Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Eligibility Criteria
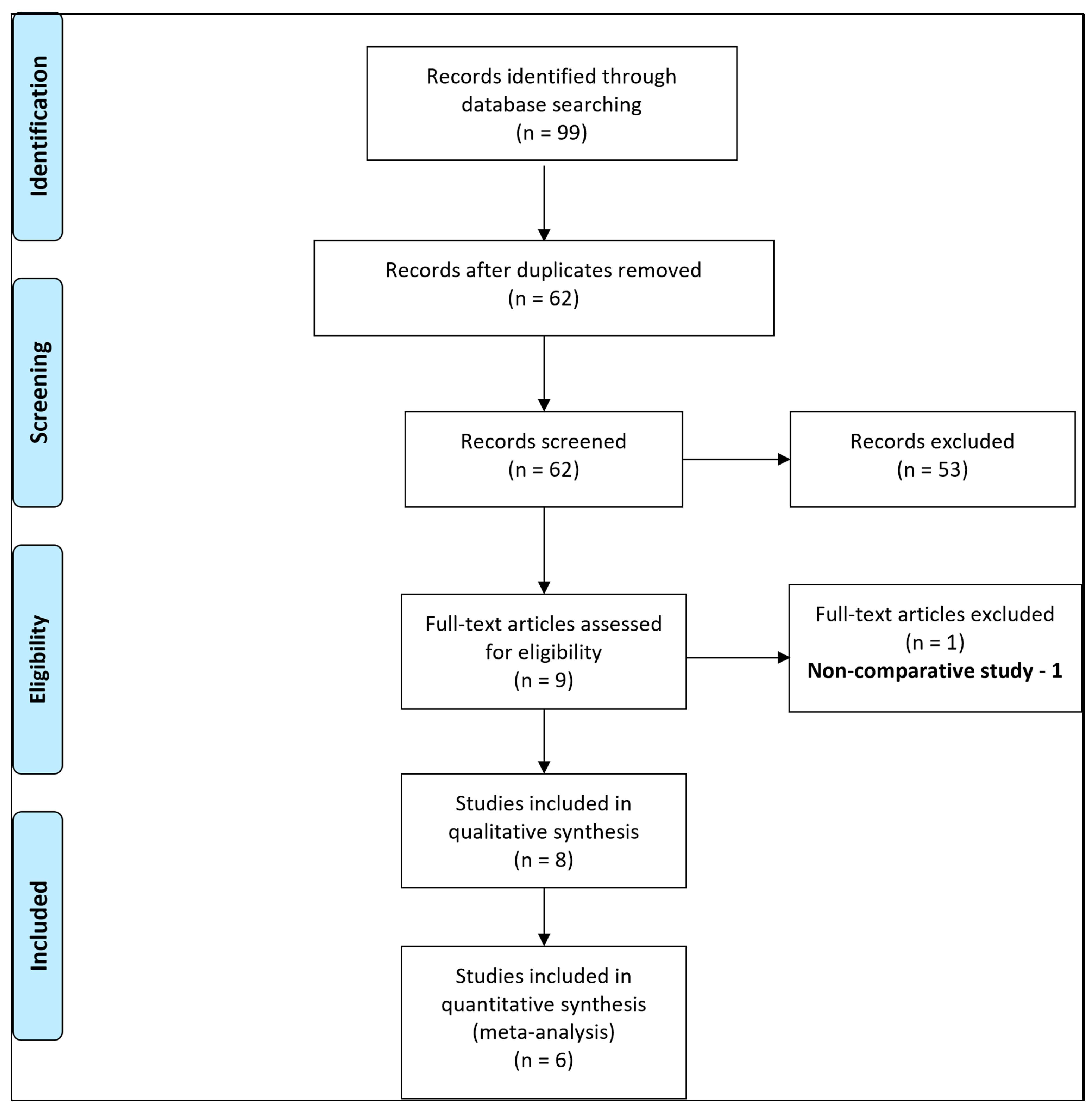
2.4. Data Synthesis
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Summary of the Studies Included in Meta-Analysis
3.2.1. Dumlu et al., 2014 [5]
3.2.2. Kılıç et al., 2017 [27]
3.2.3. Nazik et al., 2017 [28]
3.2.4. Sarac et al., 2019 [29]
3.2.5. Ünsal et al., 2022 [30]
3.2.6. Hakkoymaz et al., 2019 [31]
3.3. Quality Assessment
3.4. Meta-Analysis
- (a)
- Serum IMA in patients with acute appendicitis vs. controls
- (b)
- Serum IMA in patients with complicated vs. non-complicated acute appendicitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- PubMed—(‘ischemia modified albumin’) AND (appendicitis)
- Embase—All Fields: ‘ischemia modified albumin’ AND ‘appendicitis’
- Scopus—ALL (“ischemia modified albumin”) AND ALL (“appendicitis”)
- Web of Science—(ALL = (ischemia modified albumin)) AND ALL = (appendicitis)
| Database | Studies |
|---|---|
| PubMed | 9 |
| EMBASE | 11 |
| Web of Science | 10 |
| Scopus | 69 |
| Total | 99 |
| Duplications | 37 |
| After duplication removal | 62 |
References
- Pogorelić, Z.; Rak, S.; Mrklić, I.; Jurić, I. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr. Emerg. Care 2015, 31, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, A.B.; Vazquez-Benitez, G.; Ballard, D.W.; Vinson, D.R.; Chettipally, U.K.; Kene, M.V.; Dehmer, S.P.; Bachur, R.G.; Dayan, P.S.; Kuppermann, N.; et al. Development and Validation of a Novel Pediatric Appendicitis Risk Calculator (pARC). Pediatrics 2018, 141, e20172699. [Google Scholar] [CrossRef] [PubMed]
- Awayshih, M.M.A.; Nofal, M.N.; Yousef, A.J. Evaluation of Alvarado score in diagnosing acute appendicitis. Pan Afr. Med. J. 2019, 34, 15. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsdottir, J.; Marklund, E.; Hagander, L.; Salö, M. Clinical Prediction Scores for Pediatric Appendicitis. Eur. J. Pediatr. Surg. 2021, 31, 252–260. [Google Scholar] [CrossRef]
- Dumlu, E.G.; Tokaç, M.; Bozkurt, B.; Yildirim, M.B.; Ergin, M.; Yalçin, A.; Kiliç, M. Correlation between the serum and tissue levels of oxidative stress markers and the extent of inflammation in acute appendicitis. Clinics 2014, 69, 677–682. [Google Scholar] [CrossRef]
