Abstract
Introduction: The primary objective of researchers in the biology of aging is to gain a comprehensive understanding of the aging process while developing practical solutions that can enhance the quality of life for older individuals. This involves a continuous effort to bridge the gap between fundamental biological research and its real-world applications. Purpose: In this narrative review, we attempt to link research findings concerning the hormetic relationship between neurons and germ cells, and translate these findings into clinically relevant concepts. Methods: We conducted a literature search using PubMed, Embase, PLOS, Digital Commons Network, Google Scholar and Cochrane Library from 2000 to 2023, analyzing studies dealing with the relationship between hormetic, cognitive, and reproductive aspects of human aging. Results: The process of hormesis serves as a bridge between the biology of neuron-germ cell interactions on one hand, and the clinical relevance of these interactions on the other. Details concerning these processes are discussed here, emphasizing new research which strengthens the overall concept. Conclusions: This review presents a scientifically and clinically relevant argument, claiming that maintaining a cognitively active lifestyle may decrease age-related degeneration, and improve overall health in aging. This is a totally novel approach which reflects current developments in several relevant aspects of our biology, technology, and society.
1. Introduction and Methods
From a clinical viewpoint, aging is a process which progressively diminishes the function of the person, resulting in chronic degenerative conditions, until this burden becomes incompatible with life. In this respect, one of us (Kyriazis, 2020) has defined aging as: ‘Time-Related Dysfunction’ [1], with the explanation that:
“This definition implies that, with the passage of time and for a variety of causative factors, humans are subjected to damage which is not properly repaired. As a consequence, there is degeneration and loss of utility at all levels (molecular, cellular, tissue, organismic, and societal) with a resulting failure of the normal function of a human. In other words, it is a chronologically-dependent erosion of our functions, which makes it increasingly difficult for us to manage and operate within a given, always-changing environment”.
Therefore, encouraging specific clinical interventions may positively influence time-related dysfunction and diminish the rate of decline. One such intervention is the use of digital technology which, through the neuronal processing of meaningful information, up-regulates the function of our brain, and may prevent age-related degeneration. Details of this process are discussed below.
Our everyday life (at least in developed countries) is progressively becoming less physical and more cognitive [2]. We are exposed to an environment that is becoming less of a combination of several interactive organisms but, increasingly, more of an environment of only two elements: humans and digital machines [3]. This interaction exposes us to an increased cognitive burden, which may affect the way our brain operates. It may redefine the existing energetic balance that exists between neuronal cells and the germline. In this paper, the term ‘neuronal cells’ or ‘neurons’ refers to glial cells, oligodendrocytes, astrocytes, intermediate progenitors, immature neurons, mature neurons, glutamatergic neurons, GABAergic neurons, dopaminergic neurons, cholinergic neurons, and motor neurons. The term ‘germline’ is explained as: “cells, such as spermatozoa, ova or spermatogonial stem cells (or primordial germ cells), which participate in the process of transmission of genetic material to the progeny” [4].
There is a process of both direct and indirect communication between somatic cells (specifically, neurons), and germline cells [5,6,7,8,9]. The immense relevance of the presence of these conserved pathways of communication will become clear further on in this paper. The increasing cognitive/digital nature of our environment is slowly altering the survival relationship between neurons and germline [10]. This relationship is grounded in hormetic notions which explore the effects of ‘stimulation’ (a challenge that disturbs homeostasis) and the biological response to this stimulation. The concept of hormesis and its relationship with neuronal and germline survival is relevant here, and will be discussed below.
In this narrative review we examined publications listed in PubMed, Embase, PLOS, Digital Commons Network, Google Scholar and Cochrane Library from 2000 to 2023 for the terms ‘hormesis’, ‘hormesis in aging’, ‘hormesis in health’, ‘germline survival’, ‘neuronal stress response’, and ‘neuron/germline communication’. Our aim is to examine relevant (albeit sometimes seemingly unrelated) concepts, in one narrative paper describing the complex relationship between cognition, reproduction, and aging.
We used a generative pre-trained transformer (ChatGPT-4) to generate a small amount of original background text, in less than 5% of the paper. The text suggested by GPT-4 has been verified and methodically checked. In addition, GPT-4 has been used in places (Section 2.1, Section 2.4 and Section 2.5), in order to support ideation and concept checking of the manuscript. The feedback originated by ChatGPT-4 was discussed, modified, and verified by two of us (M.K. and T.O.).
2. Results and Discussion
2.1. Hormesis
During the past several years, there have been many attempts to understand the process of hormesis [11]. Hormesis is a phenomenon in which a low dose of a stressor, such as a chemical or radiation, can stimulate a beneficial response in an organism. Several researchers have proposed that hormesis plays a role in the process of aging [12,13,14]. Hormesis is present in many aspects of the biological world where there is ‘activation’ of a function during application of a low-dose stimulus, and ‘inhibition’ if the dose is increased beyond a certain threshold. It is a non-linear, ‘inverted U’-shaped relationship between dose–effect (Figure 1). Therefore, the principle underlying hormesis is relatively simple: Low dose of a stimulus can positively challenge the organism eliciting a controlled stress response, and result in health benefits, whereas an excessive, suboptimal, or prolonged exposure to the same stimulus can result in damage and disease [15,16]. It should be emphasized that several researchers consider hormesis as a controversial subject, because the original research on hormesis and radiation has not been proven. Nevertheless, an increasing number of other experts, study hormesis as a relevant and valuable subject in health and, particularly, in aging.
