Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve
Abstract
1. Introduction
2. Materials and Methods
2.1. First Study
2.2. Second Study
2.3. Statistical Analysis
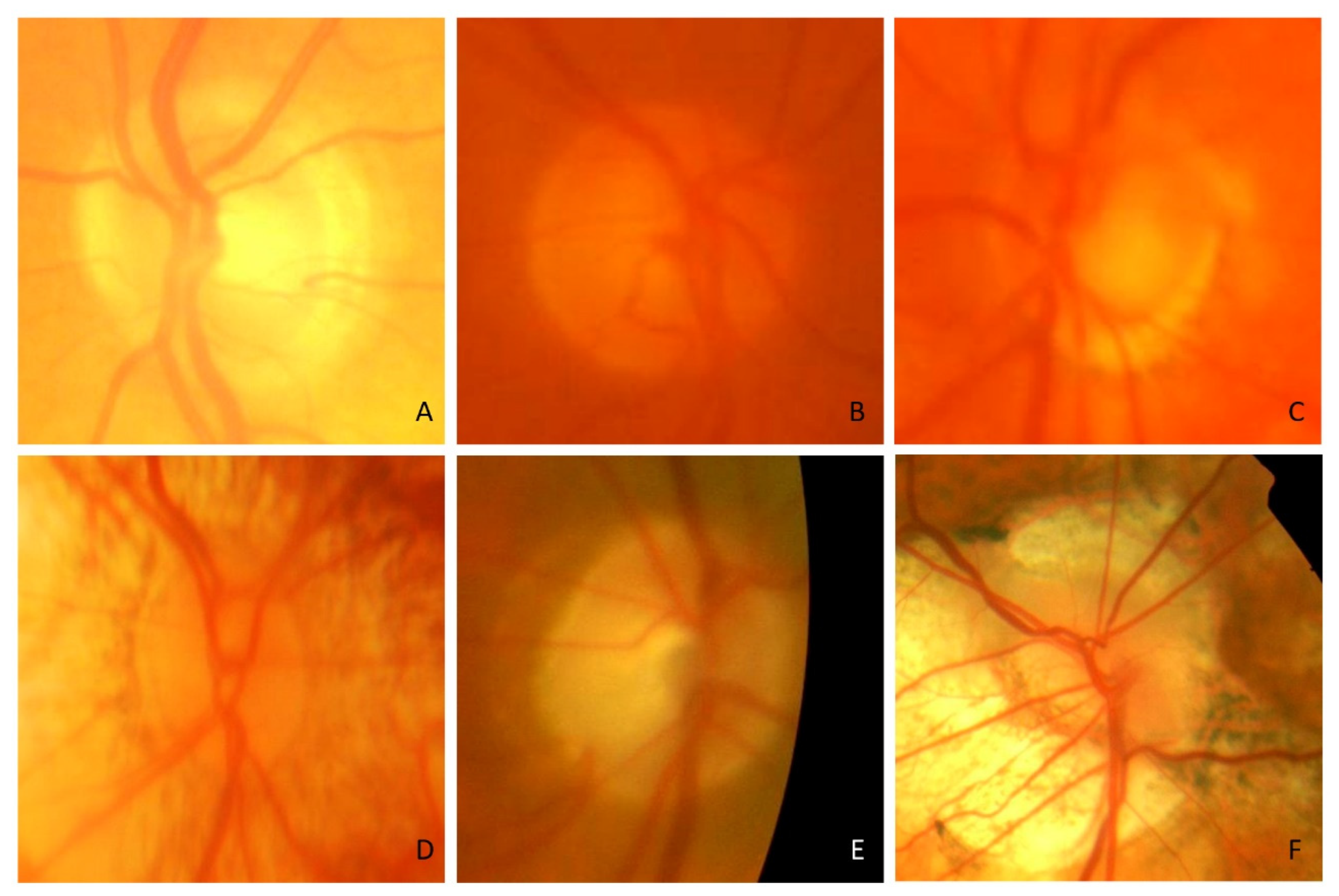
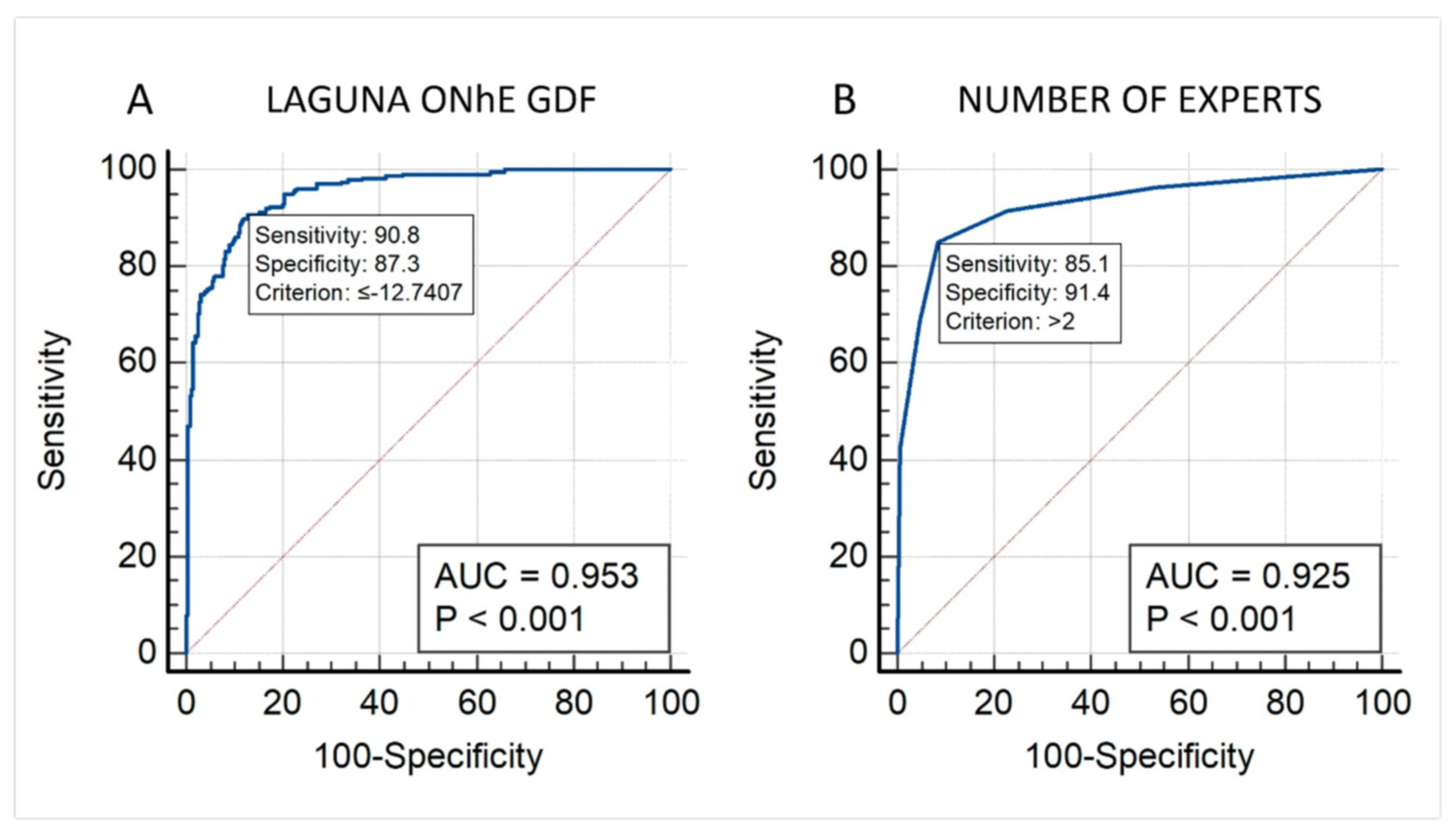
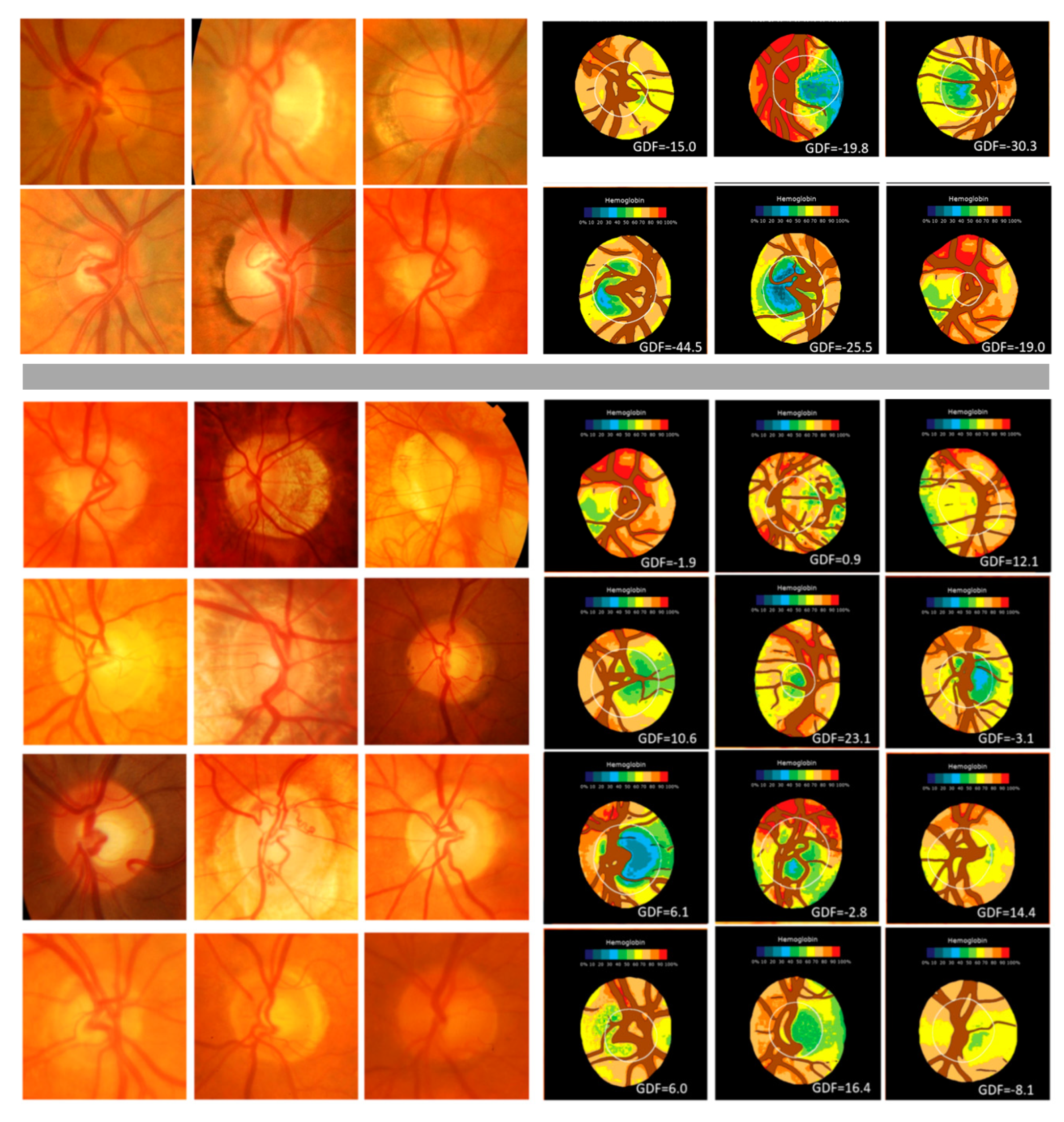
3. Results
3.1. First Study
3.2. Second Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Zedan, M.J.M.; Zulkifley, M.A.; Ibrahim, A.A.; Moubark, A.M.; Kamari, N.A.M.; Abdani, S.R. Automated Glaucoma Screening and Diagnosis Based on Retinal Fundus Images Using Deep Learning Approaches: A Comprehensive Review. Diagnostics 2023, 13, 2180. [Google Scholar] [CrossRef]
- Diaz-Pinto, A.; Morales, S.; Naranjo, V.; Köhler, T.; Mossi, J.M. NACNNs for Automatic Glaucoma Assessment Using Fundus Images: An Extensive Validation. Biomed. Eng. Online 2019, 18, 29. [Google Scholar] [CrossRef]
- de la Rosa, M.G.; Gonzalez-Hernandez, M.; Sigut, J.; Alayon, S.; Radcliffe, N.; Mendez-Hernandez, C.; García-Feijoo, J.; Fuertes-Lazaro, I.; Perez-Olivan, S.; Ferreras, A. Measuring Hemoglobin Levels in the Optic Nerve Head: Comparisons with Other Structural and Functional Parameters of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 482–489. [Google Scholar] [CrossRef]
- Pena-Betancor, C.; Gonzalez-Hernandez, M.; Fumero-Batista, F.; Sigut, J.; Medina-Mesa, E.; Alayon, S.; De La Rosa, M.G. Estimation of the Relative Amount of Hemoglobin in the Cup and Neuroretinal Rim Using Stereoscopic Color Fundus Images. