Abstract
The mainstay treatments for Parkinson’s Disease (PD) have been limited to pharmacotherapy and deep brain stimulation. While these interventions are helpful, a new wave of research is investigating noninvasive neuromodulation methods as potential treatments. Some promising avenues have included transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), electroconvulsive therapy (ECT), and focused ultrasound (FUS). While these methods are being tested in PD patients, investigations in animal models of PD have sought to elucidate their therapeutic mechanisms. In this rapid review, we assess the available animal literature on these noninvasive techniques and discuss the possible mechanisms mediating their therapeutic effects based on these findings.
1. Introduction
Parkinson’s Disease (PD) is a debilitating movement disorder usually diagnosed in individuals over 60 years of age. Its core symptoms are bradykinesia, rigidity, and impaired motor control in the limbs, as evidenced by tremors in the hands and a shuffling gait. However, the importance of cognitive, mood, and digestive symptoms associated with the disorder is being increasingly appreciated [1]. Indeed, non-motor symptoms are often more debilitating for patients and caregivers [2]. Considering the life expectancy in most industrialized nations [3], individuals diagnosed with PD may have many remaining years of life, which underscores the importance of effective treatment and management of this disease.
The approach to the treatment of PD has remained relatively stable for many years. The mainstay treatment is dopamine replacement therapy. Deep brain stimulation (DBS) is also an effective treatment but requires invasive surgery. Non-invasive neuromodulation methods, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), and focused ultrasound (FUS), are currently being explored as potential treatment options in PD with early evidence of efficacy in human patients [4,5,6]. Studies in animal models have helped elucidate the possible neurobiological mechanisms of these therapeutic effects, outlining future directions for human research. In this rapid review, we surveyed the current literature on major forms of non-invasive brain modulation in animal models of PD: repetitive transcranial stimulation (rTMS), transcranial direct current stimulation (tDCS), electroconvulsive therapy (ECT), and focused ultrasound (FUS). Because this review focuses on brain modulation methods, other forms of non-invasive stimulation that have been considered for the treatment of PD, such as transcutaneous nerve stimulation or electro-acupuncture, are beyond the scope of this review.
2. Materials and Methods
In this rapid review, we performed searches using the advanced search function in the National Institute of Health’s Pubmed website for keywords related to non-invasive methods targeting PD in animal models. We set our range of publication dates from 1980 to 2023, but no study we found was older than 1992. The keywords varied slightly for each category of non-invasive method. All searches included “Parkinson’s Disease” AND “animal model”, with the following terms also included: TMS—“AND transcranial magnetic stimulation”, “AND TMS”; tDCS—“AND transcranial direct current stimulation”, “AND transcranial alternating current stimulation”, “AND tDCS”, “AND tACS”; ECT—“AND electroconvulsive therapy”, “AND ECT”; and FUS—“AND focused ultrasound”, “AND transcranial ultrasound”, “AND FUS”, “AND TUS”, “AND LIFU”. These initial searches yielded 91 articles. Additional more inclusive searches using keywords “Parkinson’s disease” combined with “AND transcranial magnetic stimulation”, “AND TMS”; “AND transcranial direct current stimulation”, “AND tDCS”, “AND tACS”; “AND electroconvulsive therapy”, “AND ECT”; “AND focused ultrasound”, “AND FUS”, “AND TUS”, “AND LIFU” and with the filters set to “Other Animals” (i.e., excluding humans) yielded another 200 articles, some of which were duplicates of those resulting from the initial searches. Criteria for exclusion consisted of human studies, studies only using in vitro methods, review articles, meta-analyses, conference papers or presentations, and animal studies investigating neurodegenerative disorders generally instead of focusing on PD specifically. After reviewing the 291 articles for content in their abstracts (performed by the first author) and applying the exclusion criteria, we excluded 243 articles that did not meet the criteria for inclusion or were duplicates. This screening yielded 36 articles included in this review. Additional eligible articles were discovered in the references of the articles found by the literature search: two in the TMS category, eleven in the FUS category, and one in the ECT category. This made for a total of 50 articles included in this review.
3. Results
In the following sections, we will summarize studies by category of non-invasive neuromodulatory technique.
3.1. Transcranial Magnetic Stimulation (TMS)
3.1.1. Background
TMS generates a high-intensity magnetic field by discharging an electrical current through a magnetic coil. This creates a magnetic field perpendicular to the coil that passes through the skull without attenuation. This induces an electrical field perpendicular to the magnetic field. The induced electrical field modulates the ongoing activity of neurons at the site of stimulation as well as in neural circuits encompassing the stimulation site. Single TMS pulses cause the temporally restricted modulation of neural activity and are used experimentally to study regional brain function. TMS pulses applied repetitively can produce lasting changes in neuronal excitability by inducing long-term potentiation (LTP)- or long-term depression (LTD)-like plasticity in stimulated regions and related circuits [7]. Owing to these lasting effects, repetitive transcranial magnetic stimulation (rTMS) has been used therapeutically in several disorders, most notably major depressive disorder and obsessive-compulsive disorder. The neuromodulatory effects of rTMS differ by frequency, with low-frequency (lf) rTMS causing a decrease in cortical excitability and high-frequency (hf) rTMS causing an increase in cortical excitability [8].
A considerable body of work has examined rTMS as a potential treatment for motor and cognitive symptoms of PD, with several meta-analyses and reviews on this topic providing evidence of efficacy in treatment of motor, depressive, and cognitive symptoms [9], as shown in Table 1. A number of mechanisms have been proposed to underlie the therapeutic effects of rTMS in PD based on both clinical and preclinical literature, including the induction of plasticity in the stimulated cortical areas and related circuits; a release of dopamine, which may modulate synaptic activity and plasticity; and molecular mechanisms bringing about neuroprotective effects [10,11]. Studies in animal models of PD make an important contribution to elucidating these mechanisms, especially those occurring at cellular and molecular levels.

Table 1.
Summary of evidence from reviews and meta-analyses on alleviation of PD-related symptoms in humans. Hf-rTMS = high-frequency rTMS; lf-rTMS = low-frequency rTMS; cTBS = continuous theta burst stimulation; iTBS = intermittent theta burst stimulation; M1 = primary motor cortex; SMA = supplementary motor area; DLPFC = dorsolateral prefrontal cortex.
3.1.2. Animal Studies
The neural mechanisms underlying the therapeutic effects of TMS in PD were initially studied in wild-type (WT) rats prior to being investigated in PD models. We note two studies that investigated the effects of TMS on dopamine release [21,22]. Both studies found increases in striatal dopamine following hf-rTMS administration to the PFC, corroborating findings of Strafella et al. [23] in healthy human volunteers without PD. Notably, Kanno, et al. found that the acute administration of the 1-s trains of rTMS at 60% of maximum stimulator output achieved the greatest increases in dopamine compared to both 20% and 80% of stimulator output, indicating an inverted U-shape relationship between TMS intensity and striatal dopamine release [22].
A summary of TMS studies in animal models of PD is given in Table 2. All but three studies used a unilateral chemical lesioning model of PD with 6- hydroxydopamine (6-OHDA) [24,25,26,27,28,29,30,31,32] or lactacystin [33] in rats. Two of the remaining studies used bilateral lesioning via systemic injection of the toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mice [34,35], and one additional study used unilateral 6-OHDA lesioning in mice [36]. Some of the studies used intermittent theta burst stimulation (iTBS), which is a form of excitatory hf-rTMS [26,27,28,29,30], while others utilized non-theta-burst low-frequency (inhibitory) or high-frequency (excitatory) rTMS [24,25,31,32,33]. The positioning of the TMS coil was typically over the dorsal surface of the animal’s head but was not neuroanatomically specific. However, there was a degree of functional specificity for the motor cortex, as the stimulation was reported by some of the studies to produce motor responses in limb muscles. The two iTBS studies achieved greater functional specificity, optimizing the positioning of the coil for eliciting motor-evoked potentials (MEPs) from the contralesional forelimb.
Behavioral and Neuroprotective Effects. The studies that examined the therapeutic effects of rTMS in the 6-OHDA rat model all found amelioration of toxin-induced motor disturbances by the chronic (several weeks, see Table 2) administration of rTMS or iTBS compared to sham control. Motor function was most frequently evaluated using the rotational behavior test, and all studies using this test reported improvements. The rotational behavior test assesses apomorphine- or amphetamine-induced rotational behavior in unilaterally lesioned animals, manifesting as vigorously turning in circles in the direction of the lesioned side. A slowing-down of these turns indicates the effectiveness of an intervention (in this case, rTMS) [37]. Other types of motor improvements were also noted by several of the studies (Table 2), as was improvement in memory performance and reductions in anxiety-like and depressive-like behavior [29]. All of these studies also reported that relative to sham treatment, chronic rTMS or iTBS produced an attenuation of the loss of tyrosine hydroxylase positive (TH+)—i.e., dopamine-producing—neurons in the substantia nigra (SN) on the lesioned side. One of the studies reported that the attenuation of SN cell loss was correlated with improvements in motor symptoms as measured by the rotational test [26]. This suggests that neuroprotection mediates the motor function benefits of rTMS.
The studies in mice using the MPTP model yielded findings consistent with those of the rat models, reporting rTMS-induced improvements in motor [34,35] and memory performance [35], an increase in neuronal preservation in the SN and dopamine in the striatum [34,35], decreases in inflammatory markers TNFα and interlukin-6 (IL-6) [35], and increases in NFs in the SN [34]. One of the studies [35] also showed that the rTMS-induced downregulation of microRNA miR-195a-5p (a putative biomarker for PD) with a resultant upregulation of cyclic AMP-response element-binding protein (CREB) may be a mechanism for the observed neuroprotection. The behavioral and neuroprotective benefits were observed with lf- [34,35] and hf-rTMS [35]. Another study in mice [36] using 6-OHDA rather than MPTP for lesioning investigated the effects of both lf- and hf-rTMS on amyloid pathology as a putative biomarker of PD [38]. In addition to improving memory performance and attenuating SN neuronal loss caused by 6-OHDA, lf- and hf-rTMS increased the levels of amyloid β1–42 (Aβ1–42) in the cerebrospinal fluid (CSF) while decreasing levels in the brain. This suggests that one of the therapeutic effects of rTMS may be the facilitation of Aβ1–42 clearance.
Plasticity. Studies have also pointed to synaptic plasticity as a possible therapeutic mechanism of iTBS. Ghiglieri, et al. (2012) [28] investigated the acute effects of iTBS on cortico-striatal plasticity and found a moderate restoration of LTD in cortico-striatal slices, which was lost following 6-OHDA lesions. The restoration of LTD was associated with an enhanced excitability of striatal neuronal populations. Using the same iTBS protocol, Cacace, et al. (2017) [30] investigated its acute effects on motor function, plasticity, and inflammation and reported the amelioration of gait disturbances and akinesia, as well as increased levels of striatal dopamine. Neither LTP nor LTD could be induced in striatal projection neurons of sham-treated 6-OHDA animals. iTBS normalized both LTP and LTD, and the rescue of plasticity was accompanied by c-fos activation in striatal spiny neurons. Finally, Jovanovic, et al. [29] reported that iTBS altered the composition of NMDA receptor subunits in the striatum, reversing the 6-OHDA-induced pathogenic changes to subunit composition. The change to subunits included an increased expression of the GluN2A subunit, which would be expected to facilitate synaptic plasticity. Together, these findings suggest that iTBS is capable of restoring deficient synaptic plasticity in Parkinsonian animals, helping normalize the basal ganglia circuit function.
Anti-inflammatory Effects. Several studies have reported on the anti-inflammatory effects of rTMS. Two studies examined the effects of lf-rTMS on inflammatory markers. Using a lactacystin-induced model of PD, Ba, et al. [33] found that 4 weeks of lf-rTMS administration reduced the levels of the pro-apoptotic enzyme caspace-3 and inflammatory markers tumor necrosis factor-α (TNFα), and cyclooxygenase-2 (COX-2). Yang, et al. [24] likewise found decreased TNFα and COX-2 levels with the same rTMS protocol in a 6-OHDA rat model. Cacace, et al. [30] found that their iTBS protocol significantly reduced astrogliosis and microglial activation produced by the 6-OHDA lesion in the ipsilesional striatum. Investigating the endocannabinoid system’s role in neuroinflammation in PD, Kang, et al. [31] found that hf-rTMS (but not lf-rTMS) downregulated the cannabinoid CB2 receptor in reactive astrocytes and its ligands in the ventral midbrain of 6-OHDA- and lipopolysaccharide (LPS)-lesioned rats while reducing inflammatory markers and preventing cell loss in the SN, as well as improving rotational performance. The selective agonism of the CB2 receptor blocked the rTMS-induced suppression of astrocyte activity. The CB2 receptor has been implicated in neuroinflammatory mechanisms of PD [39].
Neurotrophic Factors. rTMS-induced increases in neurotrophic factors (NFs) have also been reported and proposed to mediate neuroprotective effects of rTMS. Lee, et al. [25] found that rTMS-treated 6-OHDA rats had higher levels of NFs in the SN, including brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) amongst other trophic factors. Jovanovic, et al. [29] also reported increased BDNF in the striatum following chronic iTBS, and Ba et al. [32] reported increased GDNF in the SN following lf-rTMS.
Levodopa-induced dyskinesias. In addition to investigating the effects of rTMS on the cardinal symptoms of PD in animal models, one study investigated the effects lf-rTMS on levodopa-induced dyskinesia (LID) in a 6-OHDA rat model of PD. Ba, et al. [32] found decreases in abnormal involuntary movements, as well as reductions in levodopa-induced dopamine fluctuations in the striatum of 6-OHDA-lesioned rats that had received lf-rTMS in addition to levodopa. rTMS also attenuated the loss of TH+ cells and produced an increase in GDNF in the SN. As a possible mechanism, rTMS decreased the levels of the NR2B NMDA receptor subunit tyrosine phosphorylation in the ipsilesional striatum, which has been implicated in LID [40,41].
Cortical Excitability. Lastly, one of the iTBS studies evaluated the effect of 6-OHDA lesioning on the acute potentiation of motor cortical excitability by iTBS rather than evaluating the therapeutic effects of iTBS in this PD model [26]. A loss of enhancement of MEPs by iTBS was reported in the lesioned animals (relative to intact controls). The degree of MEP enhancement was inversely associated with the loss of dopaminergic neurons in the SN and their fibers in the striatum, as well as with the degree of motor impairment. These findings suggest that 6-OHDA lesioning impairs motor cortical plasticity, which is consistent with reports in human patients [42] and with the reported loss of LTP and LTD in cortico-striatal neurons of 6-OHDA-lesioned rats [28,30].
In summary, studies in animal models of PD have demonstrated that lf-rTMS, hf-rTMS, and iTBS attenuate the PD-related motor deficits and concomitantly reduce the loss of dopamine-producing SN neurons. The effects of hf-rTMS were superior to those of lf-rTMS for some outcomes. Notably, the effects of rTMS on the cognitive and affective symptoms of PD in animal models have received little attention. Considering that rTMS produces changes in cortical excitability of all stimulated regions, effects beyond those on motor function are expected. Therefore, evaluating TMS effects on cognition and affect may be a promising future research direction for studies in PD animal models.
Two findings emerging prominently from this still limited literature are rTMS-induced decreases in inflammatory markers and increases in NFs. None of the studies thus far have causally linked the anti-neuroinflammatory effects of rTMS to NF increases, which could be addressed by future research. Because lesioning itself causes inflammation, utilizing genetic models of PD to study the effects of rTMS on inflammation would strengthen the evidence of anti-inflammatory effects.