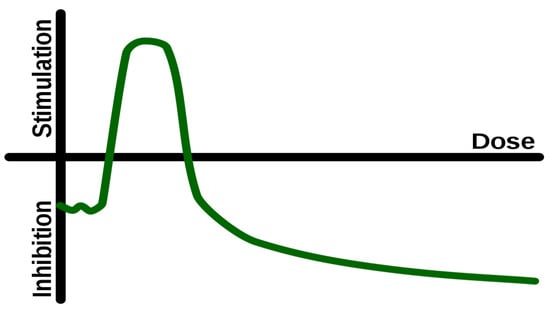
Figure 1.
Hormesis: After an initial inhibition at a very low dose, as the dose increases there is a ‘window’ of stimulation, followed again by inhibition at higher doses.
Single or multiple exposure to low doses of otherwise harmful agents, such as irradiation, food restriction, heat stress, hypergravity, reactive oxygen species, and other challenges, has a variety of anti-aging and longevity-extending effects [17]. Detailed molecular mechanisms that bring about the hormetic effects are still not very clear but are being increasingly understood, and comprise a cascade of stress response and other pathways of maintenance and repair [18].
Although the extent of immediate hormetic effects after exposure to a particular stress may only be moderate, the chain of events following initial hormesis leads to biologically amplified effects that are much larger, synergistic and pleiotropic. A consequence of hormetic amplification is an increase in the homeodynamic space (Box 1) of a living system in terms of increased defense capacity and reduced load of damaged macromolecules [19]. Hormetic strengthening of the homeodynamic space provides wider margins for metabolic fluctuation, stress tolerance, adaptation and survival. Hormesis thus counter-balances the progressive shrinkage of the homeodynamic space, which is the ultimate cause of aging, diseases and death. Healthy aging may be achieved by hormesis through mild and periodic (but not severe or chronic) physical and mental challenges [16], and by the use of nutritional hormesis incorporating mild stress-inducing molecules called hormetins. The increasingly established scientific foundations of hormesis are ready to pave the way for new and effective approaches in aging research and intervention.
Thus, hormetic stimuli can be nutritional (dietary restriction, intermittent fasting), physical (exercise, heat, cold), chemical (nutritional or pharmaceutical compounds, hormetins), and mental (brain exercises, meditation, cognitive ‘positive-stress’). These may cause a slight injury to the organism, i.e., they disturb homeostasis, thus activating stress response pathways which aim to restore homeostasis, and up-regulate repair mechanisms. During the process of repairing this hormetically-induced damage, any coincidental age-related damage may also be repaired [20].
Box 1. What is the homeodynamic space?
- Rattan explains the concept of ‘homeodynamic space’ as encompassing “three characteristics: stress response, damage control, and constant remodeling, which provide measurable biomarkers reflecting the survival ability, robustness, and resilience of a biological system. A biological definition of health thus involves measures of functionality, tolerance and adaptation” [21].
- The homeodynamic space refers to the range of physiological states and processes that an organism can tolerate and maintain without suffering harm or death. This range is determined by the organism’s capacity to adapt to and cope with stress [22].
- The concept of homeodynamic space is related to the concept of homeostasis. However, while homeostasis refers to a narrow range of physiological states that are optimal for survival, homeodynamic space refers to a much broader range of physiological states and processes that an organism can tolerate. It highlights the dynamic nature of physiological systems and the importance of resilience and adaptability in maintaining health and well-being.
Hormesis may, for example, be involved in the process of calorie restriction, which has been shown to extend lifespan in animals [23,24]. Calorie restriction has been proposed to work, in part, by causing mild stress in cells, which may stimulate the production of stress-response proteins and other molecules that have anti-aging effects [25].
The role of hormesis within the context of aging is thought to be very relevant, particularly when we consider neuronal hormetic stimulation. Exposure to new information, most likely derived from digital sources (internet, social media) maintains our neurons in a state of novelty [26], creating positive effects on health. This activation of neuronal stress response follows hormetic exposure (to meaningful ‘information-that-requires-action’) and thus up-regulates neuronal health [27].
Despite the promising evidence for the role of hormesis in aging, it is important to note that the mechanisms by which it may work are not fully understood, and more research is needed to determine the extent to which hormesis is involved in the aging process. It is also important to note that hormesis is a complex phenomenon, and the effects of any given stressor will depend on the specific dose, duration, and timing of exposure, as well as the genetic and environmental background of the organism.
A crude way to establish if clinical hormesis is taking place:
A basic characteristic of a hormetic event is the novelty of information. Hormesis is present when the challenge is of sufficient magnitude and appropriate quality as to satisfy the definition of ‘novelty’. Novelty is defined as ‘the quality of being new, original, or unusual’, and this includes both unfamiliarity and unconventionality. Routine and monotony generally do not invoke a hormetic response. The assessment and response to the new challenge leads to adaptation and thus, eventually, improvement of function within a particular environment (the environment where the challenges have originated from). Therefore, when a certain stimulus appears ‘novel’ to us, then it is likely that this stimulus is eliciting a hormetic response. When the stimulus appears boring or monotonous, it is less likely that hormesis is taking place.