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Medina-Mesa, E.; Gonzalez-Hernandez, M.; Sigut, J.; Fumero-Batista, F.; Pena-Betancor, C.; Alayon, S.; Gonzalez de la Rosa, M. Estimating the Amount of Hemoglobin in the Neuroretinal Rim Using Color Images and OCT. Curr. Eye Res. 2016, 41, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Hernandez, C.; Rodriguez-Uña, I.; Gonzalez-de-la Rosa, M.; Arribas-Pardo, P.; Garcia-Feijoo, J. Glaucoma Diagnostic Capacity of Optic Nerve Head Haemoglobin Measures Compared with Spectral Domain OCT and HRT III Confocal Tomography. Acta Ophthalmol. 2016, 94, 697–704. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.; Gonzalez-Hernandez, D.; Perez-Barbudo, D.; Rodriguez-Esteve, P.; Betancor-Caro, N.; de la Rosa, M.G. Fully Automated Colorimetric Analysis of the Optic Nerve Aided by Deep Learning and Its Association with Perimetry and Oct for the Study of Glaucoma. J. Clin. Med. 2021, 10, 3231. [Google Scholar] [CrossRef]
- Rocha, J.A.G.; Dias, D.T.; Lemos, M.B.C.; Kanadani, F.N.; Paranhos, A.; Gracitelli, C.P.B.; Prata, T.S. Optic Nerve Head Hemoglobin Levels in Glaucoma: A Structural and Functional Correlation Study. J. Ophthalmol. 2021, 2021, 9916102. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.; Gonzalez-Hernandez, D.; Perez-Barbudo, D.; De La Rosa, M.G. Optic Disc Area Frequency Distribution in a Large Sample of Retinographic Images. BMJ Open Ophthalmol. 2022, 7, e000972. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, J.B.; Rutjes, A.W.S.; Khan, K.S.; Coomarasamy, A.; Bossuyt, P.M. A Review of Solutions for Diagnostic Accuracy Studies with an Imperfect or Missing Reference Standard. J. Clin. Epidemiol. 2009, 62, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, L.; Wormstone, I.M.; Qiao, C.; Zhang, C.; Liu, P.; Li, S.; Wang, H.; Mou, D.; Pang, R.; et al. Development and Validation of a Deep Learning System to Detect Glaucomatous Optic Neuropathy Using Fundus Photographs. JAMA Ophthalmol. 