- Nance, M.L.; Adamson, W.T.; Hedrick, H.L. Appendicitis in the young child: A continuing diagnostic challenge. Pediatr. Emerg. Care 2000, 16, 160–162. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Janković Marendić, I.; Čohadžić, T.; Jukić, M. Clinical Outcomes of Daytime Vs. Nighttime Laparoscopic Appendectomy in Children. Children 2023, 10, 750. [Google Scholar] [CrossRef]
- Lee, S.L.; Stark, R.; Yaghoubian, A.; Shekherdimian, S.; Kaji, A. Does age affect the outcomes and management of pediatric appendicitis? J. Pediatr. Surg. 2011, 46, 2342–2345. [Google Scholar] [CrossRef]
- Albu, E.; Miller, B.M.; Choi, Y.; Lakhanpal, S.; Murthy, R.N.; Gerst, P.H. Diagnostic value of C-reactive protein in acute appendicitis. Dis. Colon Rectum 1994, 37, 49–51. [Google Scholar] [CrossRef]
- Uludağ, S.S.; Akıncı, O.; Güreş, N.; Tunç, E.; Erginöz, E.; Şanlı, A.N.; Zengin, A.K.; Özçelik, M.F. Effectiveness of pre-operative routine blood tests in predicting complicated acute appendicitis. Turk. J. Trauma Emerg. Surg. 2022, 28, 1590–1596. [Google Scholar]
- Dooki, M.E.; Nezhadan, M.; Mehrabani, S.; Osia, S.; Hadipoor, A.; Hajiahmadi, M.; Mohammadi, M. Diagnostic accuracy of laboratory markers for diagnosis of acute appendicitis in children. Wien. Med. Wochenschr. 2022, 172, 303–307. [Google Scholar] [CrossRef]
- Krishnan, N.; Anand, S.; Pakkasjärvi, N.; Bajpai, M.; Dhua, A.K.; Yadav, D.K. Mean Platelet Volume in the Diagnosis of Acute Appendicitis in the Pediatric Population: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1596. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Krishnan, N.; Jukić, M.; Križanac, Z.; Llorente Muñoz, C.M.; Pogorelić, Z. Utility of Red Cell Distribution Width (RDW) as a Noninvasive Biomarker for the Diagnosis of Acute Appendicitis: A Systematic Review and Meta-Analysis of 5222 Cases. Diagnostics 2022, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.A.; Pascual, C.B.; Antona, G.; Anaut, M.B.; López-Andrés, N.; Martín-Calvo, N. Diagnostic performance of calprotectin and APPY-1 test in pediatric acute appendicitis: A systematic review and a meta-analysis. Eur. J. Trauma Emerg. Surg. 2023, 49, 763–773. [Google Scholar] [CrossRef]