2.2. Autophagy and Hormesis
Autophagy is one of the markers of hormesis, i.e., the presence of efficient autophagy indicates that hormesis is occurring, and enhancing the processes of biological repair. Autophagy is a protein turnover pathway, a catalytic process, which aims to degrade and recycle cellular components. This process maintains cellular function during (or after) stress, when damaged material accumulates and it has to be eliminated [28].
The process of autophagy can be enhanced via hormetic stress, such as:
- Exercise. This can enhance autophagy in liver, muscles, pancreas and adipose tissue, as well as in the brain [29].
- Moderate hot/cold exposure [30] via activation of Heat Shock Proteins (HSP) [31]. Hormetic stress in addition to HSP involvement, also reduces the progressive accumulation of PolyQ aggregates [32].
- Intermittent fasting (IF) is a nutritional hormetic stress. Alirezaei et al. [33] conducted a study to investigate the effects of food restriction and short-term fasting on autophagy. Their findings revealed that food restriction induces autophagy in mouse livers, challenging the conventional belief of the brain’s metabolic privilege. Moreover, their research suggests that sporadic fasting could be a cost-effective approach to promote a therapeutic neuronal response. In a separate study, Pietrocola et al. [34] emphasized the significance of autophagy in cancer treatment. They highlighted that impairment of autophagy reduces the effectiveness of chemotherapy and radiotherapy. These findings underscore the importance of understanding autophagic mechanisms to enhance cancer treatment strategies. Additionally, Kim and Lemasters [35] observed the occurrence of autophagy in liver cells during fasting, providing further insights into its role in cellular recycling. Their study demonstrated that liver cells form phagophores and autophagosomes, which encapsulate and capture mitochondria for recycling. This process leads to the breakdown of mitochondria and their contents, including DNA. In addition to physical stimuli, autophagy can be modulated by hormetins, i.e., substances that can induce health-beneficial physiological hormesis [36] and this is an appropriate opportunity to discuss some more details of hormetins (Table 1).
 Table 1. Examples of hormetins and their main actions.
Table 1. Examples of hormetins and their main actions.
There are specific stress-induced pathways for enhancing autophagy in neurons [52,53], and it is known that the stress response activates autophagy [54,55,56]. This shows the direct relationship that exists between autophagy and stress. It is therefore reasonable to infer that, if this wide range of hormetic stresses improves autophagy, it could well be that other hormetic stresses, such as a cognitive stress may also have similar effects.
2.3. Environmental Enrichment
A concept relevant to hormesis is environmental enrichment (EE). This refers to a varied and stimulating environment that promotes physical and psychological well-being. It involves creating an environment that is stimulating and challenging, both physically and mentally. This can include activities such as exercise, social interaction, and learning new skills, as well as exposure to novel and varied stimuli, such as music, art, and digital information that requires a response [26]. The concept of EE has gained prominence in recent years as increasingly more research has demonstrated its benefits for both animals and humans. It can improve cognitive function, reduce stress, and promote natural behaviors. It also improves mood, reduces stress and anxiety, and increases overall quality of life [57].
Studies have shown that animals raised in enriched environments demonstrate better learning and memory, as well as improved problem-solving abilities [58,59]. This is likely because the complexity of an enriched environment provides greater opportunities for cognitive stimulation and growth. In the case of aging, environmental enrichment is becoming increasingly recognized as an effective tool for promoting healthy aging and, specifically, improving cognition [60].
Cognitive stimulation is another key component of environmental enrichment for older adults. This can include activities such as learning a new language, playing a musical instrument, engaging in brain-training exercises, or other virtual or digitally-derived cognitive activities. These activities can help keep the brain active and engaged, promoting cognitive function and reducing the risk of cognitive decline [61]. The new information reaching the brain acts as a hormetic stimulus or a challenge, that activates the neuronal stress response and requires the brain to act in order to deal with this new challenge, through remodeling and increase robustness [26].
It was shown that an environment which is rich in cognitive stimuli, has indirect effects on tissues and organs other than the brain. For instance, some authors have argued that an enriched environment improves vision [62,63], while others reported the benefits of a cognitively enriched environment on:
- Immunity [64];
- Wound healing [65,66];
- The retina [67];
- Muscle strength, without the need to physically exercise (!) [68];
- Inflammatory response [69,70] and other physical parameters [71], such as vitality, physical functioning and bodily pain, as well as social and emotional functioning [72]. Many of these effects may persist for several years, in some cases even after a 10 year period [73].
2.4. Neuronal Stress Response
The neuronal stress response is the set of molecular and cellular changes that occur in neurons in response to stress or injury [74]. These changes help neurons adapt to, and survive, stress, and they can also have important consequences for the function and health of the nervous system [75]. One of the key factors in the neuronal stress response are the stress-response proteins. These proteins are activated in response to various stressors, including heat, cold, radiation, and certain chemicals, as mentioned above. Once activated, stress-response proteins support neurons overcome stress by modifying their gene expression, protein synthesis, and other cellular processes [76,77], such as ATP generation in times of stress [78] and the modulation of the signaling molecule cyclic AMP [79]. It is important to note that the neuronal stress response is not a uniform process, and different neurons may respond to stress in different ways depending on their specific function and location in the nervous system. Additionally, the response to stress can vary depending on the severity and duration of the stressor, as well as the genetic and environmental background of the organism.