2019, 137, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, A.; Govindaiah, A.; Smith, R.T. An Artificial-Intelligence- And Telemedicine-Based Screening Tool to Identify Glaucoma Suspects from Color Fundus Imaging. J. Ophthalmol. 2021, 2021, 6694784. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.; Gonzalez-Hernandez, D.; Betancor-Caro, N.; Guedes-Guedes, I.; Guldager, M.K.; Gonzalez de la Rosa, M. Glaucoma Incidence and Progression in Diabetics: The Canary Islands Study Using the Laguna ONhE Application. J. Clin. Med. 2022, 11, 7294. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Luo, X.; Wang, C.; Liu, W.; Lin, X. Optic Disc and Optic Cup Segmentation Based on Anatomy Guided Cascade Network. Comput. Methods Programs Biomed. 2020, 197, 105717. [Google Scholar] [CrossRef]
- Fu, H.; Cheng, J.; Xu, Y.; Zhang, C.; Wong, D.W.K.; Liu, J.; Cao, X. Disc-Aware Ensemble Network for Glaucoma Screening From Fundus Image. IEEE Trans. Med. Imaging 2018, 37, 2493–2501. [Google Scholar] [CrossRef]
- Jin, B.; Liu, P.; Wang, P.; Shi, L.; Zhao, J. Optic Disc Segmentation Using Attention-Based U-Net and the Improved Cross-Entropy Convolutional Neural Network. Entropy 2020, 22, 844. [Google Scholar] [CrossRef]
- An, G.; Omodaka, K.; Tsuda, S.; Shiga, Y.; Takada, N.; Kikawa, T.; Nakazawa, T.; Yokota, H.; Akiba, M. Comparison of Machine-Learning Classification Models for Glaucoma Management. J. Healthc. Eng. 2018, 2018, 6874765. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, L.; Cheng, J.; Gu, Z.; Xia, H.; Fu, H.; Li, C.; Liu, J. JointRCNN: A Region-Based Convolutional Neural Network for Optic Disc and Cup Segmentation. IEEE Trans. Biomed. Eng. 2020, 67, 335–343. [Google Scholar] [CrossRef]