Neuroinflammation is recognized as playing a role in the pathophysiology of PD with several reviews on the topic [43,44,45]. However, the human literature has rarely considered TMS as a potential anti-neuroinflammatory treatment. One study reported that rTMS reduced proinflammatory cytokines in serum of PD patients [46]. Although neuroinflammation is not easily studied in humans, such studies are possible using positron emission tomography (PET) with tracers targeting the proxy markers of neuroinflammation. The most commonly used have been tracers targeting the translocator protein (TSPO), which is upregulated in activated microglia and is therefore considered to be a proxy measure of microglial activation [47]. There is a growing body of TSPO PET imaging literature in human PD patients [48], and tracers targeting other proxies of neuroinflammation are being tested in PD (e.g., [49]). Imaging the effect of rTMS on neuroinflammatory markers in human PD patients using such tracers may be a promising future direction. The link between NFs and the alleviation of PD symptoms is well characterized, and BDNF is now being considered as a potential treatment for PD [50]. However, the idea that TMS may be able to increase NFs is novel and worth further investigation.
Finally, although the induction of plasticity is a well-accepted therapeutic mechanism of rTMS, the animal studies reviewed here have provided considerable insight into the molecular mechanisms pertaining to the treatment of PD.

Table 2.
Specifications of TMS studies. SN = substantia nigra; 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; LPS = lipopolysaccharide; MT = motor threshold; MEP = motor-evoked potential (induced by single TMS pulses); TNFα = tumor necrosis factor-α; COX-2 = cyclooxygenase-2; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; PDNF = platelet-derived neurotrophic factor; VEGF = vascular endothelial growth factor; NMDA = N-methyl-D-aspartate; CSF = cerebrospinal fluid; miR-195a5p = micro RNA-195a5p; CREB = cyclic AMP-response element-binding protein; Aβ = amyloid β; LTP = long-term potentiation; LTD = long-term depression; AEA = anandamide; 2-AG = 2-arachidonoylglycerol.
Table 2.
Specifications of TMS studies. SN = substantia nigra; 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; LPS = lipopolysaccharide; MT = motor threshold; MEP = motor-evoked potential (induced by single TMS pulses); TNFα = tumor necrosis factor-α; COX-2 = cyclooxygenase-2; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; PDNF = platelet-derived neurotrophic factor; VEGF = vascular endothelial growth factor; NMDA = N-methyl-D-aspartate; CSF = cerebrospinal fluid; miR-195a5p = micro RNA-195a5p; CREB = cyclic AMP-response element-binding protein; Aβ = amyloid β; LTP = long-term potentiation; LTD = long-term depression; AEA = anandamide; 2-AG = 2-arachidonoylglycerol.
| Study | PD Model | Sham Control | TMS Parameters | Outcome Measures and Findings |
|---|---|---|---|---|
| Ba, et al. (2017) [33] | Lactacystin-induced (rat) |
|
|
|
| Yang, et al. (2010) [24] | 6-OHDA (rat) |
|
|
|
| Ghiglieri, et al. (2012) [28] | 6-OHDA (rat) |
|
|
|
| Lee, et al. (2013) [25] | 6-OHDA (rat) |
|
|
|
| Dong, et al. (2015) [34] | MPTP (mouse) |
|
|
|
| Hsieh, et al., 2015 [26] | 6-OHDA (rat) |
|
|
|
| Cacace, et al. (2017) [30] | 6-OHDA (rat) |
|
|
|
| Ba, et al. (2019) [36] | 6-OHDA (mouse) |
|
|
|
| Hsieh, et al. (2021) [27] | 6-OHDA (rat) |
|
|
|
| Ba, et al. (2016) [32] | 6-OHDA + levodopa (rat) |
|
|
|
| Sun, et al. (2023) [35] | MPTP (mouse) |
|
|
|
| Kang, et al. (2022) [31] | 6-OHDA or LPS (rat) |
|
|
|
| Jovanovic, et al. (2023) [29] | 6-OHDA (rat) |
|
|
|
3.1.3. Limitations
One limitation that was noted in most studies surveyed was the lack of regional specificity of TMS in the rodent models. Physical constraints on the size of the TMS coil make it difficult to target a particular brain area in rats and mice. This limits the translatability of the findings to human patients, who receive TMS to focal brain regions, as well as precluding the study of TMS effects at the circuit level. Another limitation to note is the stress experienced by the animals during the TMS procedure [22] since they are restrained and awake in most studies. Stress has been shown to increase dopamine release in the striatum [51] and could therefore be a confounding factor, although sham controls experienced similar levels of stress. It should be noted that not all studies reviewed here used sham control for rTMS. Finally, the studies in rodent models of PD have rarely evaluated the possible interactions between rTMS and dopamine replacement therapy. The literature in human patients has suggested that the effects of rTMS may be modulated by pharmacotherapy [47,48].
3.2. Transcranial Direct Current Stimulation (tDCS) and Transcranial Alternating Current Stimulation (tACS)
3.2.1. Background
Although several forms of electrical brain stimulation have long been practiced as a therapy for psychiatric disorders, transcranial direct current stimulation (tDCS) has become increasingly widespread in recent years, transcending medical and scientific use. Communities of individuals now exist that practice do-it-yourself tDCS for self-improvement [52]. tDCS has emerged as a promising treatment for several psychiatric and neurological disorders, including PD [53,54,55,56,57]. The application of a constant direct current induces a subthreshold shift in resting membrane potentials of cortical neurons at the stimulated site [58]. Depending on the direction of current flow in relation to axonal orientation, this can be either a depolarizing or a hyperpolarizing effect. Anodal tDCS has been found to increase cortical excitability, whereas cathodal tDCS decreases cortical excitability [59]. Similar to rTMS, the lasting effects of tDCS are attributed to synaptic plasticity [58], although tDCS may potentiate concurrently ongoing plasticity rather than generate it in weakly active synapses that are not already undergoing plasticity [60]. The most common targets of tDCS for PD have been the dorsolateral prefrontal cortex (DLPFC) and the primary motor cortex (M1). One of the first studies to utilize tDCS in PD patients with concurrent depression found that anodal tDCS over the DLPFC improved performance on executive function tasks compared to sham tDCS (same stimulator delivering very low current) [56]. However, application of anodal tDCS to M1 has not generally produced improvement in motor symptoms according to a recent meta-analysis [61] though some individuals studies, (e.g., Fregni et al. [62]) have reported the amelioration of motor symptoms. In addition to tDCS, a variation on it called transcranial alternating current stimulation (tACS) has also been investigated in PD patients. tACS uses an oscillatory pattern of electrical current that is known to entrain the oscillatory activity of resonant neurons in the stimulated regions. This has been shown to modulate/reduce bradykinesia, normalize oscillations in M1 and other regions, and improve cognitive performance [63,64]. Furthermore, tACS over the motor cortex has been successfully used to accomplish the phase cancellation of tremor rhythm, which produced nearly a 50% reduction in tremor amplitude [65]. Studies in animal models of PD have contributed to elucidating the cellular and molecular mechanisms of the therapeutic effects of tDCS and tACS.
3.2.2. Animal Models
The study of the neural mechanisms underlying the therapeutic effects of tDCS in PD started with the application of tDCS to WT rats to assess the effects on dopamine release in the striatum and striatal activity after stimulating the frontal cortex [66,67]. Tanaka, et al. [66] found that after cathodal (but not anodal) tDCS to frontal regions of the cortex, extracellular dopamine levels in the striatum increased relative to sham tDCS (with very low current). In contrast, Takano, et al. [67] found that anodal tDCS to the frontal cortex increased neural activity in the nucleus accumbens assessed by rodent fMRI. This group reasoned that because of lesioning concerns related to cathodal tDCS, anodal tDCS may be preferable, although stimulation intensity in Tanaka, et al. [66] fell within the safety guidelines put forth by Liebetanz, et al. [68]. Both of these initial studies, producing seemingly contradictory findings, only applied tDCS once for 10 min, and the animals were anesthetized during the stimulation.
Behavioral and Neuroprotective Effects. More recently, studies have addressed the effects of chronic tDCS (daily, for multiple days or weeks) over M1 in PD rodent models (for a summary of findings and tDCS parameters, see Table 3). All the studies using an MPTP bilateral lesioning model of PD in mice found that anodal tDCS over M1/frontal cortex was sufficient to improve motor performance, with tDCS effects being comparable to those of levodopa [69,70]. The majority of these studies also reported attenuated TH+ cell loss in the SN and/or the striatum, or increases in TH and dopamine levels in MPTP mice [69,70,71]. One study additionally reported a tDCS-induced decrease in alpha-synuclein in the SN pars compacta (SNc) [70], while another saw an increase in GDNF in the SNc in addition to the attenuation of TH+ cell loss and motor deficits following the application of tACS [72]. Studies in the 6-OHDA rat models analogously reported improvements in motor behavior following the application of anodal tDCS to the motor cortex, with one study additionally reporting an attenuation of TH+ cell loss in the SN [71]. Notably, tDCS in this study was initiated early—24 h following lesion.
In addition to improving motor function, tDCS may also improve anxiety-like behavior in rodent PD models. A study of cognitive- and neuropsychiatric-like symptoms in a 6-OHDA rat model of PD found that chronic anodal tDCS over M1 decreased anxiety-like behaviors in addition to ameliorating motor performance [71]. However, this study did not observe changes in depression-like behaviors or recognition memory. Utilizing a rotenone-induced rat model of PD, Kade, et al. [73] showed that transcranial electrostimulation—a variation on tACS developed by this group that is similar to cranial electrostimulation therapy—was able to reduce neurodegeneration in the SN and anxiety-like behavior when applied over the frontal cortex. It is important to note that this study did not include a sham electrical stimulation group, unlike the studies discussed thus far.
Antioxidant Effects and Inhibition of Autophagy. Furthermore, work in MPTP mice suggested that tDCS may decrease markers of oxidative stress and autophagy. Oxidative stress plays an important role in the degeneration of dopamine neurons in PD [74]. Lu, et al. [69] assessed the effects of chronic tDCS on markers of oxidative stress, including nonenzymatic malonaldehyde (MDA), antioxidant enzymes superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px). MDA decreased while SOD and GSH-PX increased in the brains of MPTP mice that were treated with chronic anodal tDCS over the left frontal cortex [69]. These findings were correlated with improved performance on the rotarod test. Lee, et al. [70] showed that after 5 days of treatment, anodal tDCS over M1 inhibited autophagy, which has also been implicated in the pathophysiology of PD [75].
Stem-cell Transplantation. tDCS has also been investigated as a potential supporting therapy for embryonic cell transplantation into the striatum to alleviate PD-like symptoms in a 6-OHDA rat model [76]. The rats that received anodal tDCS over the motor cortex for 2 weeks after implantation showed higher levels of BDNF, enlarged graft volume, and improved motor function relative to rats that received sham tDCS. Conversely, cathodal tDCS was associated with a slight reduction in TH+ cell count in the striatum, although this was not significant.
Non-human primates. Finally, there has been one tDCS study in a nonhuman primate PD model [77]. This study used an aged (20 years old) advanced model of MPTP-induced parkinsonism [78]. Anodal tDCS applied over M1 produced a substantial improvement in walking ability and a reduction in tremor frequency and PD-like signs compared to sham tDCS. The acute effects of tDCS were transient (approximately 30 min). An improvement in PD signs was correlated with ‘accumulated stimulation’, representing a product of duration and intensity summed across treatment sessions. Post-mortem, tDCS of the right M1 was found to have produced increased c-fos expression in M1 and in the SNc on the treated side relative to the untreated side, suggesting increased neuronal activation.
To summarize, tDCS and tACS studies in animal models have generally corroborated the results of human studies demonstrating motor function improvement with anodal tDCS and tACS over frontal cortical regions. In addition, there is some initial evidence of the amelioration of anxiety-like behavior, although there has been no evidence of improvement in depressive-like behavior or cognitive performance thus far. tDCS has also been found to have neuroprotective effects, promoting the preservation of dopaminergic neurons in the SNc, decreasing levels of alpha-synuclein in these neurons [70,71], and increasing levels of NFs [72]. The study by Lu, et al. [69] additionally points to antioxidant effects of tDCS. These findings largely parallel findings reported by the TMS literature described earlier. Furthermore, some of this work yielded evidence of an adjunctive benefits of tDCS for stem cell transplantation, which is an intriguing future direction [76].

Table 3.
Specifications of tDCS and tACS studies. TES = transcranial electrostimulation; 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH = tyrosine hydroxylase; MDA = malonaldehyde; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; SN = substantia nigra; SNc = substantia nigra pars compacta; M1 = primary motor cortex; PFC = prefrontal cortex; LC3 = microtubule-associated protein 1 light chain 3; p62 = sequestosome1/p62; PI3K = phosphoinositide 3-kinase; AMPK = AMP-activated protein kinase; mTOR = mechanistic target of rapamycin; ULK1 = unc-51-like kinase; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; ↑ = increase, ↓ = decrease.
Table 3.
Specifications of tDCS and tACS studies. TES = transcranial electrostimulation; 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TH = tyrosine hydroxylase; MDA = malonaldehyde; SOD = superoxide dismutase; GSH-Px = glutathione peroxidase; SN = substantia nigra; SNc = substantia nigra pars compacta; M1 = primary motor cortex; PFC = prefrontal cortex; LC3 = microtubule-associated protein 1 light chain 3; p62 = sequestosome1/p62; PI3K = phosphoinositide 3-kinase; AMPK = AMP-activated protein kinase; mTOR = mechanistic target of rapamycin; ULK1 = unc-51-like kinase; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; ↑ = increase, ↓ = decrease.
| Study | PD Model | Sham Control | tDCS Parameters | Outcome Measures and Findings |
|---|---|---|---|---|
| Li, et al. (2011) [79] | 6-OHDA (rat) |
|
|
|
| Li, et al. (2015) [77] | MPTP (monkey) |
|
|
|
| Lu, et al. (2015) [69] | MPTP (mouse) |
|
|
|
| Winkler, et al. (2017) [76] * | 6-OHDA (rat) |
|
|
|
| Lee, et al. (2018) [70] ** | MPTP (mouse) |
|
|
|
| Kade, et al. (2019) [73] | Rotenone (rat) |
|
|
|
| Feng, et al. (2020) [71] | 6-OHDA (rat) |
|
|
|
| Lee, et al. (2022) [72] | MPTP (mouse) |
|
|
|
* This study utilized tDCS to enhance dopaminergic cell transplantation. ** This study also performed in-vitro investigations, results not discussed in this review.
3.2.3. Limitations
Compared to TMS, tDCS and tACS techniques have somewhat improved spatial resolution in small animal models because of the use of electrodes, which facilitates targeting specific cortical areas. Unlike TMS, tDCS in rodents necessitates the removal of the scalp which requires surgery, causing additional stress that is not associated with this treatment in humans and non-human primates [77]. Another limitation is the variation in stimulation protocols and parameters in the literature we found. Some studies, such as the initial WT animal studies [66,67] only applied tDCS acutely. Some others [69,70] administered tDCS while the animals were still being treated with MPTP to induce the PD-like phenotype and continued tDCS administration for weeks after MPTP treatment had ended. In contrast, Feng et al. [71] only started tDCS after PD lesioning had concluded and administered tDCS chronically for four weeks. Treatment duration is an important variable when considering the translational utility of these findings for clinical applications. In addition, few studies have addressed the effects of tDCS on the non-motor symptoms of PD, with the existing evidence not demonstrating an improvement in depressive-like behavior or cognition. Finally, tDCS studies have yet to evaluate interactions between tDCS and dopamine replacement therapy in PD animal models. Overall, the animal literature on tDCS positions it as a promising treatment that, in addition to modulating circuit activity to improve motor and non-motor symptoms, may have neuroprotective/regenerative effects. For some of these effects parallel those seen with rTMS, however, we note that at the present time, the rTMS animal model literature is more extensive. On the other hand, the cost-effectiveness of tDCS promises enhanced versatility in clinical use.