2.5. Digital Information, Cognition, and Neuronal Stress Response
The advent of information technology has brought about a significant rise in the cognitive load imposed on our brains, primarily due to the sheer volume of information we now encounter [80]. The internet and social media platforms offer us access to an overwhelming abundance of information, making it increasingly difficult to sift through and identify what is truly important and relevant. As a result, individuals often struggle to concentrate on productive and meaningful tasks as they grapple with the challenge of filtering out the noise and distractions surrounding them [81].
This can lead to cognitive overload, which can impair cognitive function and lead to feelings of stress and fatigue. Our neurons are subjected to the phenomenon of ‘neuronal fatigue’ and they stop responding to an unchanged, continual monotonic stimulation. Such a stimulation causes the neuron to lose its ability to transmit activation to other neurons [82]. On the other hand, a moderate (in other words, hormetic) amount of information that requires us to act, may impact positively on the brain, up-regulating the neuronal stress response and thus enhancing the robustness of neuronal function [83]. In essence, we are living in an enriched environment, as described above.
We know that digital cognitive training improves cognition and may reduce the risk of dementia [84]. Studies have repeatedly shown that ‘serious games’ have a positive impact on dementia patients [85,86]. ‘Serious games’ are participative digital/electronic games designed for purposes other than entertainment. Specifically, Yang et al. [87] state that there is:
“…evidence that video game interventions could be considered for the elderly for improving performance and cognitive function, especially general cognitive scores and processing speed. Games with better interactivity and visual stimulation have better curative effects…”.
In addition, electronic games used generally for entertainment also have positive effects on the memory of older people [88,89].
By being exposed to a judicious, ever-changing, novel and positive amount of information, it becomes necessary for our neurons to acquire additional repair resources and thus function for longer, with a consequent overall improvement in healthy lifespan. These additional energetic resources are subjected to a trade-off: as a balancing (trade-off) measure, germline repair mechanisms need to be down-regulated to accommodate a corresponding escalation of repairs in neurons [90]. This is because there is a close and very relevant relationship between neurons and germline cells that will be explored in detail below.
2.6. The Bidirectional Communication (Cross-Talk) between Neurons and the Germline
Some years ago, Ermolaeva et al. [91], suggested that genetic injury in germline cells, may act as a stimulus to initiate protective effects in somatic cells. In other words, elements in the germline up-regulate the function of somatic cells. This may happen through up-regulation of the stress resistant mechanisms in such somatic cells. Others have confirmed and elaborated on this [92,93]. Khodakarami et al. [94] have suggested that this germ-initiated somatic protective mechanism reveals a conserved tendency to reverse the trade-offs that exist between germ cell and somatic cell repair.
Data increasingly suggest that there is open communication between the soma (i.e., all cells in the body which are not involved in reproduction—here, specifically the neuron) and the germline [95,96,97]. Information is transferred through ‘cross-talk’ from the soma to the germline (and in reverse), and this information may negatively affect the aging of the germline or the aging of the soma [95,98]. There is an increasing indication to show that the process of resources flowing from the soma to the germline is not unidirectional. It is possible to experience the reverse, whereby resources could move from the germline back to somatic cells, up-regulating their function [99,100]. We also know that there exist carriers of epigenetic information from the soma to the germline including microRNAs or even extracellular vesicles which move from the soma to the germline environment [101,102].
Some examples of neuron-to-germline communication are described below, with a summary in Box 2.
- DNA damage in germ cells increases resilience in somatic cells via the ERK MAP kinase MPK-1 pathway [91]. Furthermore, when somatic cells experience stress, there is an increased demand (by somatic cells) for repair resources, which are diverted from the germline [90].
- Germline cells have the innate capacity to become neurons following suitable natural (or artificial) reprogramming by transcription factors, even though there are several conserved mechanisms that safeguard against this. This is an intriguing situation because it shows the direct relationship between the germline and the brain [103]. In addition, multipotent neural and glial precursors can be derived from multipotent adult germ line stem cells [104]. These multipotent neural precursors are able to mature and integrate within the existing neural network. It is necessary to mention that, although these effects have been found in experiments conducted in vitro, clinical tests in vivo are still lacking. It is, however, remarkable to realize that the germline acts as a source of fully functional neurons [105,106,107].
- More specifically, we know that germline (spermatogonial) stem cells may act as a source of neuron-like cells [108], and definitive neural stem cells [109].
- A more detailed direct communication pathway between germline cells and the soma has been studied by Levi-Feber et al. [110] who showed that this pathway depends on the endoplasmic reticulum stress factor inositol requiring enzyme-1 (IRE-1).
- On certain situations, ectopic germline cells can be found in the brain, and could contribute to altered neuronal development, resulting in neurodevelopmental disorders. This indicates not only the close relationship between neurons and germ cells, but also the continual struggle for equilibrium, between these two [111].