- Ahn, J.M.; Kim, S.; Ahn, K.-S.; Cho, S.-H.; Lee, K.B.; Kim, U.S. A Deep Learning Model for the Detection of Both Advanced and Early Glaucoma Using Fundus Photography. PLoS ONE 2018, 13, e0207982. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Hernandez, C.; Wang, S.; Arribas-Pardo, P.; Salazar-Quiñones, L.; Güemes-Villahoz, N.; Fernandez-Perez, C.; Garcia-Feijoo, J. Diagnostic Validity of Optic Nerve Head Colorimetric Assessment and Optical Coherence Tomography Angiography in Patients with Glaucoma. Br. J. Ophthalmol. 2021, 105, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.A.; Gunn, P.J.G.; Spry, P.G.D.; Fenerty, C.H.; Lawrenson, J.G. Care Pathways for Glaucoma Detection and Monitoring in the UK. Eye 2020, 34, 89–102. [Google Scholar] [CrossRef]
- Varma, R.; Steinmann, W.C.; Scott, I.U. Expert Agreement in Evaluating the Optic Disc for Glaucoma. Ophthalmology 1992, 99, 215–221. [Google Scholar] [CrossRef]
- Spalding, J.M.; Litwak, A.B.; Shufelt, C.L. Optic Nerve Evaluation among Optometrists. Optom. Vis. Sci. 2000, 77, 446–452. [Google Scholar] [CrossRef]
- Harper, R.; Radi, N.; Reeves, B.C.; Fenerty, C.; Spencer, A.F.; Batterbury, M. Agreement between Ophthalmologists and Optometrists in Optic Disc Assessment: Training Implications for Glaucoma Co-Management. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 342–350. [Google Scholar] [CrossRef]
- Nicolela, M.T.; Drance, S.M.; Broadway, D.C.; Chauhan, B.C.; McCormick, T.A.; LeBlanc, R.P. Agreement among Clinicians in the Recognition of Patterns of Optic Disk Damage in Glaucoma. Am. J. Ophthalmol. 2001, 132, 836–844. [Google Scholar] [CrossRef]