3.3. Electroconvulsive Therapy (ECT)
3.3.1. Background
ECT has been used to treat psychiatric disorders since the early 1940s and is still in use today as an effective treatment option for those who suffer from refractory depression [80]. Aside from its use in psychiatry, ECT has also been shown to be effective for treatment of motor disturbances in PD and other movement disorders [81], although it is rarely used in clinical practice. The exact mechanisms underlying the therapeutic effects of this technique in PD are yet to be fully elucidated, which is why we turn to animal models (see Table 4 for a summary of studies).
3.3.2. Animal Models
Behavioral, Dopaminergic System, and Neuroprotective Effects. The earliest ECT study (Garcia & Sotelo, 1993) examined the effect of ECT on the levels of striatal dopamine in the MPTP mouse model [82], finding no difference between the ECT-treated and -untreated mice. Following a considerable gap in the literature after this initial discouraging finding, two studies from a single group reported improvement in motor functioning following ECT in 6-OHDA-lesioned rats [83,84]. Strome, et al. [84] applied ECT (or sham: animals anesthetized, electrodes placed, but no current delivered, see Table 4) for 10 days after 6-OHDA lesioning and found an improvement in hind limb motor functioning in ECT-treated animals. This group also investigated the binding of dopamine receptors in the striatum using autoradiography and found significant increases in binding for D1 and D3 receptors but not D2 receptors in ECT-treated animals. Anastasía, et al. [83] found that the daily administration of ECT (relative to sham ECT) for 7 or 14 days concurrently with 6-OHDA lesioning reduced motor impairment and the loss of TH+ neurons in the SNc; ECT also increased the amount of GDNF in the striatum of both lesioned and un-lesioned rats. A subsequent study by the same group demonstrated that GDNF increases mediated the preservation of dopaminergic neurons in the SNc by ECT [85].
A study in MPTP-lesioned non-human primates found no significant changes in either dopamine D2/3 receptor or the dopamine transporter availability after ECT was delivered 6 times in 3 weeks [86]. However, there were significant increases in dopamine D1 receptor and the vesicular monoamine transporter (VMAT) availability. In un-lesioned animals, ECT increased dopamine transporter, D1 receptor, and VMAT binding, which is consistent with earlier findings in intact monkeys, and the effect of ECT on VMAT and D1 binding was less robust in the lesioned than in the un-lesioned striata. This suggests that while dopaminergic response to ECT may be weakened, it is to some degree preserved in MPTP-lesioned striatum.
Stem Cell Transplantation. Approximately a decade later, three additional studies evaluated ECT in the MPTP mouse model. Yang, et al. [87] combined ECT with the transplantation of mesenchymal stem cells (MSCs) in an MPTP mouse model of PD. ECT was delivered for 8 days following MSC transplantation. While they found that ECT and the MSCs on their own slightly increased dopamine concentrations in the whole brain, when combined, there was a significant increase in dopamine and TH levels [87]. This study also found improvements in motor behavior in the MSC + ECT group relative to animals that received either ECT or MSCs alone.
Autophagy. Huh, et al. [88] investigated the role of ECT in autophagy in the MPTP mouse model and found that after two weeks of ECT treatment (once a day, three times a week), mice showed an improvement in motor deficits; increased count of TH+ neurons in the SNc; and the normalization of LC3-II levels in the PFC, striatum, and midbrain. LC3-II is an autophagy marker that has been shown to be dysregulated in PD [89] and was found to be modulated by tDCS [17], as reported above.
In summary, relatively few studies have evaluated the effects of ECT in PD animal models. The extant studies have demonstrated an amelioration in motor deficits, the preservation of dopaminergic neurons in the SNc, the enhancement of dopaminergic function, and increases in NFs [83,84], paralleling findings with rTMS and tDCS. In addition, like tDCS, ECT may affect autophagy pathways and has shown promise as an adjunctive treatment for stem cell transplantation [87].

Table 4.
Specifications of ECT studies. 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNc = substantia nigra pars compacta; PFC = prefrontal cortex; TH = tyrosine hydroxylase; GDNF = glial-derived neurotrophic factor; MSCs = mesenchymal stem cells; VMAT = vesicular monoamine transporters; LC3-II = microtubule-associated protein 1 light chain 3 conjugate; DAT = dopamine transporter; ↑ = increase, ↓ = decrease.
Table 4.
Specifications of ECT studies. 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNc = substantia nigra pars compacta; PFC = prefrontal cortex; TH = tyrosine hydroxylase; GDNF = glial-derived neurotrophic factor; MSCs = mesenchymal stem cells; VMAT = vesicular monoamine transporters; LC3-II = microtubule-associated protein 1 light chain 3 conjugate; DAT = dopamine transporter; ↑ = increase, ↓ = decrease.
| Study | PD Model | Sham Control | ECT Parameters | Outcome Measures |
|---|---|---|---|---|
| Garcia & Sotelo (1993) [82] | MPTP (mouse) |
|
|
|
| Anastasia, et al. (2007) [83] | 6-OHDA (rat) |
|
|
|
| Strome, et al. (2007) [84] | 6-OHDA (rat) |
|
|
|
| Anastasia, et al. (2011) [85] | 6-OHDA (rat) |
|
|
|
| Landau, et al. (2012) [86] | MPTP (monkey) |
|
|
|
| Yang, et al. (2020) [87] * | MPTP (mouse) |
|
|
|
| Huh, et al. (2023) [88] | MPTP (mouse) |
|
|
|
* This study utilized ECT as a supplementary therapy to enhance transplantation of mesenchymal stem cells into a mouse model of PD.
3.3.3. Limitations
Although the studies in animal models have clarified some of the mechanisms whereby ECT improves motor functioning, the literature is sparse. Furthermore, effects on non-motor PD symptoms and cognition have not been evaluated, the latter being especially important considering documented transient cognitive side effects resulting from ECT in humans [90]. Such side effects may be concerning in patients already experiencing cognitive impairment. The fact that the animals in most of the above studies were not under general anesthesia during ECT limits the translational utility of the findings. In humans, ECT is only performed under general anesthesia. Although important for seizure confirmation, the absence of anesthesia likely results in stress. Another variable to consider is the placement of the electrodes. Some studies [83,85] utilized corneal electrodes which stimulate the brain through the eyes, while the others utilized earclip [84,87,88] or temple [86] electrodes. Considering that ECT and general anesthesia used to deliver it in humans both come with risks, rTMS and tDCS may be preferable for use in clinical practice, as they offer similar benefits based on the animal literature reviewed.
3.4. Focused Ultrasound (FUS)
3.4.1. Background
FUS relies on ultrasound waves guided to target focal regions in the body or the brain. Transcranial FUS has been studied and used for the treatment of brain disorders. High-intensity FUS (HIFU) has been used to produce focal brain lesions [4], whereas low-intensity FUS (LIFU) has been studied as a method of modulating neural activity [91,92] or of aiding drug delivery to the brain by focally and transiently disrupting the integrity of the blood–brain barrier (BBB) through the sonication of injected microbubbles (MBs) [93], (Figure 1). In contrast to the other non-invasive neuromodulation techniques reviewed above, FUS can directly target deep subcortical regions and has a superior spatial resolution on the order of millimeters [94,95].
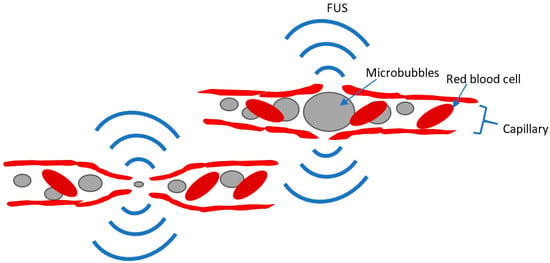
Figure 1.
Depiction of FUS-MB mechanism for BBB opening. MBs expand and contract within the capillary in response to FUS and cause a brief disruption of the BBB, which allows systemically injected substances to enter through the BBB. Adapted from Wu, et al. [20].
In human PD patients, HIFU has been utilized to ablate the ventral intermediate nucleus of the thalamus, the subthalamic nucleus (STN), the internal segment of the globus pallidus (GPi), and the pallidothalamic tract [96,97]. These ablation treatments have been encouraging, demonstrating improvement of PD motor signs with relatively few and mild side effects [96]. Although LIFU has not yet been trialed as a form of neuromodulation for PD treatment, LIFU (without microbubbles/BBB disruption) has been tested in proof-of-concept studies in healthy volunteers, as well as initial studies in epilepsy and generalized anxiety disorder [92,96]. A trial in opioid use disorder (NCT04197921) is currently underway and initial results are promising [98]. The use of low-intensity FUS with microbubbles (FUS-MBs) to disrupt the BBB has received considerable attention in Alzheimer’s disease [99,100,101] and is an emerging application in PD with a potential for targeted non-invasive delivery of therapeutics to the brain and/or for enhancing the clearance of protein aggregates (Figure 1). Thus far, a phase-1 open-label trial has investigated the safety and feasibility of applying FUS-MBs in PD patients with dementia, demonstrating that FUS-MBs safely and reversibly opened the BBB in the parieto-occipito-temporal cortex (targeted pathological site), as evidenced by gadolinium enhancement on MRI in this region [102]. The same group subsequently reported the safe and successful opening of the BBB in the putamen and midbrain of three PD patients (as evidenced by uptake of a PET tracer that does not cross the BBB), along with focal delivery of adeno-associated virus serotype 9 vectors into PD-relevant brain regions of macaque monkeys [103]. Although this is only an emerging area of research in human PD patients, there are many animal studies that have investigated the underlying mechanisms of FUS-MBs and have advanced the joint use of this technique with delivery of therapeutics. In the following sections, we discuss the application of LIFU with and without the use of microbubbles.
3.4.2. LIFU Animal Models
Behavioral and Neuroprotective Effects. Studies of LIFU in animal models of PD began rather recently, with earliest studies published in 2019 [104,105,106]. Two of the earliest studies from the same group utilized an MPTP mouse model of PD and applied LIFU to the basal ganglia nuclei (STN and GP) [105] or the motor cortex [106]. Both studies utilized a wearable ultrasound device in awake behaving animals, but LIFU parameters differed between the two studies, with [106] employing a lower stimulation frequency (Table 5). In both studies, MPTP mice treated with LIFU showed improved motor performance in comparison to animals that were given sham LIFU (animals handled, stimulator placed, but no ultrasound given; Table 5). C-fos expression was increased in the sonicated regions, suggesting that LIFU resulted in increased neural activity. Zhou, et al. [105] additionally found more TH+ neurons in the SN following LIFU, which was likely mediated by the suppression of apoptosis in the SN evidenced by a decrease in apoptosis markers. Zhou, et al. [106] reported that LIFU of the motor cortex increased the levels of antioxidant enzymes in the striatum. Neither study found any tissue damage in the brains of the mice that had received LIFU. Xu, et al. (2020) [107] corroborated the improvement of motor signs on behavioral tests and neuroprotection, reporting increased activity during the open field test and better performance on the pole test after 10 days of LIFU to the whole brain. TH+ neuron count in the SNc and striatal dopamine levels were increased in the MPTP mice that had received LIFU relative to those that did not.
Anti-inflammatory Effects. In a subsequent study, Zhou and colleagues further investigated the effects of LIFU on neuroinflammation in their MPTP model, with LIFU applied to the STN acutely (1 time for 30 min) [108]. In addition to replicating their earlier findings of motor performance improvement, the preservation of SN neurons, and increases in antioxidant enzymes, Zhou et al. (2021) [108] found reductions in the levels of proinflammatory cytokines and downstream inflammatory markers, as well as decreased microglial and astrocyte activity in the SN and striatum of MPTP mice that were treated with LIFU. There was also a decrease in alpha-synuclein in the SNc and the striatum [108]. Song, et al. [109] built upon this work by assessing neuroinflammation in a 6-OHDA rat model of PD after treatment with low-intensity pulsed ultrasound for 6 weeks targeting the lesion. Relative to no treatment, ultrasound prevented glial activation caused by 6-OHDA, decreased inflammatory markers, and produced an increase in GDNF (with no change in BDNF). Ultrasound also restored the integrity of the BBB in the lesioned and sonicated SN region.
Additional evidence of the anti-inflammatory and antioxidant effects of LIFU came from Dong, et al. (2021) [110], who utilized rodent functional magnetic resonance imaging (fMRI) to assess T2* relaxation time as a proxy measure of iron deposition in a 6-OHDA rat model. The study also examined the effects of LIFU on fractional anisotropy (FA) as a measure of neuronal integrity. Consistent with previous studies, there was an attenuation of the loss of TH+ neurons and an increase in GDNF of 6-OHDA rats treated with LIFU relative to untreated rats. After 5 weeks, the LIFU group showed higher T2* values, which was interpreted as resulting from decreased iron deposition into the surrounding tissues. Because iron deposition is a marker of oxidation and inflammation [111], LIFU was proposed to have reduced free radicals and inflammation through improving microcirculation in the targeted brain areas [110]. FA values were increased during the first week of treatment, which was interpreted as suggestive of neuroprotection, although this effect was reversed after 5 weeks of treatment, with FA values becoming smaller in treated relative to the untreated rats. Because MRI is non-invasive and routinely used in humans, the study raises the possibility of using these MRI-based measures in human patients to assess LIFU effects.
Effects on Cognition. Finally, one of the earlier studies utilized a variation of the focused ultrasound technique called transcranial magneto-acoustic stimulation (TMAS) in an MPTP mouse model of PD [104]. TMAS is performed by delivering focused ultrasound in a static magnetic field to further increase its spatial resolution [112]. Wang, et al. [104] delivered TMAS to the SN daily over 2 weeks and examined the effects on spatial learning and memory, hypothesizing that TMAS of the SN would produce the beneficial downstream modulation of basal ganglia—hippocampal circuits. TMAS improved performance on the Morris water maze and increased the dendritic spine densities in the dentate gyrus of the hippocampus, with a concomitant increase in proteins mediating plasticity (postsynaptic density protein 95 (PSD-95), BDNF, CREB, and protein kinase B).

Table 5.
Specifications of LIFU studies. 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PRF = pulse repetition frequency; TBD = tone burst duration; SD = sonication duration; ISI = interstimulus interval; SN = substantia nigra; SNc = substantia nigra pars compacta; STN = subthalamic nucleus; GP = globus pallidus; V1 = primary visual cortex; Bcl2 = B-cell lymphoma 2; Bax = bcl-2-like protein 4; Cyt C = cytochrome C; GSH-PX = glutathione peroxidase; SOD = superoxide dismutase; IL1β = interleukin-1β; NF-κBp65 = nuclear factor kappa-light-chain-enhancer of activated B cells p65; FA = fractional anisotropy; LTP = long-term potentiation; DG = dentate gyrus; PSD-95 = postsynaptic density protein 95; CREB = cyclic AMP response element-binding protein; BDNF = brain derived neurotrophic factor; PKB = protein kinase B; DAT = dopamine transporter; ↑ = increase, ↓ = decrease.
Table 5.