- Furthermore, there is another fact that underlines the close relationship between neurons and germ cells. Progesterone, which modulates sperm function, acts (via intermediate steps) by interacting with “a sperm membrane receptor which resembles the neuronal GABA(A) receptor” in the brain [112].
- It was shown that eradication of germ cells in Drosophila, has a positive impact on its lifespan, possibly through modulation of the nutrient sensing insulin/insulin-like (IIS) growth factor signaling [113]. This strengthens the general argument that that somatic lifespan is under the control of the germ line, and vice versa.
- The repressor element 1-silencing transcription factor REST which modulates multipotent stem cells, is present in testes, but, intriguingly, regulates target genes in neurons [114]. REST activity has been associated with cognitive impairment and dementia, whereas a potent activity of REST is associated with modulating the balance of neuroprotection vs. neurodegeneration—i.e., acts in a hormetic way [115,116]. Thus, there exist a conserved mechanism of modulation of neural development regulated by REST which is present in spermatogonial cells, indicating another possible mechanism of neuro-germline communication.
- It is known that the germline may influence the function of distant somatic cells, including neurons. For instance, germline stem cells influence proteostasis and thus prevent abnormal protein accumulation in neurons [117]. Thus, at least theoretically, the risk of neurodegenerative diseases is reduced.
- Spermidine is a natural polyamine compound with effects on heart disease, brain degeneration, cancer, and inflammation, among others. It also extends lifespan and health span [43], and it modulates autophagy both in the germline and in the neuron [118]. It was originally found in sperm and this begs the question: how and why does a compound in semen benefit the neuron? Spermidine is a mediator of the complex relationship that exists between neurons and the germline. The concentration of spermidine (apart from its high concentration in the sperm) is also high in the human brain. This must be because it has important actions to perform there [119]. It has positive actions on neuronal mitochondria [118], improves autophagy in neurons [120], and in germline stem cells [121], protects against synaptic degeneration [122] and exhibits general neuroprotective actions [123]. There are studies linking consumption of spermidine with a reduced risk of cognitive impairment in humans [124].
Box 2. Summary of some examples of neuron-to-germline communication (References are given in the text description).
- DNA damage in germ cells increases resilience in somatic cells [91].
- Neuronal stress causes an increased demand (by neurons) for repair resources, which are diverted from the germline [90].
- Germline cells have the capacity to become neurons. Neural precursors from the germline are able to mature and integrate within the existing neural network [103,104,105,106,107].
- A direct communication pathway between germline cells and the soma depends on the endoplasmic reticulum stress factor inositol requiring enzyme-1 (IRE-1) [110].
- Ectopic germline cells can be found in the brain, and could contribute to altered neuronal development, resulting in neurodevelopmental disorders [111].
- Progesterone, which modulates sperm function, acts by interacting with a membrane receptor which resembles the neuronal GABA(A) receptor in the brain [112].
- Eradication of germ cells in Drosophila, has a positive impact on its lifespan, possibly through modulation of the nutrient sensing insulin/insulin-like (IIS) growth factor signaling [113].
- The repressor element 1-silencing transcription factor (REST) which modulates multipotent stem cells, is present in both neonatal and adult testes, and regulates target genes in neurons [114].
- There is a conserved mechanism of modulation of neural development regulated by REST which is present in spermatogonial cells [115,116].
- The germline may influence the function of distant somatic cells, including neurons. For instance, germline stem cells influence proteostasis and thus control abnormal protein accumulation in neurons [117].
- We mention the example of the hormetin spermidine, which modulates autophagy both in the germline and in the neuron [43,118,119,120,121,122,123,124].
2.7. Neurons vs. Germline
There is a balance between allocation of resources to the different organs in any given organism. These resources could be allocated for damage repair or for growth of the organism [125]. Specifically, at the current stage of human evolution, nature has a propensity to favor allocation of repair resources to the germline [126], in order to assure the survival of the species, even if this means that allocation of resources to other organs (including the brain) will have to be suboptimal [127,128].
Some years ago, we proposed the Indispensable Soma Hypothesis (www.indispensablesoma.info accessed on 28 June 2023). We suggested that there is a direct competition for survival between neurons (cognition) and the germline (reproduction) [129], where neurons try to survive and function well by diverting repair resources from the germline. This means that a healthy neuron may live longer (and therefore we too live longer), when at the same time, the germ cells remain without adequate repairs, become defective and this results in a reduced reproduction. It may be possible to manipulate this relationship through hormetically increasing the function of the neurons, and thus be able to reduce age-related degeneration [130].
The suggestion that energetic trade-offs exist between organs that are costly to repair and others that are less costly, has been made several years ago, under the term ‘The Expensive Tissue Hypothesis’ [131]. Owing to the fact that repair resources are finite, there are preferred energy investments in organs that are evolutionarily ‘important’ followed by reduced investments in other organs [132]. Several studies have supported this general principle [133,134,135].
Here, the general meaning of the term ‘trade-off’ is taken to be: improvement in one aspect tends to be counterbalanced by deterioration in another aspect.