- Breusegem, C.; Fieuws, S.; Stalmans, I.; Zeyen, T. Agreement and Accuracy of Non-Expert Ophthalmologists in Assessing Glaucomatous Changes in Serial Stereo Optic Disc Photographs. Ophthalmology 2011, 118, 742–746. [Google Scholar] [CrossRef]
- Kong, Y.X.G.; Coote, M.A.; O’Neill, E.C.; Gurria, L.U.; Xie, J.; Garway-Heath, D.; Medeiros, F.A.; Crowston, J.G. Glaucomatous Optic Neuropathy Evaluation Project: A Standardized Internet System for Assessing Skills in Optic Disc Examination. Clin. Exp. Ophthalmol. 2011, 39, 308–317. [Google Scholar] [CrossRef]
- Han, J.W.; Cho, S.Y.; Kang, K.D. Correlation between Optic Nerve Parameters Obtained Using 3D Nonmydriatic Retinal Camera and Optical Coherence Tomography: Interobserver Agreement on the Disc Damage Likelihood Scale. J. Ophthalmol. 2014, 2014, 931738. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, D.M.; De Moraes, C.G.; Liebmann, J.M.; Garg, R.; Chen, C.; Theventhiran, A.; Hood, D.C. Technology and the Glaucoma Suspect. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT80–OCT85. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Nolivos, K.; Pazos, M.; Fatti, G.; Herranz, A.; Ayala-Fuentes, M.E.; Martínez-Prats, E.; Peral, O.; Vega-Lopez, Z.; Monleon-Getino, A.; et al. Interobserver and Intertest Agreement in Telemglaucoma Screening with Optic Disk Photos Ancoherence Tomography. J. Clin. Med. 2021, 10, 3337. [Google Scholar] [CrossRef] [PubMed]
- Tielsch, J.M.; Katz, J.; Quigley, H.A.; Miller, N.R.; Sommer, A. Intraobserver and Interobserver Agreement in Measurement of Optic Disc Characteristics. Ophthalmology 1988, 95, 350–356. [Google Scholar] [CrossRef]