Specifications of LIFU studies. 6-OHDA = 6-hydroxydopamine; MPTP = 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PRF = pulse repetition frequency; TBD = tone burst duration; SD = sonication duration; ISI = interstimulus interval; SN = substantia nigra; SNc = substantia nigra pars compacta; STN = subthalamic nucleus; GP = globus pallidus; V1 = primary visual cortex; Bcl2 = B-cell lymphoma 2; Bax = bcl-2-like protein 4; Cyt C = cytochrome C; GSH-PX = glutathione peroxidase; SOD = superoxide dismutase; IL1β = interleukin-1β; NF-κBp65 = nuclear factor kappa-light-chain-enhancer of activated B cells p65; FA = fractional anisotropy; LTP = long-term potentiation; DG = dentate gyrus; PSD-95 = postsynaptic density protein 95; CREB = cyclic AMP response element-binding protein; BDNF = brain derived neurotrophic factor; PKB = protein kinase B; DAT = dopamine transporter; ↑ = increase, ↓ = decrease.
| Study | PD Model | Sham Control | Ultrasound Parameters | Outcome Measures |
|---|---|---|---|---|
| Wang, et al. (2019) [104] | MPTP (mouse) |
|
|
|
| Zhou, et al. (2019) (2) [106] | MPTP (mouse) |
|
|
|
| Zhou, et al. (2019) (1) [105] | MPTP (mouse) |
|
|
|
| Xu, et al. (2020) [107] | MPTP (mouse) and cell line (not discussed) |
|
|
|
| Dong, et al. (2021) [110] | 6-OHDA (rat) |
|
|
|
| Zhou, et al. (2021) [108] | MPTP (mouse) |
|
|
|
| Song, et al. (2022) [109] | 6-OHDA (rat) |
|
|
|
3.4.3. FUS-MB Animal Models
Although NFs, such as BDNF and GDNF, have been identified as potentially promising therapeutics for neurodegenerative disorders, the BBB has presented an obstacle preventing their non-invasive delivery to the brain [113]. While the BBB’s permeability to BDNF is comparable to that of insulin [114], the BBB is impermeable to GDNF, necessitating intracerebral administration. A growing body of literature in animal models of neurodegenerative disorders is providing increasing support for the use of the FUS-MB BBB disruption technique as a non-invasive method of delivering therapeutics, such as NFs, to the brain, with encouraging findings regarding therapeutic efficacy.
Delivery of GDNF. One group of FUS studies in PD models yielded by our search have investigated whether FUS can be used to facilitate the non-invasive delivery of NFs or NF genes. This technique entails the systemic administration of a therapeutic (e.g., NF) and microbubbles, either in isolation or coupled together as a complex. FUS is then used to sonicate the focal brain area to which the therapeutic is targeted. FUS induces a rapid expansion and contraction of the MBs, which transiently disrupts the BBB, allowing for the delivery of the therapeutic to the parenchyma (Figure 1). The majority of these studies investigated the use of the FUS-MB technique for the delivery of GDNF through either protein or gene delivery to the SNc and/or striatum, including Fan, et al. [115], Lin, et al. [116], Mead, et al. [117], Yue, et al. [118], and Karakatsani, et al. [119] (see Table 6 for details of each study). All of these studies demonstrated significant improvements in motor function in their respective PD models, in some cases even reporting an almost complete restoration of motor function, with motor performance approaching un-lesioned levels, e.g., [117]. Every study also demonstrated increases in dopaminergic neurons and in the levels of dopamine (and/or other markers of dopaminergic signaling) in the targeted areas, with some reporting almost complete restoration to un-lesioned levels [116,117,118]. Yue, et al. [118] additionally found that behavioral improvements from GDNF delivery in FUS-MB-treated animals were associated with the increased expression of nuclear receptor-related factor 1 (Nurr1), which has been previously found to enhance the integrity of dopaminergic cells [120]. Some of the studies directly compared the therapeutic efficacy of injecting the NF genes together with, but uncoupled from, MBs versus injecting conjugated MB-gene complexes. Their findings suggested that conjugates produced superior therapeutic effects, although uncoupled delivery also produced significant benefits for some outcomes (as did FUB-MBs without gene delivery) [115,116,121]. One study aimed to increase the efficiency of GDNF gene delivery by designing a novel type of microbubbles loaded with polyethylenimine–superparamagnetic iron oxide plasmid DNA, used in conjunction with FUS and two-step magnetic navigation [122]. In cell culture, each component of this delivery system was reported to increase the transfection rate. In a genetic mouse PD model (MitoPark), the delivery of the GDNF gene to the SN using this system resulted in a 3.2-fold increase in the recovery of TH+ neurons and a 3.9-fold improvement in motor function relative to untreated PD mice. Although utilized in this study for GDNF gene delivery in the mouse model, this technology could be used for other types of gene delivery/therapy.
Delivery of Other Neurotrophic Factors. Besides GDNF, studies have investigated the FUS-MB-assisted delivery of other trophic factors, including neurturin (NTN) [119], BDNF [123], and fibroblast growth factor 20 (FGF20) [124]. Karakatsani et al. [119] investigated the protein delivery of NTN, as well as the gene delivery of GDNF, finding increases in dopamine neurons and dopamine levels in the targeted areas (SN and striatum). As NTN belongs to the GDNF family, the effects were similar to those observed after the FUS-MB-assisted gene delivery of GDNF, although they tended to be less pronounced with protein NTN delivery (some were not significant following a single NTN treatment and only observed after 3 deliveries). Ji, et al. [123] investigated FUS-MB-assisted delivery of BDNF in an MPTP mouse model using a unique approach; while the other studies injected both MBs and therapeutics intravenously, Ji et al. only injected MBs and administered BDNF intranasally. The intranasal administration of BDNF coupled with FUS-MBs increased the expression of TH in the SNc and striatum relative to the untreated hemisphere. FUS-MBs without intranasal BDNF administration produced no effect. At the behavioral level, amphetamine-induced rotational behavior towards the untreated side was observed, indicating increased dopaminergic activity on the treated side. Niu, et al. [125] used FUS-MBs to deliver FGF20, which is preferentially expressed in the SNc and has been found to enhance dopamine neuron differentiation from embryonic stem cells [126] and the survival of dopamine neurons in vitro [124]. Genetic variability in FGF20 is associated with risk for PD in humans [124], and in rodent PD models, FGF20 was found to be protective against the loss of dopamine neurons and concomitant motor dysfunction [124,127]. Therefore, FGF20 is a promising therapeutic for PD. Niu, et al. [125] utilized the FUS-assisted delivery of human recombinant FGF20 in proteoliposomes over two weeks to the brain of 6-OHDA-lesioned rats and found improvements on the rotational behavior test, as well as the alleviation of TH+ cell loss in the SN.
Delivery of Antioxidants. FUS-assisted delivery of other therapeutics has also been investigated in PD models. A study by Long, et al. [121] investigated the FUS-assisted delivery to the SN of nuclear factor E2-related factor 2 (Nrf2) gene coupled to nanomicrobubbles in the 6-OHDA rat model. Nrf2 is a transcription factor that activates an antioxidant response, which could be beneficial considering that oxidative stress plays an important role in the degeneration of dopamine neurons in PD [74]. In addition to increased Nrf2 expression, FUS-MBs also produced increases in TH and dopamine transporter (DAT) levels, as well as an increase in SOD and a decrease in reactive oxygen species in the SN.
Delivery of Medicinal Herbs. In addition, a series of studies examined the use of the FUS-MB technique to deliver medicinal herbs to the brain in PD mouse models. Two studies examined the FUS-assisted curcumin delivery to the striatum of MPTP-treated mice [128,129]. Curcumin may have neuroprotective effects in neurological disorders [130] and has also been shown to decrease alpha-synuclein aggregation in-vitro [131]. The two studies manufactured curcumin-loaded nanobubbles using different technologies and used FUS to sonicate these particles in order to facilitate curcumin delivery. Both studies reported motor improvements, while Zhang et al. [129] additionally reported increased dopamine and dopamine metabolites in the striatum and the preservation of TH+ cells in the SN. We note, however, that the therapeutic effects reported by Zhang et al. [129] were also observed in control groups that received nanobubbles without FUS. Feng, et al. [132] used FUS to facilitated the delivery of Triptolide (T10) into the brains of alpha-synuclein-overexpressing mice. Triptolide is a compound found in a Chinese medicinal herb that has been found to increase autophagy of alpha-synuclein in vitro [133] but does not easily cross the BBB [134]. Delivery of Triptolide with the aid of FUS promoted autophagic clearance of alpha-synuclein, attenuated the loss of TH+ cells in the SN, and resulted in an improvement of motor impairments. Another study [135] examined FUS-MB-assisted delivery of Gastrodin, a compound from another traditional Chinese medicinal herb, Gastrodia elata, which may offer neuroprotection through anti-oxidant, anti-inflammatory, and anti-apoptotic effects [135,136]. The FUS-MB technique was used to open the BBB in the area of the striatum to allow the entry of injected Gastrodin. This had protective effects on TH+ cells and DAT in the nigrostriatal pathway of MPTP mice as well as enhanced expression of synaptic-related proteins and anti-apoptotic activity, with some of the latter effect also evident with FUS or Gastrodin alone. However, no significant motor function improvement resulted from this treatment, as motor function auto-recovered in all lesioned animals with this sub-acute MPTP model.
Gene Silencing. A unique study by Xhima, et al. [137] utilized FUS-MBs to facilitate the non-invasive delivery a virally expressed short hairpin RNA (shRNA) to silence the alpha-synuclein gene in transgenic mice expressing human alpha-synuclein. Several brain areas known to be vulnerable to Lewy body pathology were targeted: SNc, hippocampus, olfactory bulb, and the dorsal motor nucleus of the vagus. Alpha-synuclein expression was decreased 1 month following treatment in mice that received FUS with active shRNA relative to mice that received FUS with scrambled shRNA. In contrast, neuronal markers, cell death, and glial activation remained unchanged following treatment [137]. This study demonstrates that the FUS-MB technique holds promise for the non-invasive delivery of brain-targeted gene therapy to curb the spread of alpha-synuclein and therefore alter disease progression.
Creation of PD model. Conversely, the same FUS-MB technique was employed to create a novel mouse model of PD by introducing the alpha-synuclein gene into the SN dopamine neurons [138]. The delivery of the alpha-synuclein gene accomplished a PD-like spread of alpha-synuclein with an accompanying loss of 50% of SN dopamine, which was associated with motor impairment. Although this study does not meet the inclusion criteria for the current review, we mention it as relevant to the present discussion.
Electrical Stimulation. Finally, one study utilized high-intensity FUS to non-invasively generate direct current in the STN by sonicating systemically administered piezoelectric nanoparticles releasing nitric oxide [139]. The release of nitric oxide disrupted the BBB, allowing for the entry of the nanoparticles into the parenchyma. Kim, et al. [139] reported that in-vitro, the piezoelectrically-induced current stimulated dopamine release from dopaminergic-like neurons. In MPTP mice, the injection of the nanoparticles coupled with the application of HIFU to the STN improved motor performance and fear learning, enhanced neural activity in the STN, and attenuated TH+ cell loss in the SN. No tissue damage was observed. This treatment was proposed to be analogous to DBS with the advantage of acting non-invasively.
In summary, studies utilizing LIFU and FUS-MBs detected no brain tissue damage, suggesting the procedure is safe at low intensities. Even without the delivery of exogenous therapeutics via FUS-MBs, studies employing variations of LIFU showed that this treatment increased the density and/ or integrity of dopaminergic SNc neurons and produced improvements in motor functioning. In addition, some of these studies produced evidence of increases in NFs [104,109,110] and reduction in inflammatory markers [108,109], oxidative stress [106,108], and apoptosis [105], pointing to some possible therapeutic mechanisms. These effects are similar to the ones produced by other electrical stimulation methods reviewed earlier. Studies of the FUS-MB-assisted delivery of therapeutics reported similar effects, including increases in dopaminergic cells, dopaminergic transmission and improvements in motor function. However, in some cases, this treatment produced an almost complete restoration of motor function and dopaminergic signaling [116,117,118,125,128,129,135], even in the 6-OHDA model. Furthermore, the study by Xhima, et al. [137] demonstrated that this approach can be used successfully to curb the spread of alpha-synuclein in brain areas that are vulnerable to Lewy body pathology, raising the possibility that this application of the FUS-MB approach has the potential to alter disease progression. However, this technique has yet to be applied in human patients.

Table 6.
Specifications of FUS-MB studies. PRF = pulse repetition frequency; SN = substantia nigra; SNc = substantial nigra pars compacta; SNr = substantia nigra pars reticulata; OB = olfactory bulb; DMN = dorsal motor nucleus; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; pGDNF = plasmid glial cell-line-derived neurotrophic factor; α-syn = alpha-synuclein; AAV = adeno-associated viral vector; shRNA = short hairpin RNA; BPN = brain-penetrating nanoparticles; NTN = neurturin; DAT = dopamine transporter; TH = tyrosine hydroxylase; Nurr1 = nuclear receptor-related factor 1; Nrf2 = nuclear factor E2-related factor 2; ROS = reactive oxygen species; SOD = superoxide dismutase; PSp-MBs = polyethylenimine–superparamagnetic iron oxide plasmid DNA load microbubbles; PLGA = poly(lactide-co-glycolide); Bcl2 =B-cell lymphoma 2; PSD-95 = postsynaptic density protein 95.
Table 6.
Specifications of FUS-MB studies. PRF = pulse repetition frequency; SN = substantia nigra; SNc = substantial nigra pars compacta; SNr = substantia nigra pars reticulata; OB = olfactory bulb; DMN = dorsal motor nucleus; BDNF = brain-derived neurotrophic factor; GDNF = glial-derived neurotrophic factor; pGDNF = plasmid glial cell-line-derived neurotrophic factor; α-syn = alpha-synuclein; AAV = adeno-associated viral vector; shRNA = short hairpin RNA; BPN = brain-penetrating nanoparticles; NTN = neurturin; DAT = dopamine transporter; TH = tyrosine hydroxylase; Nurr1 = nuclear receptor-related factor 1; Nrf2 = nuclear factor E2-related factor 2; ROS = reactive oxygen species; SOD = superoxide dismutase; PSp-MBs = polyethylenimine–superparamagnetic iron oxide plasmid DNA load microbubbles; PLGA = poly(lactide-co-glycolide); Bcl2 =B-cell lymphoma 2; PSD-95 = postsynaptic density protein 95.
| Study | PD Model | Sham Control | FUS-MB Parameters | Outcome Measures |
|---|---|---|---|---|
| Fan, et al. (2016) [115] | 6-OHDA (rat) |
|
|
|
| Lin, et al. (2016) [116] | MPTP (mouse) |
|
|
|
| Long, et al. (2017) [121] | 6-OHDA (rat) |
|
|
|
| Mead, et al. (2017) [117] | 6-OHDA (rat) |
|
|
|
| Xhima, et al. (2018) [137] | Transgenic humanized alpha-synuclein (mouse) |
|
|
|
| Yue, et al. (2018) [118] | 6-OHDA (rat) |
|
|
|
| Zhang, et al. (2018) [129] ** | MPTP (mouse) |
|
|
|
| Niu, et al. (2018) [125] ** | 6-OHDA (rat) |
|
|
|
| Ji, et al. (2019) [123] | MPTP (mouse) |
|
|
|
| Karakatsani, et al. (2019) [119] | MPTP (mouse, early PD model) |
|
|
|
| Wu, et al. (2020) [122] ** | MitoPark (mouse, genetic) |
|
|
|
| Yan, et al. (2021) [128] | MPTP (mouse) |
|
|
|
| Feng, et al. (2022) [132] ** | rAAV2/5-wild type α-syn or A53T α-syn (mouse) |
|
|
|
| Wang, et al. (2022) [135] | MPTP (mouse) |
|
|
|
| Kim, et al. (2023) [139] | MPTP (mouse) |
|
|
|
** This study also performed in-vitro investigations; results not discussed in this review.
3.4.4. Limitations
Although findings regarding the effects of FUS in animal models of PD have been promising, several limitations must be noted regarding its potential for use in human patients. As with the tDCS studies, the FUS delivery protocols have differed substantially across studies. With the FUS-MB technique, the risk of inflammation resulting from the disruption of the BBB in the targeted areas presents a concern [140]. However, the studies reviewed herein did not report evidence of inflammation, and some studies in other animal models have suggested that inflammation may be transient [141,142,143]. On the other hand, LIFU was found to help restore the integrity of the BBB [108], although more studies are needed to replicate this finding. As noted for the other forms of neuromodulation reviewed here, possible interactions of FUS with dopamine replacement therapy remain to be studied, and its effects on non-motor symptoms of PD remain to be investigated. In addition, despite its superior spatial resolution of FUS, it may be less anatomically precise in small animal models considering the small brain size of these animals. Finally, the optimal frequency and duration for sonication of human patients remains to be determined.