As an extension of this general hypothesis, a more specific hypothesis has been suggested: ‘The Expensive Germline Hypothesis’ [136]. Evidence supports the view that germline maintenance is costly (the expensive germline hypothesis) and that there are direct trade-offs with somatic maintenance [90]. This is why time-related degenerative damage to all organs is not repaired properly (and we have loss of function, age-related degeneration and death), whereas damage to the germline is as optimal as it could be [137].
However, we are now witnessing a general shift from this situation. Due to the fact that there is so much useful, relevant and actionable information reaching our brains (via sharing of digital information), we are witnessing, for the first time in human history, a shifting of priorities: from the germline, to the neuron (from the survival of the species, to the survival of the individual) [138]. By redirecting resources from the germline, neurons are able to maintain their structural and functional integrity over a long period of time. This process is thought to involve the up-regulation of certain genes and pathways that are involved in the maintenance and repair of neurons, as well as the down-regulation of genes and pathways that are involved in the production of gametes.
It is important to note that this process of resource redirection is not unique to neurons, and many other types of cells are also able to redirect resources from the germline in order to maintain their structural and functional integrity. However, the specialized nature of neurons and their long lifespan make them particularly dependent on this process.
3. Conclusions
In this paper, we essentially make the first steps in describing a new biology. A biology not based on reproduction and aging, but based on cognition and indefinite survival without chronic degenerative diseases. It represents a shift from a physical model of aging to a more cognitive one.
Specifically, it appears increasingly relevant that there is a connection between brain function and longevity. This is a complex process, influenced by a large number of factors. Germline elements may transmit somatic factors to somatic cells in order to increase somatic function. The success of this process depends on hormetic constraints (“too little is bad, too much is also bad”).
Our current enriched environment depends less on physical abilities, and more on cognitive ones. The continual exposure to new, meaningful, digitally-derived information that requires us to act, has typical hormetic characteristics, whereby neurons are challenged by this input of information, and need to adapt in order to process it. This process of adaptation takes place at the expense of resources allocated to the germline, which are diverted to the neuron, in order to execute successfully the neuronal stress response. The balance of repair resources shifts from the germline to the neuron, resulting in reduced resources for reproduction but increased somatic (neuronal) repairs, leading to reductions in age-related degeneration, longer, healthier lifespans and reduced reproduction rates, just as we increasingly see in developed, technological societies. A schematic representation of these general concepts is given in Figure 2.
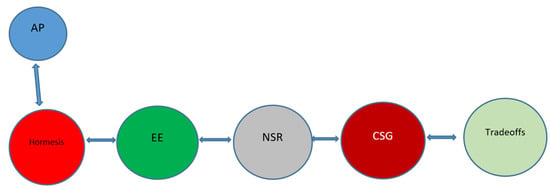
Figure 2.
Graphical summary of the subjects discussed. The logical progression of the discussion of these, perhaps seemingly unrelated subjects is schematically depicted below. The discussion starts from the concept of hormesis (and the example of autophagy (AP), and then progresses to the subjects of environmental enrichment (EE), neuronal stress response (NSR), the communication between somatic and germline cells (CSG), and the war of trade-offs between the neuron and the germline. The result may be an improvement of the clinical parameters of the aging patient.
Based on the discussion above, there is one important piece of clinical advice we can give to the public: We should intentionally expose ourselves to meaningful and novel digitally-derived information, information that requires us to act constructively and creatively, in other words, maintain our brain in a state of hormetic ‘positive stress’. Hormesis is an important concept here. There is solid recent research describing the role of hormesis in health [139,140,141,142,143,144,145,146] and also studies concentrating specifically on hormesis in aging—in addition to those studies already mentioned above [147,148,149,150,151,152,153].
An effort should be made to use digital technology as a means of enhancing our cognitive abilities, which will eventually reflect on a reduction in age-related degenerative diseases, through diversion of repair resources from the germline to somatic cells, and particularly, to the neuron.
Author Contributions
M.K. devised the concept and prepared the review; L.S. and T.O. critically reviewed the paper and provided, insights, extensive comments, and corrections. In addition, T.O. operated the AI platform (ChatGPT) as described above. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The paper contains a small amount of text derived from GPT-4 (https://chat.openai.com/chat (accessed on 28 June 2023)) which automatically generated original background text. The text suggested by GPT4 has been humanly verified and meticulously checked. In addition, GPT-4 has been used in places in order to support ideation and concept checking of the manuscript. No other content generated by generative pre-trained transformers or large language model (LLM) tools was included in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kyriazis, M. Aging as “Time-Related Dysfunction”: A Perspective. Front. Med. 2020, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Clinical Effects of a ‘Human-Computer’ Interaction. 21 June 2016. Available online: https://ssrn.com/abstract=2798529 (accessed on 20 June 2023).
- Digital Watch. Available online: https://dig.watch/trends/digital-and-environment (accessed on 20 June 2023).
- Germline: National Human Genome Research Institute. Available online: https://www.genome.gov/genetics-glossary/germ-line (accessed on 20 June 2023).