- Abrams, L.S.; Scott, I.U.; Spaeth, G.L.; Quigley, H.A.; Varma, R. Agreement among Optometrists, Ophthalmologists, and Residents in Evaluating the Optic Disc for Glaucoma. Ophthalmology 1994, 101, 1662–1667. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, M.; Saavedra, J.S.; De La Rosa, M.G. Relationship between Retinal Nerve Fiber Layer Thickness and Hemoglobin Present in the Optic Nerve Head in Glaucoma. J. Ophthalmol. 2017, 2017, 2340236. [Google Scholar] [CrossRef]
- De La Rosa, M.G.; De-La-Huerga-Moreno, S.; Alfonso-Lopez, F.; Cabrera-Lopez, F.; Pareja-Rios, A.; Gonzalez-Hernandez, D.; Gonzalez-Hernandez, M. Comparison of Age-Related Vascular Changes in the Optic Disc of Patients with Diabetes, with Glaucomatous and Non-Glaucomatous Features. BMJ Open Ophthalmol. 2022, 7, e001100. [Google Scholar] [CrossRef]
- Meneses, L.S.D.; Ciarlini, L.R.; Ayub, G.; Vasconcellos, J.P.C.; Costa, V.P. Discrimination Between Healthy Eyes and Those With Mild Glaucoma Damage Using Hemoglobin Measurements of the Optic Nerve Head. J. Glaucoma 2022, 31, 567–573. [Google Scholar] [CrossRef]

| % GLAUCOMA | |
|---|---|
| ACRIMA | 57.2 |
| Laguna ONhE GDF | 45.1 |
| EXPERT 1 | 43.7 |
| EXPERT 2 | 48.4 |
| EXPERT 3 | 46.4 |
| EXPERT 4 | 57.4 |
| EXPERT 5 | 27.0 |
| MAJORITY | 43.1 |
| Kappa | ACRIMA | Laguna ONhE GDF | EXPERT 1 | EXPERT 2 | EXPERT 3 | EXPERT 4 | EXPERT 5 |
|---|---|---|---|---|---|---|---|
| Laguna ONhE GDF | 0.58201 | ||||||
| EXPERT 1 | 0.58374 | 0.72118 | |||||
| EXPERT 2 | 0.32329 | 0.41305 | 0.41957 | ||||
| EXPERT 3 | 0.57691 | 0.67523 | 0.74768 | 0.37506 | |||
| EXPERT 4 | 0.55935 | 0.61430 | 0.60882 | 0.37541 | 0.60211 | ||
| EXPERT 5 | 0.35803 | 0.53714 | 0.59214 | 0.34525 | 0.53898 | 0.37599 | |
| Average | 0.49722 | 0.59218 | 0.51167 | ||||
| t test | ACRIMA | Laguna ONhE GDF | |||||
| Laguna ONhE GDF | 0.11566 | ||||||