4. Discussion and Future Directions
Animal models have offered important insights regarding possible therapeutic mechanisms of non-invasive neuromodulation treatments for PD. Our literature review has identified some therapeutic endpoints and mechanisms that have remained elusive or understudied in human patients (Figure 2).
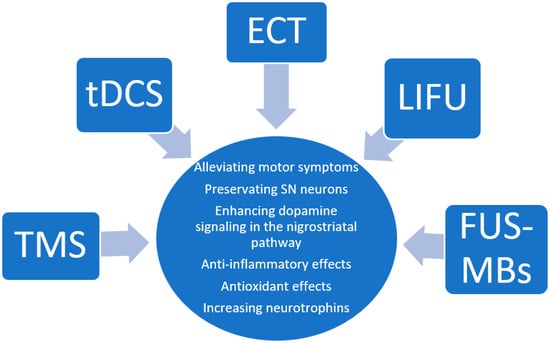
Figure 2.
Insights gathered from the animal literature regarding the therapeutic mechanisms of the neuromodulation techniques reviewed.
Specifically, the capacity of most neuromodulation methods reviewed here (TMS, tDCS/tACS, ECT, and FUS) to influence neuroinflammation deserves more attention in human patient research. PET tracers exist for imaging neuroinflammation [47,144]. While neuroinflammation imaging in PD is novel [145], evidence is accumulating for the role of neuroinflammation in PD pathophysiology [44,146]. Leveraging PET imaging to observe changes in neuroinflammation in response to non-invasive neuromodulation treatments may be a promising future direction. However, the PET imaging of neuroinflammation comes with methodological challenges, such as low binding specificity of tracers to their targets, variable tracer kinetics, and genetic variation in translocator protein, determining the affinity to TSPO tracers for this protein in activated microglia [47,147]. However, improvements in radiotracer signal to noise ratio, kinetics, and binding affinity will decrease these limitations in the future [47].
Another prominent set of findings that emerged from the studies reviewed here are neuromodulation-induced increases in NFs. This is a valuable mechanistic insight made possible uniquely by research in animal models. Furthermore, several studies have employed FUS to aid in the delivery of NFs to brain areas affected by PD pathology as therapeutics with encouraging results. This presents an intriguing avenue for future research in PD patients. However, a major limitation in this research is that NFs are not currently measurable in the brains of human patients in-vivo. While it is possible to quantify BDNF in the body through blood serum analysis and in the cerebrospinal fluid, these values do not directly reflect levels in the PD-related brain regions. Although it will not be possible to measure changes in the levels of NFs in human PD patients as a result of interventions such as FUS-assisted NF delivery, therapeutic effects of such interventions may be assessed as behavioral changes, as well as PET measures of pre- and postsynaptic dopamine function. This major limitation will make it difficult to compare NF levels between the human and animal literature as the majority of the animal literature quantifies NFs in the brain post-mortem.
TMS research in animal models of PD is limited by the low spatial resolution in small animals. rTMS studies in non-human primate models may be more informative regarding regional effects, as they would allow for more focal applications. The existing TMS animal literature suggests that rTMS improves motor function, restores synaptic plasticity, enhances the survival of SN dopamine neurons, and may increase NFs and reduce neuroinflammation in models of PD. However, these studies utilized a lesioning model of PD, and lesioning itself produces inflammation and oxidative stress. The replication of these findings using a transgenic PD model would enhance the translational validity of these findings.
tDCS studies in animal models of PD showed similar effects to those of rTMS; namely tDCS resulted in the improvement of motor function, as well as increases in dopamine cells and anti-neuroinflammatory effects. Notably, findings regarding the effects of tDCS on neuropsychiatric symptoms associated with PD were scarce and mixed, with one study reporting improvements in anxiety-like but not in depressive-like behaviors [71]. The effects of tDCS on non-motor symptoms require further study. Unlike TMS, tDCS has reached the non-human primate literature and has shown efficacy in decreasing tremor [77]. This study was in an advanced model of PD, suggesting that tDCS may still be effective even at later stages of the disease. Overall, considering the relative ease of use and accessibility of tDCS combined with its capacity to produce similar therapeutic effects to those of rTMS at behavioral, cellular, and molecular levels, tDCS is poised to become a helpful and accessible adjunctive treatment for PD patients.
ECT studies in animal models of PD were few, and their translatability to human patients was limited by the absence of anesthesia accompanying the procedure in most of the reviewed animal studies. The reported effects were similar to the ones observed with TMS and tDCS, namely the improvement of motor deficits, the preservation of dopamine neurons, and increases in NFs. Intriguingly, one study [88] found evidence that ECT may impact autophagy pathways in PD, although it is unclear whether this finding would translate to human patients. Considering that other forms of non-invasive neuromodulations are likely to offer similar benefits based on the studies reviewed here, while being associated with fewer risks, ECT may be less preferable in clinical practice than other forms of non-invasive neuromodulation.
FUS is the neuromodulation technique that has been most studied in animal models of PD. While some of these studies employed FUS to achieve the focal modulation of neural activity in the areas affected by PD pathology, others used this technique in combination with injected microbubbles to disrupt the BBB and aid the delivery of therapeutics. The former group of studies reported effects that were similar to those of the other neuromodulation techniques reviewed, including improvements in motor performance, the improved survival of dopamine neurons, increases in neural activity in the targeted circuits, decreased neuroinflammation, antioxidant effects, and increases in NFs along with facilitation of structural plasticity. The group of studies using FUS to enable the delivery of NFs or gene therapy have demonstrated effective delivery of these therapeutics to focal brain regions with beneficial (and sometimes fully restorative) effects on motor function and key aspects of PD pathophysiology. This application of FUS for the focal delivery of therapeutics is a unique capability of this technique, which could open avenues for ground-breaking and even disease-altering treatments for PD. Another the clear advantage of FUS is its superior spatial resolution and capacity to precisely target deep as well as cortical brain areas. This allows for a more precise and targeted modulation of dysfunctional circuits. However, the relative novelty of this technique and the paucity of studies in human patients present barriers to its imminent widespread use in clinical practice.
Many of the studies reviewed focused on motor symptoms as an outcome, which does not represent the full spectrum of PD symptoms. Cognitive and neuropsychiatric symptoms are important parts of the pathology and a worthwhile focus for future studies in animal models of PD. However, investigation into neuropsychiatric symptoms in animals is more challenging than studying the motor signs, with some questioning the validity of tests, such as the forced swim test used to assess states like depression in rodents [148]. For example, the forced swim test may actually be measuring a rodent’s motivation rather than despair [148]. Administering multiple tests assessing non-motor symptoms as a battery may help increase confidence in the measurement of cognitive phenotypes and boost translational validity [149]. In addition, certain non-motor symptoms such as feelings of dizziness or orthostasis are not measurable in animal models.
In addition, the motor symptoms investigated in the studies reviewed here over-represented rotational behavior as an outcome. Fewer studies utilized other tests like gait analysis, open field locomotion, or tests of motor coordination. Although the rotational behavior test is considered the gold standard, especially for unilateral lesion models (6-OHDA), other tests would give a more complete picture of the alleviation of motor symptoms.
Most of the studies reviewed utilized lesioning models of PD, most commonly the unilateral 6-OHDA lesioning model in rats and the bilateral MPTP lesioning model in mice. Lesioning models come with a set of limitations and concerns (e.g., surgery risks, inflammation, and stress on the animals). First, lesioned animals have a high mortality rate (6-OHDA model = 16% [150,151], chronic MPTP model~15% [152] mortality rate), leading to their use to acutely model a rapid onset of severe PD, instead of the chronic, progressive condition that human patients face. Second, both the 6-OHDA and MPTP models lack Lewy body inclusions, which is the cardinal feature of PD. Transgenic animal models of PD would avoid some of these complications and allow for better evaluations of these neuromodulation methods in aged animals (e.g., parkin null mice can live up to 24 months [153]). However, transgenic animals model genetic forms of PD, not idiopathic PD, which accounts for the majority of PD cases. Idiopathic PD and genetic forms of the disease differ in their molecular mechanisms [154].
Differences between the 6-OHDA and MPTP models must be noted when considering findings across studies employing the two models. 6-OHDA does not cross the BBB and needs to be administered intracerebrally. It enters the dopamine cells through the dopamine transporter and oxidizes rapidly producing reactive oxygen species and mitochondrial dysfunction causing the degeneration of neurons [155]. Usually, 6-OHDA is injected in the SN, medial forebrain bundle, or striatum [151]. The majority of 6-OHDA studies reviewed here (i.e., 12 studies total) injected 6-OHDA in the medial forebrain bundle, which is known to cause the long-lasting relatively slow degeneration of SN neurons over up to 5 weeks [156]. Seven studies injected 6-OHDA into the striatum, which is known to cause the rapid degeneration of local terminals, followed by a more gradual degeneration of nigral cell bodies (for up to several weeks), which is believed to more accurately reflect the pathophysiology of PD [151,157]. The lesioning site could affect response to the non-invasive brain stimulation. MPTP is a lipophilic molecule that can be administered systemically. It is oxidized into a dopaminergic neurotoxin 1-methyl-4-phenylpyridinium ion (MPP+), which enters the cell through the dopamine transporter and causes a progressive loss of dopamine neurons. Unlike the 6-OHDA model, the MPTP model requires repeated lesioning [155]. Thus, in the studies using the MPTP model, the neuromodulation treatments sometimes occurred concurrently with the lesioning [70,72,82,87,108,116,128,129], in which case the prevention rather than the remediation of damage was assessed. In the studies using the 6-OHDA model, treatments occurred following the lesioning and produced remediation rather than prevention of damage. In unilaterally lesioned animals (mainly 6-OHDA) comparisons were typically made between the lesioned and the un-lesioned side for both behavioral and neural effects, whereas in bilaterally lesioned animals, comparisons with sham-lesioned animals were typically employed. Finally, different behavioral tests were used to assess motor function depending on the model. As mentioned earlier, the rotational behavior test predominated in the studies employing unilateral lesioning (mainly 6-OHDA) models, whereas bilateral lesioning models (such as MPTP) more often used the rotarod test to test motor functions. Despite these differences, the findings pertaining to the neurobiological effects of neuromodulation were qualitatively similar between the 6-OHDA and MPTP model studies, including improvement in motor function, the attenuation of dopaminergic cell loss in the SN, increases in NFs in key brain regions, and decreases in pro-inflammatory markers in PD-related brain regions.
Finally, the results of the studies reviewed do not speak to whether/how the different neuromodulation methods may be combined with each other or with other PD treatments. We note two studies that combined stem cell transplantation with tDCS [76] and ECT [87], showing benefits from the adjunctive neuromodulation. Evaluating how different forms of non-invasive neuromodulation may be combined with more conventional or breakthrough treatments is an important future direction.
4.1. Applications to Clinical Practice
Although the animal studies reviewed here have produced important insights into the therapeutic mechanisms of the brain modulation techniques, they provide little information that is pertinent to the application of these techniques in clinical practice. For a discussion of clinical applications of these brain modulation techniques in PD, we refer the reader to the following reviews: [14,96,158,159]. The literature in animal models aligns with the human patient literature in that the majority of studies have focused on and provided evidence for the amelioration of motor symptoms as the primary outcome. Hence, the literature primarily supports the clinical use of these techniques for the treatment of the motor symptoms, although this may be an artifact of motor symptoms being most commonly used as the primary outcome. More studies of these methods for non-motor symptoms are needed to inform clinical practice. Considering that non-motor symptoms can be the most debilitating aspect of the disease [160], this is an important future direction.
Besides motor function improvement, the studies in animal models have revealed the neuroprotective/neuroregenerative effects of the brain modulation methods reviewed here. To the degree that these effects may translate to human PD patients, they may be disease-modifying. Given the challenges of demonstrating neuroprotective or anti-neuroinflammatory effects in human patients, such findings in animal models alone may provide some rationale for prophylactic use of these techniques, especially considering their rather benign side effect profile. However, the duration of the neuroprotective effects following treatment discontinuation remains unknown. Perhaps with the exception of FUS-assisted gene therapy, the effects are likely to be transient. Importantly, the effects of long-term chronic administration of these forms of neuromodulation (as would be done for prophylaxis) are also unknown.
Qualitatively similar therapeutic effects were observed across all forms of neuromodulation reviewed at both the behavioral and neurobiological levels. As noted in the Limitations subsection, this review did not attempt to quantify effect sizes and compare them across the different brain modulation approaches. We are, therefore, unable to compare the therapeutic efficacy of the different neuromodulation techniques. Relative to the other methods, the FUS-MB technique has had the clear advantage of enabling the delivery of therapeutics to the brain and hence shows the most promise as a disease-modifying therapy. In addition, some of the studies reviewed here reported therapeutic benefits following a single treatment with FUS-MBs or LIFU, whereas repeated treatments with rTMS, tDCS, and ECT were employed by studies demonstrating more than transient therapeutic effects. On the other hand, FUS-MBs is the least studied (in humans) of the methods reviewed and has not yet been adopted into clinical practice, although the initial studies demonstrate that this technique is well-tolerated and can be safely used in humans [99,100] which is in agreement with the animal studies reporting no tissue damage.
Besides efficacy, important considerations for clinical applications are safety, tolerability, and accessibility. rTMS, tDCS/ tACS, and ECT all have favorable safety profiles. The delivery of rTMS, tDCS, and tACS is generally painless. The most common side effects of these treatments are headaches [161,162,163], and patients also report itching, tingling or burning sensations on the scalp with tDCS and tACS [162]. In addition, in patients with a history of seizure disorders, rTMS may induce seizures, which are otherwise a rare side effect of rTMS [164]. Therefore, this treatment is not suitable for those with a history of seizures. ECT has the least favorable side effect profile as it may produce transient cognitive side effects, including confusion and delirium (in addition to headaches and nausea being common side effects) [161]. Hence, this treatment is less appropriate for patients already experiencing cognitive difficulties or lacking strong family or social support systems. Regarding accessibility, tDCS is poised to become the most accessible form of neuromodulation. Although not currently FDA-approved for any condition, this treatment has regulatory approval in the European Union and other countries, with some devices approved for home use. One such device has been recently granted an FDA Breakthrough designation. The devices are lightweight, portable, easy to use, and relatively inexpensive. rTMS and ECT both necessitate frequent visits to treatment facilities. Although ECT requires less frequent sessions and fewer total treatments than TMS, it requires short-acting general anesthesia, which may be undesirable or contraindicated for some patients because of other medical conditions.
In addition, some PD patients may already be undergoing DBS, and there is minimal literature that speaks to whether the non-invasive brain modulation methods reviewed are compatible with DBS. In a brain phantom model of PD, researchers found that combining DBS and TMS will cause overstimulation [165]. A case report has found that two patients who had undergone DBS did not show any substantial side effects when receiving tDCS [166]. Similarly, several case reports investigating use of ECT on PD patients with a DBS stimulator (stimulator off during ECT) did not show any adverse effects, but showed an amelioration of depressive symptoms [167,168,169,170,171]. For FUS combined with DBS, one study using a brain phantom did not find any dangerous increases in brain tissue temperature that would pose a safety concern in patients that have a DBS stimulator [172].
Finally, we note that the lesions induced by neurotoxins to produce the PD models for the majority of the studies we reviewed are considered severe and thought to mimic late-stage neurodegeneration (albeit in young animals) [173]. Therefore, the therapeutic mechanisms reported by these studies may be more pertinent for late-stage PD, although studies in human patients are required to determine this definitively.
4.2. Limitations
This is not a systematic review but rather a rapid review, whose goal was to survey the literature. As such, we employed a less exhaustive search strategy than would be expected of a systematic review. Crucially, we did not perform a risk of bias or quality assessment of the included studies and hence cannot make judgements about the quality of the articles reviewed; although, we make note of methodological issues, such as the absence of controls. Furthermore, we did not calculate the effect sizes in the included studies. Therefore, we cannot compare the effects across the different non-invasive brain modulation methods and evaluate their relative efficacy for specific outcomes. A meta-analysis or a meta-analytic review would be a valuable future addition to the literature. Additionally, we acknowledge that we have not covered every non-invasive method currently available. We have focused on the most promising brain modulation techniques in the human PD literature that have been studied in animal models of PD. Therefore, we have not discussed methods such as electro-acupuncture or transcutaneous nerve stimulation.