- Gaddy, M.A.; Kuang, S.; Alfhili, M.A.; Lee, M.H. The soma-germline communication: Implications for somatic and reproductive aging. BMB Rep. 2021, 54, 253–259. [Google Scholar] [CrossRef]
- Sharma, A. Transgenerational epigenetics: Integrating soma to germline communication with gametic inheritance. Mech. Ageing Dev. 2017, 163, 15–22. [Google Scholar] [CrossRef]
- Qi, W.; Xu, F.; Heimbucher, T.; Baumeister, R. Protection of germline immortality by the soma via a secreted endoribonuclease. BioEssays 2021, 43, 2100195. [Google Scholar] [CrossRef] [PubMed]
- Conine, C.C.; Rando, O.J. Soma-to-germline RNA communication. Nat. Rev. Genet. 2022, 23, 73–88. [Google Scholar] [CrossRef]
- Levi-Ferber, M.; Shalash, R.; Le-Thomas, A.; Salzberg, Y.; Shurgi, M.; Benichou, J.I.; Ashkenazi, A.; Henis-Korenblit, S. Neuronal regulated ire-1-dependent mRNA decay controls germline differentiation in Caenorhabditis elegans. Elife 2021, 10, e65644. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Biological ageing and clinical consequences of modern technology. Biogerontology 2017, 18, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020, 64, 101142. [Google Scholar] [CrossRef]
- Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C.; Traverso, N. Hormesis and Oxidative Distress: Pathophysiology of Reactive Oxygen Species and the Open Question of Antioxidant Modulation and Supplementation. Antioxidants 2022, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, T.; Sun, Z. Hormesis in Health and Chronic Diseases. Trends Endocrinol. Metab. 2019, 30, 944–958. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly Generalizable and Beyond Laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef]
- Pande, S.; Raisuddin, S. The Underexplored Dimensions of Nutritional Hormesis. Curr. Nutr. Rep. 2022, 11, 386–394. [Google Scholar] [CrossRef]
- Demirovic, D.; Rattan, S.I. Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Exp. Gerontol. 2013, 48, 94–98. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. How does hormesis impact biology, toxicology, and medicine? Aging. Mech. Dis. 2017, 3, 13. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Biological Health and Homeodynamic Space. In Explaining Health Across the Sciences; Healthy Ageing and Longevity; Sholl, J., Rattan, S.I., Eds.; Springer: Cham, Switzerland, 2020; Volume 12. [Google Scholar]
- Rattan, S.I. Aging is not a disease: Implications for intervention. Aging Dis. 2014, 5, 196–202. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Solanki, P.; Singh, P. Oxidative stress, antioxidants, hormesis and calorie restriction: The current perspective in the biology of aging. Arch. Gerontol. Geriatr. 2021, 95, 104413. [Google Scholar] [CrossRef]
- Toussaint, O.; Remacle, J.; Dierick, J.F.; Pascal, T.; Frippiat, C.; Royer, V.; Chainiaux, F. Approach of evolutionary theories of ageing, stress, senescence-like phenotypes, calorie restriction and hormesis from the view point of far-from-equilibrium thermodynamics. Mech. Ageing Dev. 2002, 123, 937–946. [Google Scholar] [CrossRef]
- Testa, G.; Biasi, F.; Poli, G.; Chiarpotto, E. Calorie restriction and dietary restriction mimetics: A strategy for improving healthy aging and longevity. Curr Pharm Des. 2014, 20, 2950–2977. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Challenging Aging: The Anti-Senescence Effects of Hormesis, Environmental Enrichment, and Information Exposure; Frontiers in Aging Sciences; Bentham Science Publishers: Sharjah, United Arab Emirates, 2016; Volume 1, ISSN 2468-5933. [Google Scholar]
- Kyriazis, M. Systems neuroscience in focus: From the human brain to the global brain? Front. Syst. Neurosci. 2015, 9, 7. [Google Scholar] [CrossRef][Green Version]
- Barbosa, M.C.; Grosso, R.A.; Fader, C.M. Hallmarks of Aging: An Autophagic Perspective. Front. Endocrinol. 2019, 9, 790. [Google Scholar] [CrossRef]
- He, C.; Sumpter, R.; Levine, B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 2012, 8, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, M.; Bi, Y.; Zhu, H.; Zhou, Z. Autophagy and Apoptosis act as partners to induce germ cell death after heat stress in mice. PLoS ONE 2012, 7, e41412. [Google Scholar] [CrossRef]
- Penke, B.; Bogár, F.; Crul, T.; Sántha, M.; Tóth, M.E.; Vígh, L. Heat Shock Proteins and Autophagy Pathways in Neuroprotection: From Molecular Bases to Pharmacological Interventions. Int. J. Mol. Sci. 2018, 19, 325. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Alirezaei, M.; Kemball, C.C.; Flynn, C.T.; Wood, M.R.; Whitton, J.; Kiosses, W.B. Short-term fasting induces profound neuronal autophagy. Autophagy 2010, 6, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Pol, J.; Vacchelli, E.; Rao, S.; Enot, D.P.; Baracco, E.E.; Levesque, S.; Castoldi, F.; Jacquelot, N.; Yamazaki, T.; et al. Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 2016, 30, 147–160. [Google Scholar] [CrossRef]
- Kim, I.; Lemasters, J.J. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am. J. Physiol. Cell Physiol. 2011, 300, C308–C317. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Hormetins as drugs for healthy aging. In Anti-Aging Drugs: From Basic Research to Clinical Practice; Vaiserman, M., Ed.; The Royal Society of Chemistry: London, UK, 2017; pp. 170–180. [Google Scholar]
