From pathophysiology to practice. J. Bone Miner. Res. 2019, 34, 996–1013. [Google Scholar] [CrossRef]
- Brautbar, A.; Elstein, D.; Pines, G.; Abrahamov, A.; Zimran, A. Effect of enzyme replacement therapy on gammopathies in Gaucher disease. Blood Cells Mol. Dis. 2004, 32, 214–217. [Google Scholar] [CrossRef]
- Rajkumar, S.V. MGUS and smoldering multiple myeloma: Update on pathogenesis, natural history, and management. Hematol. Am. Soc. Hematol. Educ. Program. 2005, 2005, 340–345. [Google Scholar] [CrossRef]
- Mizukami, H.; Mi, Y.; Wada, R.; Kono, M.; Yamashita, T.; Liu, Y.; Werth, N.; Sandhoff, R.; Sandhoff, K.; Proia, R.L. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J. Clin. Investig. 2002, 109, 1215–1221. [Google Scholar] [CrossRef]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef]
- Nair, S.; Bar, N.; Xu, M.L.; Dhodapkar, M.; Mistry, P.K. Glucosylsphingosine but not Saposin C, is the target antigen in Gaucher disease-associated gammopathy. Mol. Genet. Metab. 2020, 129, 286–291. [Google Scholar] [CrossRef]
- Preuss, K.D.; Hollak, C.E.M.; Fadle, N.; van Oers, M.; Regitz, E.; Pfreundschuh, M. Saposin C is a frequent target of paraproteins in Gaucher disease-associated MGUS/multiple myeloma. Br. J. Haematol. 2019, 184, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Cancer Today International Agency for Research on Cancer World Health Organization. Available online: https://gco.iarc.fr/today/home (accessed on 8 May 2023).
- Taddei, T.H.; Kacena, K.A.; Yang, M.; Yang, R.; Malhotra, A.; Boxer, M.; Aleck, K.A.; Rennert, G.; Pastores, G.M.; Mistry, P.K. The underrecognized progressive nature of N370S Gaucher disease and assessment of cancer risk in 403 patients. Am. J. Hematol. 2009, 84, 208–214. [Google Scholar] [CrossRef]
- Yang, H.S.; Cho, K.S.; Park, T.S. Chronic myeloid leukemia with marked splenomegaly and pseudo-Gaucher cells. Blood Res. 2013, 48, 241. [Google Scholar] [CrossRef]
- Stewart, A.J.; Jones, R.D. Pseudo-Gaucher cells in myelodysplasia. J. Clin. Pathol. 1999, 52, 917–918. [Google Scholar] [CrossRef]
- Saito, T.; Usui, N.; Asai, O.; Dobashi, N.; Ida, H.; Kawakami, M.; Yano, S.; Osawa, H.; Takei, Y.; Takahara, S.; et al. Pseudo-Gaucher cell proliferation associated with myelodysplastic syndrome. Int. J. Hematol. 2007, 85, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Zidar, B.L.; Hartsock, R.J.; Lee, R.E.; Glew, R.H.; LaMarco, K.L.; Pugh, R.P.; Raju, R.N.; Shackney, S.E. Pseudo-Gaucher cells in the bone marrow of a patient with Hodgkin’s disease. Am. J. Clin. Pathol. 1987, 87, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Khurana, N.; Singh, T. Pseudo-Gaucher cells in Hb E disease and thalassemia intermedia. Hematology 2007, 12, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Busarla, S.V.; Sadruddin, F.A.; Sohani, A.R. Pseudo-Gaucher cells in disseminated mycobacterial infection. Am. J. Hematol. 2013, 88, 155. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Gören, Ş.D.; Üsküdar, T.H.; Karagülle, M.; Andıç, N.; Gündüz, E.; Işıksoy, S.; Balić, M.; Akay, O.M. Gaucher Cells or Pseudo-Gaucher Cells: That’s the Question. Turk. J. Haematol. 2014, 31, 428–429. [Google Scholar] [CrossRef] [PubMed]
- de Fost, M.; Vom Dahl, S.; Weverling, G.J.; Brill, N.; Brett, S.; Häussinger, D.; Hollak, C.E.M. Increased incidence of cancer in adult Gaucher disease in Western Europe. Blood Cells Mol. Dis. 2006, 36, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Markuszewska-Kuczynska, A.; Klimkowska, M.; Regenthal, S.; Bulanda, A.; Kämpe Björkvall, C.; Machaczka, M. Atypical cytomorphology of Gaucher cells is frequently seen in bone marrow smears from untreated patients with Gaucher disease type 1. Folia Histochem. Cytobiol. 2015, 53, 62–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, A.S.; Mehta, A.B.; Hughes, D.A. Diagnosing Gaucher disease: An on-going need for increased awareness amongst haematologists. Blood Cells Mol. Dis. 2013, 50, 212–217. [Google Scholar] [CrossRef]
- Beutler, E.; Saven, A. Misuse of marrow examination in the diagnosis of Gaucher disease. Blood 1990, 76, 646–648. [Google Scholar] [CrossRef]
- Cazaubiel, T.; Mulas, O.; Montes, L.; Schavgoulidze, A.; Avet-Loiseau, H.; Corre, J.; Perrot, A. Risk and Response-Adapted Treatment in Multiple Myeloma. Cancers 2020, 12, 3497. [Google Scholar] [CrossRef] [PubMed]