4.3. Conclusions
In conclusion, the literature reviewed herein has supported findings from human patient literature pointing to the clinical utility of non-invasive neuromodulation techniques as treatments for PD. Crucially, it has identified cellular and molecular mechanisms underlying the therapeutic effects of these techniques and have pointed to promising future directions for research and treatment in human patients. Further research is necessary to determine whether these therapeutic mechanisms translate to human PD patients and how they can best be leveraged to produce maximal therapeutic benefits.
Author Contributions
Conceptualization, K.M. and M.V.C.; methods, K.M. and M.V.C.; writing—original draft preparation, K.M.; writing—review and editing, K.M., M.V.C., D.M.S. and J.J.M.III. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by the National Institute of General Medical Sciences, 5U54GM104942 (West Virginia Clinical and Translation Science Institute Research Scholar award to M.V.C., D.M.S., and J.J.M.III). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldman, J.G.; Postuma, R. Premotor and Nonmotor Features of Parkinson’s Disease. Curr. Opin. Neurol. 2014, 27, 434. [Google Scholar] [CrossRef]
- Roheger, M.; Kalbe, E.; Liepelt-Scarfone, I. Progression of Cognitive Decline in Parkinson’s Disease. J. Park. Dis. 2018, 8, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Moosa, S.; Martínez-Fernández, R.; Elias, W.J.; del Alamo, M.; Eisenberg, H.M.; Fishman, P.S. The Role of High-Intensity Focused Ultrasound as a Symptomatic Treatment for Parkinson’s Disease. Mov. Disord. 2019, 34, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of Non-Invasive Brain Stimulation on Cognitive Functioning in Brain Disorders: A Meta-Analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef] [PubMed]
- Brak, I.V.; Filimonova, E.; Zakhariya, O.; Khasanov, R.; Stepanyan, I. Transcranial Current Stimulation as a Tool of Neuromodulation of Cognitive Functions in Parkinson’s Disease. Front. Neurosci. 2022, 16, 781488. [Google Scholar] [CrossRef]
- Ridding, M.C.; Rothwell, J.C. Is There a Future for Therapeutic Use of Transcranial Magnetic Stimulation? Nat. Rev. Neurosci. 2007, 8, 559–567. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, A.M. Use of Transcranial Magnetic Stimulation for Depression. Cureus 2019, 11, e4736. [Google Scholar] [CrossRef]
- Cheng, B.; Zhu, T.; Zhao, W.; Sun, L.; Shen, Y.; Xiao, W.; Zhang, S. Effect of Theta Burst Stimulation-Patterned RTMS on Motor and Nonmotor Dysfunction of Parkinson’s Disease: A Systematic Review and Metaanalysis. Front. Neurol. 2022, 12, 762100. [Google Scholar] [CrossRef]
- Somaa, F.A.; de Graaf, T.A.; Sack, A.T. Transcranial Magnetic Stimulation in the Treatment of Neurological Diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef]
- Benninger, D.H.; Hallett, M. Non-Invasive Brain Stimulation for Parkinson’s Disease: Current Concepts and Outlook 2015. NeuroRehabilitation 2015, 37, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Simon, D.K.; Wu, A.; Pascual-Leone, A. Non-Invasive Brain Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis of the Literature. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Elahi, B.; Elahi, B.; Chen, R. Effect of Transcranial Magnetic Stimulation on Parkinson Motor Function–Systematic Review of Controlled Clinical Trials. Mov. Disord. 2009, 24, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Dong, Z.; Pan, L.; Liu, Y.; Ye, Z.; Qin, L.; Liu, Q.; Qin, C. Effects of Repetitive Transcranial Magnetic Stimulation on Gait Disorders and Cognitive Dysfunction in Parkinson’s Disease: A Systematic Review with Meta-analysis. Brain Behav. 2022, 12, e2697. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.; Hickey, P.T.; Sundman, M.; Song, A.W.; Chen, N. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Symptoms in Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Neurol. 2015, 72, 432–440. [Google Scholar] [CrossRef]
- Zanjani, A.; Zakzanis, K.K.; Daskalakis, Z.J.; Chen, R. Repetitive Transcranial Magnetic Stimulation of the Primary Motor Cortex in the Treatment of Motor Signs in Parkinson’s Disease: A Quantitative Review of the Literature. Mov. Disord. 2015, 30, 750–758. [Google Scholar] [CrossRef]
- Li, S.; Jiao, R.; Zhou, X.; Chen, S. Motor Recovery and Antidepressant Effects of Repetitive Transcranial Magnetic Stimulation on Parkinson Disease: A PRISMA-Compliant Meta-Analysis. Medicine 2020, 99, e19642. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Z.; McClure, M.A.; He, L.; Mu, Q. Effect of RTMS on Parkinson’s Cognitive Function: A Systematic Review and Meta-Analysis. BMC Neurol. 2020, 20, 377. [Google Scholar] [CrossRef]
- Zhu, H.; Lu, Z.; Jin, Y.; Duan, X.; Teng, J.; Duan, D. Low-Frequency Repetitive Transcranial Magnetic Stimulation on Parkinson Motor Function: A Meta-Analysis of Randomised Controlled Trials. Acta Neuropsychiatr. 2015, 27, 82–89. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, X.; Zeng, W.; Zhai, H.; Zhang, X.; Yang, X.; Cheng, C.; Wang, J.; Yang, X.; Xu, Y. Transcranial Magnetic Stimulation Alleviates Levodopa-Induced Dyskinesia in Parkinson’s Disease and the Related Mechanisms: A Mini-Review. Front. Neurol. 2021, 12, 758345. [Google Scholar] [CrossRef]
- Keck, M.E.; Welt, T.; Müller, M.B.; Erhardt, A.; Ohl, F.; Toschi, N.; Holsboer, F.; Sillaber, I. Repetitive Transcranial Magnetic Stimulation Increases the Release of Dopamine in the Mesolimbic and Mesostriatal System. Neuropharmacology 2002, 43, 101–109. [Google Scholar] [CrossRef]
- Kanno, M.; Matsumoto, M.; Togashi, H.; Yoshioka, M.; Mano, Y. Effects of Acute Repetitive Transcranial Magnetic Stimulation on Dopamine Release in the Rat Dorsolateral Striatum. J. Neurol. Sci. 2004, 217, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, L.; Liu, Z. The Effect of Repetitive Transcranial Magnetic Stimulation on a Model Rat of Parkinson’s Disease. Neuroreport 2010, 21, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, S.H.; Ko, A.-R.; Lee, J.S.; Yu, J.H.; Seo, J.H.; Cho, B.P.; Cho, S.-R. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation in an Animal Model of Parkinson’s Disease. Brain Res. 2013, 1537, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Huang, Y.-Z.; Rotenberg, A.; Pascual-Leone, A.; Chiang, Y.-H.; Wang, J.-Y.; Chen, J.-J.J. Functional Dopaminergic Neurons in Substantia Nigra Are Required for Transcranial Magnetic Stimulation-Induced Motor Plasticity. Cereb. Cortex 2015, 25, 1806–1814. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; He, X.-K.; Liu, H.-H.; Chen, J.-J.J.; Peng, C.-W.; Liu, H.-L.; Rotenberg, A.; Chen, K.-T.; Chang, M.-Y.; Chiang, Y.-H.; et al. Early Repetitive Transcranial Magnetic Stimulation Exerts Neuroprotective Effects and Improves Motor Functions in Hemiparkinsonian Rats. Neural Plast. 2021, 2021, 1763533. [Google Scholar] [CrossRef]
- Ghiglieri, V.; Pendolino, V.; Sgobio, C.; Bagetta, V.; Picconi, B.; Calabresi, P. Θ-Burst Stimulation and Striatal Plasticity in Experimental Parkinsonism. Exp. Neurol. 2012, 236, 395–398. [Google Scholar] [CrossRef]
- Zeljkovic Jovanovic, M.; Stanojevic, J.; Stevanovic, I.; Stekic, A.; Bolland, S.J.; Jasnic, N.; Ninkovic, M.; Zaric Kontic, M.; Ilic, T.V.; Rodger, J.; et al. Intermittent Theta Burst Stimulation Improves Motor and Behavioral Dysfunction through Modulation of NMDA Receptor Subunit Composition in Experimental Model of Parkinson’s Disease. Cells 2023, 12, 1525. [Google Scholar] [CrossRef]
- Cacace, F.; Mineo, D.; Viscomi, M.T.; Latagliata, E.C.; Mancini, M.; Sasso, V.; Vannelli, A.; Pascucci, T.; Pendolino, V.; Marcello, E.; et al. Intermittent Theta-Burst Stimulation Rescues Dopamine-Dependent Corticostriatal Synaptic Plasticity and Motor Behavior in Experimental Parkinsonism: Possible Role of Glial Activity. Mov. Disord. 2017, 32, 1035–1046. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, B.; Du, W.; Zhao, R.; Liu, X.; Bai, Y.; Jiang, X.; Pang, J.; Zhao, C.; Mou, X.; et al. High-Frequency Repetitive Transcranial Magnetic Stimulation Regulates Astrocyte Activation by Modulating the Endocannabinoid System in Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 5121–5134. [Google Scholar] [CrossRef]
- Ba, M.; Kong, M.; Guan, L.; Yi, M.; Zhang, H. Repetitive Transcranial Magnetic Stimulation (RTMS) Improves Behavioral and Biochemical Deficits in Levodopa-Induced Dyskinetic Rats Model. Oncotarget 2016, 7, 58802–58812. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.; Ma, G.; Ren, C.; Sun, X.; Kong, M. Repetitive Transcranial Magnetic Stimulation for Treatment of Lactacystin-Induced Parkinsonian Rat Model. Oncotarget 2017, 8, 50921–50929. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Wang, Y.; Gu, P.; Shao, R.; Zhao, L.; Liu, X.; Wang, Z.; Wang, M. The Neuroprotective Mechanism of Low-Frequency RTMS on Nigral Dopaminergic Neurons of Parkinson’s Disease Model Mice. Park. Dis. 2015, 2015, 564095. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, F.; Han, J.; Bai, L.; Du, J. Repetitive Transcranial Magnetic Stimulation Reduces Neuronal Loss and Neuroinflammation in Parkinson?S Disease Through the MiR-195a-5p/CREB Axis. Turk. Neurosurg. 2023, 33, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ba, F.; Zhou, Y.; Zhou, J.; Chen, X. Repetitive Transcranial Magnetic Stimulation Protects Mice against 6-OHDA-Induced Parkinson’s Disease Symptoms by Regulating Brain Amyloid Β1–42 Level. Mol. Cell. Biochem. 2019, 458, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. The Amphetamine Induced Rotation Test: A Re-Assessment of Its Use as a Tool to Monitor Motor Impairment and Functional Recovery in Rodent Models of Parkinson’s Disease. J. Park. Dis. 2019, 9, 17–29. [Google Scholar] [CrossRef]
- van Dijk, K.D.; Teunissen, C.E.; Drukarch, B.; Jimenez, C.R.; Groenewegen, H.J.; Berendse, H.W.; van de Berg, W.D.J. Diagnostic Cerebrospinal Fluid Biomarkers for Parkinson’s Disease: A Pathogenetically Based Approach. Neurobiol. Dis. 2010, 39, 229–241. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D.-K. Promising Cannabinoid-Based Therapies for Parkinson’s Disease: Motor Symptoms to Neuroprotection. Mol. Neurodegener. 2015, 10, 17. [Google Scholar] [CrossRef]
- Ba, M.; Kong, M.; Ma, G. Postsynaptic Density Protein 95-Regulated NR2B Tyrosine Phosphorylation and Interactions of Fyn with NR2B in Levodopa-Induced Dyskinesia Rat Models. Drug Des. Dev. Ther. 2015, 9, 199–206. [Google Scholar] [CrossRef][Green Version]
- Oh, J.D.; Russell, D.S.; Vaughan, C.L.; Chase, T.N. Enhanced Tyrosine Phosphorylation of Striatal NMDA Receptor Subunits: Effect of Dopaminergic Denervation and L-DOPA Administration. Brain Res. 1998, 813, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Suppa, A.; Marsili, L.; Belvisi, D.; Conte, A.; Iezzi, E.; Modugno, N.; Fabbrini, G.; Berardelli, A. Lack of LTP-like Plasticity in Primary Motor Cortex in Parkinson’s Disease. Exp. Neurol. 2011, 227, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s Disease and Its Potential as Therapeutic Target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Troncoso-Escudero, P.; Parra, A.; Nassif, M.; Vidal, R.L. Outside in: Unraveling the Role of Neuroinflammation in the Progression of Parkinson’s Disease. Front. Neurol. 2018, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and Immune Dysfunction in Parkinson Disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Aftanas, L.I.; Gevorgyan, M.M.; Zhanaeva, S.Y.; Dzemidovich, S.S.; Kulikova, K.I.; Al’perina, E.L.; Danilenko, K.V.; Idova, G.V. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation (RTMS) on Neuroinflammation and Neuroplasticity in Patients with Parkinson’s Disease: A Placebo-Controlled Study. Bull. Exp. Biol. Med. 2018, 165, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Lee, J.; Lee, S.-Y. Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging in Neurological Diseases. Nucl. Med. Mol. Imaging 2017, 51, 283–296. [Google Scholar] [CrossRef]
- Zhang, P.-F.; Gao, F. Neuroinflammation in Parkinson’s Disease: A Meta-Analysis of PET Imaging Studies. J. Neurol. 2022, 269, 2304–2314. [Google Scholar] [CrossRef]
- Doot, R.K.; Young, A.J.; Nasrallah, I.M.; Wetherill, R.R.; Siderowf, A.; Mach, R.H.; Dubroff, J.G. [18F]NOS PET Brain Imaging Suggests Elevated Neuroinflammation in Idiopathic Parkinson’s Disease. Cells 2022, 11, 3081. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Keefe, K.A.; DiFrischia, D.S.; Zigmond, M.J. Differential Effect of Stress on In Vivo Dopamine Release in Striatum, Nucleus Accumbens, and Medial Frontal Cortex. J. Neurochem. 1989, 52, 1655–1658. [Google Scholar] [CrossRef]
- Wexler, A. The Practices of Do-It-Yourself Brain Stimulation: Implications for Ethical Considerations and Regulatory Proposals. J. Med. Ethics 2016, 42, 211–215. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Boggio, P.S.; Fregni, F.; Pascual-Leone, A. Treatment of Depression with Transcranial Direct Current Stimulation (TDCS): A Review. Exp. Neurol. 2009, 219, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P.; Fiori, V.; Calpagnano, M.A.; Campana, S.; Razzano, C.; Caltagirone, C.; Marini, A. TDCS over the Left Inferior Frontal Cortex Improves Speech Production in Aphasia. Front. Hum. Neurosci. 2013, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; Crinion, J. Can TDCS Enhance Treatment of Aphasia after Stroke? Aphasiology 2012, 26, 1169–1191. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Ferrucci, R.; Rigonatti, S.P.; Covre, P.; Nitsche, M.; Pascual-Leone, A.; Fregni, F. Effects of Transcranial Direct Current Stimulation on Working Memory in Patients with Parkinson’s Disease. J. Neurol. Sci. 2006, 249, 31–38. [Google Scholar] [CrossRef]