- Montero, J.A.; Antona, G.; Anaut, M.B.; Pascual, C.B.; Briones, R.R.; Fernández-Celis, A.; Marcotegui, A.R.; López-Andrés, N.; Martín-Calvo, N. Diagnostic performance of serum pentraxin-3 in pediatric acute appendicitis: A prospective diagnostic validation study. Pediatr. Surg. Int. 2022, 39, 27. [Google Scholar] [CrossRef]
- Montero, J.A.; Antona, G.; Marcotegui, A.R.; Pascual, C.B.; Anaut, M.B.; Briones, R.R.; Fernández-Celis, A.; López-Andrés, N.; Martín-Calvo, N. Discriminatory capacity of serum interleukin-6 between complicated and uncomplicated acute appendicitis in children: A prospective validation study. World J. Pediatr. 2022, 18, 810–817. [Google Scholar] [CrossRef]
- Pogorelić, Z.; Lukšić, A.M.; Mihanović, J.; Đikić, D.; Balta, V. Hyperbilirubinemia as an indicator of perforated acute appendicitis in pediatric population: A prospective study. Surg. Infect. 2021, 22, 1064–1071. [Google Scholar] [CrossRef]
- Yap, T.L.; Fan, J.D.; Ho, M.F.; Choo, C.S.C.; Ong, L.Y.; Chen, Y. Salivary biomarker for acute appendicitis in children: A pilot study. Pediatr. Surg. Int. 2020, 36, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Kentsis, A.; Ahmed, S.; Kurek, K.; Brennan, E.; Bradwin, G.; Steen, H.; Bachur, R. Detection and diagnostic value of urine leucine-rich α-2-glycoprotein in children with suspected acute appendicitis. Ann. Emerg. Med. 2012, 60, 78–83.e1. [Google Scholar] [CrossRef]
- Maghsoudi, L.H.; Soltanian, A.; Shirzadi, A.; Alizadeh-Kashani, R.; Ahmadinejad, M. Biomarker of urinary 5-HIAA as a valuable predictor of acute appendicitis. Pract. Lab. Med. 2020, 23, e00198. [Google Scholar] [CrossRef]
- Sachit, A.; Verma, A.; Agrawal, A.; Kainth, D.; Singh, A. Role of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis. PROSPERO 2023. CRD42023394470. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023394470 (accessed on 2 August 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch Losos, M.; Tugwell, P.; Ga, S.W.; Zello, G.A.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 2 August 2023).