| ALL EXPERTS | 0.41766 | 0.14364 | |||||
| Majority | 0.60925 | 0.76862 | 0.87831 | 0.46499 | 0.84629 | 0.69110 | 0.62478 |
| ACRIMA | EXPERT 1 | EXPERT 2 | EXPERT 3 | EXPERT 4 | EXPERT 5 | ||
|---|---|---|---|---|---|---|---|
| EXPERT 1 | 0.18957 | ||||||
| EXPERT 2 | −0.09091 | 0.13223 | |||||
| EXPERT 3 | 0.34783 | 0.42308 | −0.06838 | ||||
| EXPERT 4 | 0.23881 | 0.30636 | −0.03604 | 0.47368 | |||
| EXPERT 5 | 0.09174 | 0.33912 | −0.01563 | 0.32990 | 0.19263 | ||
| Average | 0.26870 | 0.18376 | |||||
| Average | |||||||
| Majority | 0.27488 | 0.68894 | 0.13223 | 0.71154 | 0.56647 | 0.45928 | 0.47222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendez-Hernandez, C.; Gutierrez-Diaz, E.; Pazos, M.; Gimenez-Gomez, R.; Pinazo-Duran, M.D. Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve. J. Clin. Med. 2023, 12, 5485. https://doi.org/10.3390/jcm12175485
Mendez-Hernandez C, Gutierrez-Diaz E, Pazos M, Gimenez-Gomez R, Pinazo-Duran MD. Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve. Journal of Clinical Medicine. 2023; 12(17):5485. https://doi.org/10.3390/jcm12175485
Chicago/Turabian StyleMendez-Hernandez, Carmen, Esperanza Gutierrez-Diaz, Marta Pazos, Rafael Gimenez-Gomez, and Maria Dolores Pinazo-Duran. 2023. "Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve" Journal of Clinical Medicine 12, no. 17: 5485. https://doi.org/10.3390/jcm12175485
APA StyleMendez-Hernandez, C., Gutierrez-Diaz, E., Pazos, M., Gimenez-Gomez, R., & Pinazo-Duran, M. D. (2023). Agreement between Five Experts and the Laguna ONhE Automatic Colourimetric Application Interpreting the Glaucomatous Aspect of the Optic Nerve. Journal of Clinical Medicine, 12(17), 5485. https://doi.org/10.3390/jcm12175485