- Ferrucci, R.; Mameli, F.; Ruggiero, F.; Priori, A. Transcranial Direct Current Stimulation as Treatment for Parkinson’s Disease and Other Movement Disorders. Basal Ganglia 2016, 6, 53–61. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, G.; Bridi, M.; Abel, T.; Bikson, M.; Parra, L.C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 2017, 10, 51–58. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Liu, Z.; Rao, J.; Wang, J.; Wang, P.; Gong, X.; Wen, Y. Transcranial Direct Current Stimulation for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 746797. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Santos, M.C.; Lima, M.; Vieira, A.L.; Rigonatti, S.P.; Silva, M.T.A.; Barbosa, E.R.; Nitsche, M.A.; Pascual-Leone, A. Noninvasive Cortical Stimulation with Transcranial Direct Current Stimulation in Parkinson’s Disease. Mov. Disord. 2006, 21, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Colella, D.; Giangrosso, M.; Cannavacciuolo, A.; Paparella, G.; Fabbrini, G.; Suppa, A.; Berardelli, A.; Bologna, M. Driving Motor Cortex Oscillations Modulates Bradykinesia in Parkinson’s Disease. Brain J. Neurol. 2022, 145, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Del Felice, A.; Castiglia, L.; Formaggio, E.; Cattelan, M.; Scarpa, B.; Manganotti, P.; Tenconi, E.; Masiero, S. Personalized Transcranial Alternating Current Stimulation (TACS) and Physical Therapy to Treat Motor and Cognitive Symptoms in Parkinson’s Disease: A Randomized Cross-over Trial. NeuroImage Clin. 2019, 22, 101768. [Google Scholar] [CrossRef]
- Brittain, J.-S.; Probert-Smith, P.; Aziz, T.Z.; Brown, P. Tremor Suppression by Rhythmic Transcranial Current Stimulation. Curr. Biol. 2013, 23, 436–440. [Google Scholar] [CrossRef]
- Tanaka, T.; Takano, Y.; Tanaka, S.; Hironaka, N.; Kobayashi, K.; Hanakawa, T.; Watanabe, K.; Honda, M. Transcranial Direct-Current Stimulation Increases Extracellular Dopamine Levels in the Rat Striatum. Front. Syst. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Takano, Y.; Yokawa, T.; Masuda, A.; Niimi, J.; Tanaka, S.; Hironaka, N. A Rat Model for Measuring the Effectiveness of Transcranial Direct Current Stimulation Using FMRI. Neurosci. Lett. 2011, 491, 40–43. [Google Scholar] [CrossRef]
- Liebetanz, D.; Koch, R.; Mayenfels, S.; König, F.; Paulus, W.; Nitsche, M.A. Safety Limits of Cathodal Transcranial Direct Current Stimulation in Rats. Clin. Neurophysiol. 2009, 120, 1161–1167. [Google Scholar] [CrossRef]
- Lu, C.; Wei, Y.; Hu, R.; Wang, Y.; Li, K.; Li, X. Transcranial Direct Current Stimulation Ameliorates Behavioral Deficits and Reduces Oxidative Stress in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Neuromodul. Technol. Neural Interface 2015, 18, 442–447. [Google Scholar] [CrossRef]
- Lee, S.-B.; Kim, H.-T.; Yang, H.O.; Jang, W. Anodal Transcranial Direct Current Stimulation Prevents Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Induced Neurotoxicity by Modulating Autophagy in an in Vivo Mouse Model of Parkinson’s Disease. Sci. Rep. 2018, 8, 15165. [Google Scholar] [CrossRef]
- Feng, X.-J.; Huang, Y.-T.; Huang, Y.-Z.; Kuo, C.-W.; Peng, C.-W.; Rotenberg, A.; Juan, C.-H.; Pei, Y.-C.; Chen, Y.-H.; Chen, K.-Y.; et al. Early Transcranial Direct Current Stimulation Treatment Exerts Neuroprotective Effects on 6-OHDA-Induced Parkinsonism in Rats. Brain Stimul. 2020, 13, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jung, D.H.; Jung, Y.J.; Shin, H.K.; Choi, B.T. Transcranial Alternating Current Stimulation Rescues Motor Deficits in a Mouse Model of Parkinson’s Disease via the Production of Glial Cell Line-Derived Neurotrophic Factor. Brain Stimul. 2022, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kade, A.K.; Kravchenko, S.V.; Trofimenko, A.I.; Chaplygina, K.Y.; Ananeva, E.I.; Poliakov, P.P.; Lipatova, A.S. [The efficacy of tes-therapy for treatment of anxiety-like behavior and motor disorders in rats with an experimental model of parkinsonism]. Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova 2019, 119, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The Role of Oxidative Stress in Parkinson’s Disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The Role of Autophagy in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357. [Google Scholar] [CrossRef]
- Winkler, C.; Reis, J.; Hoffmann, N.; Gellner, A.-K.; Münkel, C.; Curado, M.R.; Furlanetti, L.; Garcia, J.; Döbrössy, M.D.; Fritsch, B. Anodal Transcranial Direct Current Stimulation Enhances Survival and Integration of Dopaminergic Cell Transplants in a Rat Parkinson Model. eNeuro 2017, 4, ENEURO.0063-17.2017. [Google Scholar] [CrossRef]
- Li, H.; Lei, X.; Yan, T.; Li, H.; Huang, B.; Li, L.; Xu, L.; Liu, L.; Chen, N.; Lü, L.; et al. The Temporary and Accumulated Effects of Transcranial Direct Current Stimulation for the Treatment of Advanced Parkinson’s Disease Monkeys. Sci. Rep. 2015, 5, 12178. [Google Scholar] [CrossRef]
- Mounayar, S.; Boulet, S.; Tandé, D.; Jan, C.; Pessiglione, M.; Hirsch, E.C.; Féger, J.; Savasta, M.; François, C.; Tremblay, L. A New Model to Study Compensatory Mechanisms in MPTP-Treated Monkeys Exhibiting Recovery. Brain J. Neurol. 2007, 130, 2898–2914. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Qian, L.; Yu, X.; Jiang, W. Anodal Transcranial Direct Current Stimulation Relieves the Unilateral Bias of a Rat Model of Parkinson’s Disease. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 765–768. [Google Scholar]
- Pagnin, D.; de Queiroz, V.; Pini, S.; Cassano, G.B. Efficacy of ECT in Depression: A Meta-Analytic Review. J. ECT 2004, 20, 13–20. [Google Scholar] [CrossRef]
- Kennedy, R.; Mittal, D.; O’Jile, J. Electroconvulsive Therapy in Movement Disorders: An Update. J. Neuropsychiatry Clin. Neurosci. 2003, 15, 407–421. [Google Scholar] [CrossRef]
- Garcia, E.; Sotelo, J. Electroconvulsive Shock Does Not Modify Striatal Contents of Dopamine in MPTP-Treated Mice. Neurochem. Res. 1993, 18, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Anastasia, A.; De Erausquin, G.A.; Wojnacki, J.; Mascó, D.H. Protection of Dopaminergic Neurons by Electroconvulsive Shock in an Animal Model of Parkinson’s Disease. J. Neurochem. 2007, 103, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Strome, E.M.; Zis, A.P.; Doudet, D.J. Electroconvulsive Shock Enhances Striatal Dopamine D1 and D3 Receptor Binding and Improves Motor Performance in 6-OHDA-Lesioned Rats. J. Psychiatry Neurosci. 2007, 32, 193–202. [Google Scholar] [PubMed]
- Anastasía, A.; Wojnacki, J.; de Erausquin, G.A.; Mascó, D.H. Glial Cell-Line Derived Neurotrophic Factor Is Essential for Electroconvulsive Shock-Induced Neuroprotection in an Animal Model of Parkinson’s Disease. Neuroscience 2011, 195, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Clark, C.; Jivan, S.; Doudet, D.J. Antiparkinsonian Mechanism of Electroconvulsive Therapy in MPTP-Lesioned Non-Human Primates. Neurodegener. Dis. 2012, 9, 128–138. [Google Scholar] [CrossRef]
- Yang, C.; Qiu, Y.; Qing, Y.; Xu, J.; Dai, W.; Hu, X.; Wu, X. Synergistic Effect of Electric Stimulation and Mesenchymal Stem Cells against Parkinson’s Disease. Aging 2020, 12, 16062–16071. [Google Scholar] [CrossRef]
- Huh, S.; Yu, H.S.; Kang, N.; Ahn, Y.M.; Kim, Y.S.; Kim, S.H. Electroconvulsive Seizure Normalizes Motor Deficits and Induces Autophagy Signaling in the MPTP-Induced Parkinson Disease Mouse Model. Psychiatry Investig. 2023, 20, 273–283. [Google Scholar] [CrossRef]
- Robertson, D.C.; Schmidt, O.; Ninkina, N.; Jones, P.A.; Sharkey, J.; Buchman, V.L. Developmental Loss and Resistance to MPTP Toxicity of Dopaminergic Neurones in Substantia Nigra Pars Compacta of Gamma-Synuclein, Alpha-Synuclein and Double Alpha/Gamma-Synuclein Null Mutant Mice. J. Neurochem. 2004, 89, 1126–1136. [Google Scholar] [CrossRef]
- Landry, M.; Moreno, A.; Patry, S.; Potvin, S.; Lemasson, M. Current Practices of Electroconvulsive Therapy in Mental Disorders: A Systematic Review and Meta-Analysis of Short and Long-Term Cognitive Effects. J. ECT 2021, 37, 119. [Google Scholar] [CrossRef]
- Yoo, S.-S.; Bystritsky, A.; Lee, J.-H.; Zhang, Y.; Fischer, K.; Min, B.-K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused Ultrasound Modulates Region-Specific Brain Activity. Neuroimage 2011, 56, 1267–1275. [Google Scholar] [CrossRef]
- Mahdavi, K.; Jordan, K.; Haroon, J.; Habelhah, B.; Rindner, E.; Surya, R.; Venkatraman, V.; Becerra, S.; Spivak, N.; Bystritsky, A.; et al. A Pilot Study of Low-Intensity Focused Ultrasound for Treatment-Resistant Generalized Anxiety Disorder. Brain Stimul. 2023, 16, 369–370. [Google Scholar] [CrossRef]
- Wu, S.-K.; Tsai, C.-L.; Huang, Y.; Hynynen, K. Focused Ultrasound and Microbubbles-Mediated Drug Delivery to Brain Tumor. Pharmaceutics 2020, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial Focused Ultrasound Modulates the Activity of Primary Somatosensory Cortex in Humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Y.; Lan, L.; Ge, X.; Cheng, R.; Zhan, Y.; Chen, G.; Shi, L.; Wang, R.; Zheng, N.; et al. Optically-Generated Focused Ultrasound for Noninvasive Brain Stimulation with Ultrahigh Precision. Light Sci. Appl. 2022, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Natera-Villalba, E.; Matarazzo, M.; Martinez-Fernandez, R. Update in the Clinical Application of Focused Ultrasound. Curr. Opin. Neurol. 2022, 35, 525. [Google Scholar] [CrossRef] [PubMed]
- Krishna, V.; Fishman, P.S.; Eisenberg, H.M.; Kaplitt, M.; Baltuch, G.; Chang, J.W.; Chang, W.-C.; Martinez Fernandez, R.; del Alamo, M.; Halpern, C.H.; et al. Trial of Globus Pallidus Focused Ultrasound Ablation in Parkinson’s Disease. N. Engl. J. Med. 2023, 388, 683–693. [Google Scholar] [CrossRef]
- Mahoney, J.J.; Thompson Lake, D.G.Y.; Ranjan, M.; Marton, J.L.; Carpenter, J.S.; Zheng, W.; Berry, J.H.; Farmer, D.L.; D’Haese, P.F.; Finomore, V.S.; et al. Low-Intensity Focused Ultrasound Targeting the Bilateral Nucleus Accumbens as a Potential Treatment for Substance Use Disorder: A First-in-Human Report. Biol. Psychiatry, 2023; in press. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; D’Haese, P.-F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive Hippocampal Blood-Brain Barrier Opening in Alzheimer’s Disease with Focused Ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; Haut, M.W.; Carpenter, J.; D’Haese, P.-F.; Mehta, R.I.; Najib, U.; Wang, P.; Claassen, D.O.; Chazen, J.L.; et al. Focused Ultrasound-Mediated Blood-Brain Barrier Opening in Alzheimer’s Disease: Long-Term Safety, Imaging, and Cognitive Outcomes. J. Neurosurg. 2023, 139, 275–283. [Google Scholar] [CrossRef]
- Liu, X.; Naomi, S.S.M.; Sharon, W.L.; Russell, E.J. The Applications of Focused Ultrasound (FUS) in Alzheimer’s Disease Treatment: A Systematic Review on Both Animal and Human Studies. Aging Dis. 2022, 12, 1977–2002. [Google Scholar] [CrossRef]
- Gasca-Salas, C.; Fernández-Rodríguez, B.; Pineda-Pardo, J.A.; Rodríguez-Rojas, R.; Obeso, I.; Hernández-Fernández, F.; del Álamo, M.; Mata, D.; Guida, P.; Ordás-Bandera, C.; et al. Blood-Brain Barrier Opening with Focused Ultrasound in Parkinson’s Disease Dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Pineda-Pardo, J.A.; Inoue, K.-I.; Gasca-Salas, C.; Balzano, T.; Del Rey, N.L.-G.; Reinares-Sebastián, A.; Esteban-García, N.; Rodríguez-Rojas, R.; Márquez, R.; et al. BBB Opening with Focused Ultrasound in Nonhuman Primates and Parkinson’s Disease Patients: Targeted AAV Vector Delivery and PET Imaging. Sci. Adv. 2023, 9, eadf4888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, L.; Liu, S.; Zhou, X.; Yin, T.; Liu, Z.; Yang, Z. Transcranial Magneto-Acoustic Stimulation Improves Neuroplasticity in Hippocampus of Parkinson’s Disease Model Mice. Neurotherapeutics 2019, 16, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Niu, L.; Meng, L.; Lin, Z.; Zou, J.; Xia, X.; Huang, X.; Zhou, W.; Bian, T.; Zheng, H. Noninvasive Ultrasound Deep Brain Stimulation for the Treatment of Parkinson’s Disease Model Mouse. Research 2019, 2019, 1748489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Niu, L.; Xia, X.; Lin, Z.; Liu, X.; Su, M.; Guo, R.; Meng, L.; Zheng, H. Wearable Ultrasound Improves Motor Function in an MPTP Mouse Model of Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2019, 66, 3006–3013. [Google Scholar] [CrossRef]
- Xu, T.; Lu, X.; Peng, D.; Wang, G.; Chen, C.; Liu, W.; Wu, W.; Mason, T.J. Ultrasonic Stimulation of the Brain to Enhance the Release of Dopamine—A Potential Novel Treatment for Parkinson’s Disease. Ultrason. Sonochem. 2020, 63, 104955. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, L.; Xia, X.; Lin, Z.; Zhou, W.; Pang, N.; Bian, T.; Yuan, T.; Niu, L.; Zheng, H. Transcranial Ultrasound Stimulation Suppresses Neuroinflammation in a Chronic Mouse Model of Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2021, 68, 3375–3387. [Google Scholar] [CrossRef]
- Song, W.-S.; Sung, C.-Y.; Ke, C.-H.; Yang, F.-Y. Anti-Inflammatory and Neuroprotective Effects of Transcranial Ultrasound Stimulation on Parkinson’s Disease. Ultrasound Med. Biol. 2022, 48, 265–274. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, D.; Zhao, Y.; Yuan, Y.; Wang, W.; Wu, S.; Liang, X.; Wang, Z.; Liu, L. Assessment of Neuroprotective Effects of Low-Intensity Transcranial Ultrasound Stimulation in a Parkinson’s Disease Rat Model by Fractional Anisotropy and Relaxation Time T2∗ Value. Front. Neurosci. 2021, 15, 590354. [Google Scholar] [CrossRef]