- Reddy, V.S.; Perugu, B.; Garg, M.K. Ischemia-modified albumin must be evaluated as an oxidative stress marker together with albumin and bilirubin in individuals with acute appendicitis. Clinics 2015, 70, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, E.; Çitlenbik, H.; Akgül, F.; Öztürk, A.; Şık, N.; Ulusoy, O.; Küme, T.; Yılmaz, D.; Duman, M. Is Ischemia-Modified Albumin a Reliable Marker in Accurate Diagnosis of Appendicitis in Children? World J. Surg. 2020, 44, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Yeniocak, S.; Saraç, F.; Yazıcıoğlu, M.; Karabulut, N.; Ünal, A.; Yücetaş, E.; Koldaş, M.; Akkoç, İ.; Ekici, M.; Evrin, T. The Diagnostic Values of Ischemia-Modified Albumin in Patients with Acute Abdominal Pain and Its Role in Differentiating Acute Abdomen. Emerg. Med. Int. 2020, 7925975. [Google Scholar] [CrossRef]
- Kılıç, M.Ö.; Güldoğan, C.E.; Balamir, İ.; Tez, M. Ischemia-modified albumin as a predictor of the severity of acute appendicitis. Am. J. Emerg. Med. 2017, 35, 92–95. [Google Scholar] [CrossRef]
- Nazik, S.; Avci, V.; Küskü Kiraz, Z. Ischemia-modified albumin and other inflammatory markers in the diagnosis of appendicitis in children. Ulus Travma Acil Cerrahi Derg 2017, 23, 317–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sarac, F.; Yeniocak, S.; Buyukbese Sarsu, S.; Sahin, K.; Yucetas, E.; Koldas, M.; Katipoglu, B. The diagnostic value of ischaemia-modified albumin in acute abdominal pain. J. Paediatr. Child Health 2019, 55, 1247–1250. [Google Scholar] [CrossRef]
- Ünsal, A.; Turhan, V.B.; Öztürk, D.; Buluş, H.; Türkeş, G.F.; Erel, Ö. The predictive value of ischemia-modified albumin in the diagnosis of acute appendicitis: A prospective case-control study. Turk. J. Trauma Emerg. Surg. 2022, 28, 523–528. [Google Scholar] [CrossRef]
- Hakkoymaz, H.; Nazik, S.; Seyithanoğlu, M.; Güler, O.; Şahin, A.R.; Cengiz, E.; Yazar, F.M. The value of ischemia-modified albumin and oxidative stress markers in the diagnosis of acute appendicitis in adults. Am. J. Emerg. Med. 2019, 37, 2097–2101. [Google Scholar] [CrossRef]
- Bundy, D.G.; Byerley, J.S.; Liles, E.A.; Perrin, E.M.; Katznelson, J.; Rice, H.E. Does this child have appendicitis? JAMA 2007, 298, 438–451. [Google Scholar] [CrossRef]
- Yilmaz, F.M.; Yilmaz, G.; Erol, M.F.; Köklü, S.; Yücel, D. Nitric oxide, lipid peroxidation and total thiol levels in acute appendicitis. J. Clin. Lab. Anal. 2010, 24, 63–66. [Google Scholar] [CrossRef]
- Boettcher, M.; Schacker, A.L.; Esser, M.; Schönfeld, L.; Ebenebe, C.U.; Rohde, H.; Mokhaberi, N.; Trochimiuk, M.; Appl, B.; Raluy, L.P.; et al. Markers of neutrophil activation and extracellular trap formation predict appendicitis. Surgery 2022, 171, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Pakkasjärvi, N.; Bajpai, M.; Krishnan, N.; Goswami, C.; Suominen, J.S.; Yadav, D.K.; Goel, P. Utility of Pentraxin-3 as a biomarker for diagnosis of acute appendicitis: A systematic review and meta-analysis. Pediatr. Surg. Int. 2022, 38, 1105–1112. [Google Scholar] [CrossRef]