- Harder, H.; Eucker, J.; Zang, C.; Possinger, K.; Müller-Höcker, J.; Beutler, E.; Petrides, P.E. Coincidence of Gaucher’s disease due to a 1226G/1448C mutation and of an immunoglobulin G lambda multiple myeloma with Bence-Jones proteinuria. Ann. Hematol. 2000, 79, 640–643. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Greenberg, C.R.; Bernstein, K.; Schacter, B.; Fourie, T.; Seftel, M.D. Type I Gaucher disease following chemotherapy for light chain multiple myeloma. Intern. Med. 2007, 46, 1255–1258. [Google Scholar] [CrossRef][Green Version]
- de Fost, M.; Out, T.A.; de Wilde, F.A.; Tjin, E.P.; Pals, S.T.; van Oers, M.H.; Boot, R.G.; Aerts, J.F.; Maas, M.; Vom Dahl, S.; et al. Immunoglobulin and free light chain abnormalities in Gaucher disease type I: Data from an adult cohort of 63 patients and review of the literature. Ann. Hematol. 2008, 87, 439–449. [Google Scholar] [CrossRef]
- Machaczka, M.; Lerner, R.; Klimkowska, M.; Hägglund, H. Treatment of multiple myeloma in patients with Gaucher disease. Am. J. Hematol. 2009, 84, 694–696. [Google Scholar] [CrossRef]
- Regenboog, M.; van Dussen, L.; Verheij, J.; Weinreb, N.J.; Santosa, D.; Vom Dahl, S.; Häussinger, D.; Müller, M.N.; Canbay, A.; Rigoldi, M.; et al. Hepatocellular carcinoma in Gaucher disease: An international case series. J. Inherit. Metab. Dis. 2018, 41, 819–827. [Google Scholar] [CrossRef]
- Grabowski, G.A.; Kacena, K.; Cole, J.A.; Hollak, C.E.; Zhang, L.; Yee, J.; Mistry, P.K.; Zimran, A.; Charrow, J.; vom Dahl, S. Dose-response relationships for enzyme replacement therapy with imiglucerase/alglucerase in patients with Gaucher disease type 1. Genet. Med. 2009, 11, 92–100. [Google Scholar] [CrossRef]
- Abell, K.; Chadwell, S.E.; Burrow, T.A.; Becker, A.P.P.; Bailey, L.; Steele, P.; Zhang, X.; Islas-Ohlmayer, M.; Bittencourt, R.; Schwartz, I.V.D.; et al. Outcomes of screening for gammopathies in children and adults with Gaucher disease type 1 in a cohort from Brazil and the United States. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Zimran, A.; Ruchlemer, R.; Revel-Vilk, S. A patient with Gaucher disease and plasma cell dyscrasia: Bidirectional impact. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Harel, R.; Gavish, I.; Aviv, A.; Greenman Maravi, N.; Trougouboff, P.; Zimran, A.; Revel-Vilk, S. Enzyme replacement therapy leading to improvement in myeloma indices in a patient with concomitant Gaucher disease. Intern. Med. J. 2022, 52, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Lukina, E.; Watman, N.; Dragosky, M.; Lau, H.; Avila Arreguin, E.; Rosenbaum, H.; Zimran, A.; Foster, M.C.; Gaemers, S.J.M.; Peterschmitt, M.J. Outcomes after 8 years of eliglustat therapy for Gaucher disease type 1: Final results from the Phase 2 trial. Am. J. Hematol. 2019, 94, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.K.; Lukina, E.; Ben Turkia, H.; Shankar, S.P.; Baris Feldman, H.; Ghosn, M.; Mehta, A.; Packman, S.; Lau, H.; Petakov, M.; et al. Clinical outcomes after 4.5 years of eliglustat therapy for Gaucher disease type 1: Phase 3 ENGAGE trial final results. Am. J. Hematol. 2021, 96, 1156–1165. [Google Scholar] [CrossRef]
- Pavlova, E.V.; Archer, J.; Wang, S.; Dekker, N.; Aerts, J.M.; Karlsson, S.; Cox, T.M. Inhibition of UDP-glucosylceramide synthase in mice prevents Gaucher disease-associated B-cell malignancy. J. Pathol. 2015, 235, 113–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudul, P.; Piatkowska-Jakubas, B.; Pawlinski, L.; Galazka, K.; Sacha, T.; Kiec-Wilk, B. The Complexities of Diagnosis with Co-Existing Gaucher Disease and Hemato-Oncology—A Case Report and Review of the Literature. J. Clin. Med. 2023, 12, 5518. https://doi.org/10.3390/jcm12175518
Sudul P, Piatkowska-Jakubas B, Pawlinski L, Galazka K, Sacha T, Kiec-Wilk B. The Complexities of Diagnosis with Co-Existing Gaucher Disease and Hemato-Oncology—A Case Report and Review of the Literature. Journal of Clinical Medicine. 2023; 12(17):5518. https://doi.org/10.3390/jcm12175518
Chicago/Turabian StyleSudul, Paulina, Beata Piatkowska-Jakubas, Lukasz Pawlinski, Krystyna Galazka, Tomasz Sacha, and Beata Kiec-Wilk. 2023. "The Complexities of Diagnosis with Co-Existing Gaucher Disease and Hemato-Oncology—A Case Report and Review of the Literature" Journal of Clinical Medicine 12, no. 17: 5518. https://doi.org/10.3390/jcm12175518
APA StyleSudul, P., Piatkowska-Jakubas, B., Pawlinski, L., Galazka, K., Sacha, T., & Kiec-Wilk, B. (2023). The Complexities of Diagnosis with Co-Existing Gaucher Disease and Hemato-Oncology—A Case Report and Review of the Literature. Journal of Clinical Medicine, 12(17), 5518. https://doi.org/10.3390/jcm12175518