- Haacke, E.M.; Cheng, N.Y.C.; House, M.J.; Liu, Q.; Neelavalli, J.; Ogg, R.J.; Khan, A.; Ayaz, M.; Kirsch, W.; Obenaus, A. Imaging Iron Stores in the Brain Using Magnetic Resonance Imaging. Magn. Reson. Imaging 2005, 23, 1–25. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.; Li, X. Theoretical Analysis of Transcranial Magneto-Acoustical Stimulation with Hodgkin-Huxley Neuron Model. Front. Comput. Neurosci. 2016, 10, 35. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Permeability at the Blood-Brain and Blood-Nerve Barriers of the Neurotrophic Factors: NGF, CNTF, NT-3, BDNF. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-H.; Ting, C.-Y.; Lin, C.-Y.; Chan, H.-L.; Chang, Y.-C.; Chen, Y.-Y.; Liu, H.-L.; Yeh, C.-K. Noninvasive, Targeted and Non-Viral Ultrasound-Mediated GDNF-Plasmid Delivery for Treatment of Parkinson’s Disease. Sci. Rep. 2016, 6, 19579. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsieh, H.-Y.; Chen, C.-M.; Wu, S.-R.; Tsai, C.-H.; Huang, C.-Y.; Hua, M.-Y.; Wei, K.-C.; Yeh, C.-K.; Liu, H.-L. Non-Invasive, Neuron-Specific Gene Therapy by Focused Ultrasound-Induced Blood-Brain Barrier Opening in Parkinson’s Disease Mouse Model. J. Control. Release 2016, 235, 72–81. [Google Scholar] [CrossRef]
- Mead, B.P.; Kim, N.; Miller, G.W.; Hodges, D.; Mastorakos, P.; Klibanov, A.L.; Mandell, J.W.; Hirsh, J.; Suk, J.S.; Hanes, J.; et al. Novel Focused Ultrasound Gene Therapy Approach Noninvasively Restores Dopaminergic Neuron Function in a Rat Parkinson’s Disease Model. Nano Lett. 2017, 17, 3533–3542. [Google Scholar] [CrossRef]
- Yue, P.; Gao, L.; Wang, X.; Ding, X.; Teng, J. Ultrasound-Triggered Effects of the Microbubbles Coupled to GDNF- and Nurr1-Loaded PEGylated Liposomes in a Rat Model of Parkinson’s Disease. J. Cell. Biochem. 2018, 119, 4581–4591. [Google Scholar] [CrossRef]
- Karakatsani, M.E.; Wang, S.; Samiotaki, G.; Kugelman, T.; Olumolade, O.O.; Acosta, C.; Sun, T.; Han, Y.; Kamimura, H.A.S.; Jackson-Lewis, V.; et al. Amelioration of the Nigrostriatal Pathway Facilitated by Ultrasound-Mediated Neurotrophic Delivery in Early Parkinson’s Disease. J. Control. Release 2019, 303, 289–301. [Google Scholar] [CrossRef]
- Decressac, M.; Volakakis, N.; Björklund, A.; Perlmann, T. NURR1 in Parkinson Disease—From Pathogenesis to Therapeutic Potential. Nat. Rev. Neurol. 2013, 9, 629–636. [Google Scholar] [CrossRef]
- Long, L.; Cai, X.; Guo, R.; Wang, P.; Wu, L.; Yin, T.; Liao, S.; Lu, Z. Treatment of Parkinson’s Disease in Rats by Nrf2 Transfection Using MRI-Guided Focused Ultrasound Delivery of Nanomicrobubbles. Biochem. Biophys. Res. Commun. 2017, 482, 75–80. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Huang, R.-Y.; Liao, E.-C.; Lin, Y.-C.; Ho, Y.-J.; Chang, C.-W.; Chan, H.-L.; Huang, Y.-Z.; Hsieh, T.-H.; Fan, C.-H.; et al. A Preliminary Study of Parkinson’s Gene Therapy via Sono-Magnetic Sensing Gene Vector for Conquering Extra/Intracellular Barriers in Mice. Brain Stimul. 2020, 13, 786–799. [Google Scholar] [CrossRef]
- Ji, R.; Smith, M.; Niimi, Y.; Karakatsani, M.E.; Murillo, M.F.; Jackson-Lewis, V.; Przedborski, S.; Konofagou, E.E. Focused Ultrasound Enhanced Intranasal Delivery of Brain Derived Neurotrophic Factor Produces Neurorestorative Effects in a Parkinson’s Disease Mouse Model. Sci. Rep. 2019, 9, 19402. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Ohta, H. Roles of FGF20 in Dopaminergic Neurons and Parkinson’s Disease. Front. Mol. Neurosci. 2013, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xie, J.; Guo, K.; Zhang, X.; Xia, F.; Zhao, X.; Song, L.; Zhuge, D.; Li, X.; Zhao, Y.; et al. Efficient Treatment of Parkinson’s Disease Using Ultrasonography-Guided RhFGF20 Proteoliposomes. Drug Deliv. 2018, 25, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Yoshimura, N.; Tsuji, A.; Kunisada, T. Differentiation of Dopaminergic Neurons from Human Embryonic Stem Cells: Modulation of Differentiation by FGF-20. J. Biosci. Bioeng. 2009, 107, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, I.J.; Boshoff, E.L.; Duty, S. Fibroblast Growth Factor-20 Protects against Dopamine Neuron Loss in Vitro and Provides Functional Protection in the 6-Hydroxydopamine-Lesioned Rat Model of Parkinson’s Disease. Neuropharmacology 2012, 63, 1268–1277. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain Delivery of Curcumin Through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021, 16, 7433–7447. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized Delivery of Curcumin into Brain with Polysorbate 80-Modified Cerasomes by Ultrasound-Targeted Microbubble Destruction for Improved Parkinson’s Disease Therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-Loaded Nanoparticles: A Novel Therapeutic Strategy in Treatment of Central Nervous System Disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin Inhibits Aggregation of Alpha-Synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Feng, Y.; An, R.; Zhang, Y.; Chen, M.; Wang, L.; Duan, Y.; Xing, C. AHNAK-Modified Microbubbles for the Intracranial Delivery of Triptolide: In-Vitro and in-Vivo Investigations. Int. J. Pharm. 2022, 629, 122351. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gong, X.; Wang, L.; Liu, M.; Liu, Y.; Fu, X.; Wang, W.; Zhang, T.; Wang, X. Triptolide Promotes the Clearance of α-Synuclein by Enhancing Autophagy in Neuronal Cells. Mol. Neurobiol. 2017, 54, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-L.; Yang, Y.-X.; Ding, J.; Li, Y.-C.; Miao, Z.-H. Triptolide: Structural Modifications, Structure-Activity Relationships, Bioactivities, Clinical Development and Mechanisms. Nat. Prod. Rep. 2012, 29, 457–475. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, K.; Li, J.; Liao, Y.; Liao, C.; Chen, W.-S.; Chen, M.; Ao, L. Focused Ultrasound Promotes the Delivery of Gastrodin and Enhances the Protective Effect on Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease. Front. Cell. Neurosci. 2022, 16, 884788. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, X.; Wang, H.; Qu, M. Gastrodin Attenuates Pentylenetetrazole-Induced Seizures by Modulating the Mitogen-Activated Protein Kinase-Associated Inflammatory Responses in Mice. Neurosci. Bull. 2016, 33, 264–272. [Google Scholar] [CrossRef]
- Xhima, K.; Nabbouh, F.; Hynynen, K.; Aubert, I.; Tandon, A. Non-Invasive Delivery of an α-Synuclein Gene Silencing Vector with MR-Guided Focused Ultrasound. Mov. Disord. 2018, 33, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Huang, C.-Y.; Chen, C.-M.; Liu, H.-L. Focused Ultrasound-Induced Blood-Brain Barrier Opening Enhanced α-Synuclein Expression in Mice for Modeling Parkinson’s Disease. Pharmaceutics 2022, 14, 444. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.J.; Choi, W.; Lee, Y.M.; Pyo, J.H.; Lee, J.; Kim, J.; Kim, J.; Kim, J.-H.; Kim, C.; et al. Deep Brain Stimulation by Blood-Brain-Barrier-Crossing Piezoelectric Nanoparticles Generating Current and Nitric Oxide under Focused Ultrasound. Nat. Biomed. Eng. 2023, 7, 149–163. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the Blood-Brain Barrier by Focused Ultrasound Induces Sterile Inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K.; et al. Amyloid-β Plaque Reduction, Endogenous Antibody Delivery and Glial Activation by Brain-Targeted, Transcranial Focused Ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef]
- Leinenga, G.; Götz, J. Scanning Ultrasound Removes Amyloid-β and Restores Memory in an Alzheimer’s Disease Mouse Model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound Is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chaney, A.M.; Carlson, M.L.; Jackson, I.M.; Rao, A.; James, M.L. Neuroinflammation PET Imaging: Current Opinion and Future Directions. J. Nucl. Med. 2020, 61, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Lavisse, S.; Goutal, S.; Wimberley, C.; Tonietto, M.; Bottlaender, M.; Gervais, P.; Kuhnast, B.; Peyronneau, M.-A.; Barret, O.; Lagarde, J.; et al. Increased Microglial Activation in Patients with Parkinson Disease Using [18F]-DPA714 TSPO PET Imaging. Park. Relat. Disord. 2021, 82, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef]
- Shah, F.; Hume, S.P.; Pike, V.W.; Ashworth, S.; McDermott, J. Synthesis of the Enantiomers of [N-Methyl-11C]PK 11195 and Comparison of Their Behaviours as Radioligands for PK Binding Sites in Rats. Nucl. Med. Biol. 1994, 21, 573–581. [Google Scholar] [CrossRef]
- Molendijk, M.L.; de Kloet, E.R. Forced Swim Stressor: Trends in Usage and Mechanistic Consideration. Eur. J. Neurosci. 2022, 55, 2813–2831. [Google Scholar] [CrossRef]
- Stephan, M.; Volkmann, P.; Rossner, M.J. Assessing Behavior and Cognition in Rodents, Nonhuman Primates, and Humans: Where Are the Limits of Translation? Dialogues Clin. Neurosci. 2019, 21, 249–259. [Google Scholar] [CrossRef]
- Rentsch, P.; Stayte, S.; Morris, G.P.; Vissel, B. Time Dependent Degeneration of the Nigrostriatal Tract in Mice with 6-OHDA Lesioned Medial Forebrain Bundle and the Effect of Activin A on l-Dopa Induced Dyskinesia. BMC Neurosci. 2019, 20, 5. [Google Scholar] [CrossRef]
- Masini, D.; Plewnia, C.; Bertho, M.; Scalbert, N.; Caggiano, V.; Fisone, G. A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson’s Disease for the Study of Non-Motor Symptoms. Biomedicines 2021, 9, 598. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Navarro, J.A.; Casarejos, M.J.; Menéndez, J.; Solano, R.M.; Rodal, I.; Gómez, A.; de Yébenes, J.G.; Mena, M.A. Mortality, Oxidative Stress and Tau Accumulation during Ageing in Parkin Null Mice. J. Neurochem. 2007, 103, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Correia Guedes, L.; Mestre, T.; Outeiro, T.F.; Ferreira, J.J. Are Genetic and Idiopathic Forms of Parkinson’s Disease the Same Disease? J. Neurochem. 2020, 152, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Armentero, M.-T. Animal Models of Parkinson’s Disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef]
- Sarre, S.; Yuan, H.; Jonkers, N.; Van Hemelrijck, A.; Ebinger, G.; Michotte, Y. In Vivo Characterization of Somatodendritic Dopamine Release in the Substantia Nigra of 6-Hydroxydopamine-Lesioned Rats. J. Neurochem. 2004, 90, 29–39. [Google Scholar] [CrossRef]
- Stott, S.R.W.; Barker, R.A. Time Course of Dopamine Neuron Loss and Glial Response in the 6-OHDA Striatal Mouse Model of Parkinson’s Disease. Eur. J. Neurosci. 2014, 39, 1042–1056. [Google Scholar] [CrossRef]
- Pol, F.; Salehinejad, M.A.; Baharlouei, H.; Nitsche, M.A. The Effects of Transcranial Direct Current Stimulation on Gait in Patients with Parkinson’s Disease: A Systematic Review. Transl. Neurodegener. 2021, 10, 22. [Google Scholar] [CrossRef]
- Takamiya, A.; Seki, M.; Kudo, S.; Yoshizaki, T.; Nakahara, J.; Mimura, M.; Kishimoto, T. Electroconvulsive Therapy for Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2021, 36, 50–58. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The Clinical Symptoms of Parkinson’s Disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Andrade, C.; Arumugham, S.S.; Thirthalli, J. Adverse Effects of Electroconvulsive Therapy. Psychiatr. Clin. N. Am. 2016, 39, 513–530. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ugawa, Y. Adverse Events of TDCS and TACS: A Review. Clin. Neurophysiol. Pract. 2016, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.K.; McFarquhar, T.F.; Mitchell, P.B. A Review of the Safety of Repetitive Transcranial Magnetic Stimulation as a Clinical Treatment for Depression. Int. J. Neuropsychopharmacol. 2008, 11, 131–147. [Google Scholar] [CrossRef]
- Wassermann, E.M. Side Effects of Repetitive Transcranial Magnetic Stimulation. Depress. Anxiety 2000, 12, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Magsood, H.; Syeda, F.; Holloway, K.; Carmona, I.C.; Hadimani, R.L. Safety Study of Combination Treatment: Deep Brain Stimulation and Transcranial Magnetic Stimulation. Front. Hum. Neurosci. 2020, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.D.; Bang, Y.M.; Youn, J.; Cho, J.W.; Kim, Y.-H.; Chang, W.H. Feasibility of Transcranial Direct Current Stimulation in Patients with Deep Brain Stimulation: A Case Report. Brain NeuroRehabil. 2020, 13, e13. [Google Scholar] [CrossRef]
- Ducharme, S.; Flaherty, A.W.; Seiner, S.J.; Dougherty, D.D.; Morales, O.G. Temporary Interruption of Deep Brain Stimulation for Parkinson’s Disease during Outpatient Electroconvulsive Therapy for Major Depression: A Novel Treatment Strategy. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 194–197. [Google Scholar] [CrossRef]
- Cunningham, M.G.; Yadollahikhales, G.; Vitaliano, G.; van Horne, C. Administration of Electroconvulsive Therapy for Depression Associated with Deep Brain Stimulation in a Patient with Post-Traumatic Parkinson’s Disease: A Case Study. BMC Psychiatry 2016, 16, 399. [Google Scholar] [CrossRef][Green Version]
- Volkaerts, L.; Roels, R.; Bouckaert, F. Motor Function Improvement After Electroconvulsive Therapy in a Parkinson’s Disease Patient With Deep Brain Stimulator. J. ECT 2020, 36, 66–68. [Google Scholar] [CrossRef]
- Bailine, S.; Kremen, N.; Kohen, I.; Linder, H.; Schwartz, G.J.; Mogilner, A.Y.; Pourfar, M. Bitemporal Electroconvulsive Therapy for Depression in a Parkinson Disease Patient with a Deep-Brain Stimulator. J. ECT 2008, 24, 171–172. [Google Scholar] [CrossRef]
- Nasr, S.; Murillo, A.; Katariwala, N.; Mothkur, V.; Wendt, B. Case Report of Electroconvulsive Therapy in a Patient with Parkinson Disease Concomitant with Deep Brain Stimulation. J. ECT 2011, 27, 89–90. [Google Scholar] [CrossRef]
- Sarica, C.; Fomenko, A.; Nankoo, J.-F.; Darmani, G.; Vetkas, A.; Yamamoto, K.; Lozano, A.M.; Chen, R. Toward Focused Ultrasound Neuromodulation in Deep Brain Stimulator Implanted Patients: Ex-Vivo Thermal, Kinetic and Targeting Feasibility Assessment. Brain Stimul. 2022, 15, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Lewis, V.; Blesa, J.; Przedborski, S. Animal Models of Parkinson’s Disease. Park. Relat. Disord. 2012, 18, S183–S185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).