- Anand, S.; Krishnan, N.; Birley, J.R.; Tintor, G.; Bajpai, M.; Pogorelić, Z. Hyponatremia—A New Diagnostic Marker for Complicated Acute Appendicitis in Children: A Systematic Review and Meta-Analysis. Children 2022, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Tintor, G.; Jukić, M.; Šupe-Domić, D.; Jerončić, A.; Pogorelić, Z. Diagnostic Utility of Serum Leucine-Rich α-2-Glycoprotein 1 for Acute Appendicitis in Children. J. Clin. Med. 2023, 12, 2455. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Dodsworth, N.; Woodrow, J.; Tucker, A.; Harris, R. Site-specific N-terminal auto-degradation of human serum albumin. Eur. J. Biochem. 1995, 227, 524–528. [Google Scholar] [CrossRef]
- Collinson, P.O.; Gaze, D.C. Ischaemia-modified albumin: Clinical utility and pitfalls in measurement. J. Clin. Pathol. 2008, 61, 1025–1028. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Allosteric inhibition of cobalt binding to albumin by fatty acids: Implications for the detection of myocardial ischemia. J. Med. Chem. 2012, 55, 4425–4430. [Google Scholar] [CrossRef]
- Sinha, M.K.; Vazquez, J.M.; Calvino, R.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Effects of balloon occlusion during percutaneous coronary intervention on circulating Ischemia Modified Albumin and transmyocardial lactate extraction. Heart 2006, 92, 1852–1853. [Google Scholar] [CrossRef]
- Bar-Or, D.; Winkler, J.V.; Vanbenthuysen, K.; Harris, L.; Lau, E.; Hetzel, F.W. Reduced albumin-cobalt binding with transient myocardial ischemia after elective percutaneous transluminal coronary angioplasty: A preliminary comparison to creatine kinase-MB, myoglobin, and troponin I. Am. Heart J. 2001, 141, 985–991. [Google Scholar] [CrossRef] [PubMed]


| Author/Year | Groups | N | Age | Male/ Female | TLC (per µL) | CRP (mg/dL) | MPV (fL) | Serum IMA Levels (AbsU) |
|---|---|---|---|---|---|---|---|---|
| Dulmu 2014 [5] | Control | 30 | - | - | - | - | - | 0.31 +/− 0.09 |
| No Appendicitis | 4 | 30.5 +/− 11.1 (Years) | 1/3 | 9650 +/− 2154.54 | - | - | 0.65 +/− 0.09 | |
| Non-Complicated Acute Appendicitis | 37 | 32.06 +/− 10.47 (Years) | 15/22 | 12,021.63 +/− 4751.62 | - | - | 0.64 +/− 0.09 | |
| Perforated Appendicitis | 8 | 34.88 +/− 15.74 (Years) | 5/3 | 12,737.5 +/− 3601.17 | - | - | 0.67 +/− 0.09 | |
| Phlegmonous Appendicitis | 12 | 25.92 +/− 6.49 (Years) | 8/4 | 11,583.34 +/− 4683.41 | - | - | 0.67 +/− 0.09 | |
| Perforated + Phlegmonous Appendicitis | 4 | 33.5 +/− 9.82 (Years) | 1/3 | 14,475 +/− 8109.82 | - | - | 0.67 +/− 0.10 | |
| Kılıç 2017 [27] | Non-Complicated Acute Appendicitis | 33 | 29.8 +/− 10.7 (Years) | 20/13 | 15,100 +/− 3400 | - | - | 0.618 +/− 0.09 |
| Gangrenous/Perforated Appendicitis | 29 | 30.4 +/− 11.3 (Years) | 13/16 | 15,200 +/− 5900 | - | - | 0.682 +/− 0.08 | |
| Nazik 2017 [28] | Control | 33 | 105.6 +/− 30.8 (Months) | 23/10 | 7730 +/− 2100 | 7.45 +/− 9.2 | 8.08 +/− 0.9 | 0.33 +/− 0.1 |
| Acute Appendicitis | 30 | 119 +/− 27.4 (Months) | 18/12 | 12,120 +/− 4800 | 29.63 +/− 41.3 | 8.23 +/− 0.8 | 0.56 +/− 0.1 | |
| Hakkoymaz 2019 [31] | Control | 45 | 30.9 +/− 12.3 (Years) | 29/16 | 7700 +/− 2100 | 3.7 +/− 1.6 | 10.4 +/− 0.9 | 0.21 +/− 0.1 |
| Non-Complicated Acute Appendicitis | 35 | 33.6 +/− 16.2 (Years) | 29/22 | 12,400 +/− 4500 | 25.4 +/− 32.1 | 9.6 +/− 2.2 | 0.29 +/− 0.04 | |
| Complicated Appendicitis | 16 | 12,200 +/− 4700 | 43.8 +/− 52.2 | 0.40 +/− 0.05 | ||||
| Sarac 2019 [29] | Control | 40 | 8.4 +/− 4.8 (Years) | 26/16 | - | - | - | 0.36 +/− 0.08 |
| Non-Complicated Acute Appendicitis | 54 | 10.2 +/− 3.3 (Years) | 36/17 | 15,040.0 +/− 3688.7 | 2.34 +/− 5.89 | - | 0.70 +/− 0.17 | |
| Perforated Appendicitis | 16 | 9.3 +/− 3.0 (Years) | 8/8 | 18,241.3 +/− 4865.1 | 7.62 +/− 9.47 | - | 0.73 +/− 0.20 | |
| Unsal 2022 [30] | Control | 42 | 35.93 +/− 10.07 (Years) | 24/18 | 9320 +/− 3610 | 5.860 +/− 6.224 | - | 0.693 +/− 0.16 |
| Non-Complicated Acute Appendicitis | 64 | 35.75 +/− 12.13 (Years) | 53/44 | 13,630 +/− 3920 | 5.470 +/− 5.531 | - | 0.78 +/− 0.12 | |
| Acute Suppurative Appendicitis | 13 | - | 14,200 +/− 5440 | 6.675 +/− 6.076 | - | 0.78 +/− 0.14 | ||
| Perforated Appendicitis | 20 | - | 16,010 +/− 4450 | 9.269 +/− 7.612 | - | 0.79 +/− 0.12 |
| Author/ Year | Selection | Comparability | Outcomes | Total Score | Quality # | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | |||
| Dulmu 2014 [5] | * | * | * | * | - | * | * | * | 7 | Poor |
| Kılıç 2017 [27] | * | - | * | * | - | * | * | * | 6 | Poor |
| Nazik 2017 [28] | * | * | * | * | * | * | * | * | 8 | Good |
| Hakkoymaz 2019 [31] | * | * | * | * | ** | * | * | * | 9 | Good |
| Sarac 2019 [29] | * | * | * | * | * | * | * | * | 8 | Good |
| Unsal 2022 [30] | * | * | * | * | - | * | * | * | 7 | Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Pogorelić, Z.; Agrawal, A.; Muñoz, C.M.L.; Kainth, D.; Verma, A.; Jindal, B.; Agarwala, S.; Anand, S. Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5486. https://doi.org/10.3390/jcm12175486
Singh A, Pogorelić Z, Agrawal A, Muñoz CML, Kainth D, Verma A, Jindal B, Agarwala S, Anand S. Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(17):5486. https://doi.org/10.3390/jcm12175486
Chicago/Turabian StyleSingh, Apoorv, Zenon Pogorelić, Aniket Agrawal, Carlos Martin Llorente Muñoz, Deepika Kainth, Ajay Verma, Bibekanand Jindal, Sandeep Agarwala, and Sachit Anand. 2023. "Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 17: 5486. https://doi.org/10.3390/jcm12175486
APA StyleSingh, A., Pogorelić, Z., Agrawal, A., Muñoz, C. M. L., Kainth, D., Verma, A., Jindal, B., Agarwala, S., & Anand, S. (2023). Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(17), 5486. https://doi.org/10.3390/jcm12175486