- Arabit, J.G.J.; Elhaj, R.; Schriner, S.E.; Sevrioukov, E.A.; Jafari, M. Rhodiola rosea Improves Lifespan, Locomotion, and Neurodegeneration in a Drosophila melanogaster Model of Huntington’s Disease. Biomed. Res. Int. 2018, 2018, 6726874. [Google Scholar] [CrossRef]
- Liu, Z.; Li, X.; Simoneau, A.R.; Jafari, M.; Zi, X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol. Carcinog. 2012, 51, 257–267. [Google Scholar] [CrossRef]
- Yu, C.; Yu, C.; Jing, S.; Li, H.; Jiang, E.; Ju, W.; Chen, J. Effects of Schisandra total lignin on autophagy and apoptosis of mouse brain aging induced by D-galactose. J. Jilin Univ. (Med. Ed.) 2014, 40, 1210–1215. [Google Scholar]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Aβ1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yi, H.K. Schisandrin C enhances mitochondrial biogenesis and autophagy in C2C12 skeletal muscle cells: Potential involvement of anti-oxidative mechanisms. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 197–206. [Google Scholar] [CrossRef]
- Sun, J.; Jing, S.; Jiang, R.; Wang, C.; Zhang, C.; Chen, J.; Li, H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by d-galactose. Clin. Interv. Aging 2018, 13, 829–841. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, S.J.; Carmona-Gutierrez, D.; Gupta, V.K.; Bhukel, A.; Mertel, S.; Eisenberg, T.; Madeo, F. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 2014, 10, 178–179. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X.; et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic Caffeine Treatment Protects Against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front. Neurosci. 2018, 12, 301. [Google Scholar] [CrossRef]
- Hung, J.Y.; Hsu, Y.L.; Li, C.; Ko, Y.C.; Ni, W.C.; Huang, M.S.; Kuo, P.L. 6-Shogaol, an active constituent of dietary ginger, induces autophagy by inhibiting the AKT/mTOR pathway in human non-small cell lung cancer A549 cells. J. Agric. Food Chem. 2009, 57, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Chiang, B.H. 6-shogaol induces autophagic cell death then triggered apoptosis in colorectal adenocarcinoma HT-29 cells. Biomed. Pharmacother. 2017, 93, 208–217. [Google Scholar] [CrossRef]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Li, X.H.; Zeng, X.C.; Li, J.; Zhou, J.; Xiao, B.; Hu, K. Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can. J. Physiol. Pharmacol. 2017, 95, 501–509. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Plant Polyphenols for Aging Health: Implication from Their Autophagy Modulating Properties in Age-Associated Diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019, 35, 477–500. [Google Scholar] [CrossRef]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct Target Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Martinelli, S.; Anderzhanova, E.A.; Bajaj, T.; Wiechmann, S.; Dethloff, F.; Weckmann, K.; Heinz, D.; Ebert, T.; Hartmann, J.; Geiger, T.M.; et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat. Commun. 2021, 12, 4643. [Google Scholar] [CrossRef]
- Peker, N.; Gozuacik, D. Autophagy as a Cellular Stress Response Mechanism in the Nervous System. J. Mol. Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef]
- Cappucci, U.; Noro, F.; Casale, A.; Pimpinell, S. The Hsp70 chaperone is a major player in stress-induced transposable element activation. Proc. Natl. Acad. Sci. USA 2019, 116, 17943–17950. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.; Woo, C. Environmental Enrichment and Successful Aging. Front. Behav. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Colavitta, M.F.; Grasso, L.; Barrantes, F.J. Environmental Enrichment in Murine Models and Its Translation to Human Factors Improving Conditions in Alzheimer Disease. J. Prev. Alzheimers Dis. 2023, 10, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Birch, A.M.; Kelly, Á.M. Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology 2019, 145, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Balietti, M.; Conti, F. Environmental enrichment and the aging brain: Is it time for standardization? Neurosci. Biobehav. Rev. 2022, 139, 104728. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Haase, M.; Best, L.; Groth, M.; Lindner, J.; Witte, O.W.; Kaleta, C.; Frahm, C. Restoring Age-Related Cognitive Decline through Environmental Enrichment: A Transcriptomic Approach. Cells 2022, 11, 3864. [Google Scholar] [CrossRef]
- Barone, I.; Novelli, E.; Strettoi, E. Long-term preservation of cone photoreceptors and visual acuity in rd10 mutant mice exposed to continuous environmental enrichment. Mol. Vis. 2014, 20, 1545–1556. [Google Scholar]
- Levine, J.N.; Chen, H.; Gu, Y.; Cang, J. Environmental Enrichment Rescues Binocular Matching of Orientation Preference in the Mouse Visual Cortex. J. Neurosci. 2017, 37, 5822–5833. [Google Scholar] [CrossRef]
- Gurfein, B.T.; Davidenko, O.; Premenko-Lanier, M.; Milush, J.M.; Acree, M.; Dallman, M.F.; Touma, C.; Palme, R.; York, V.A.; Fromentin, G.; et al. Environmental enrichment alters splenic immune cell and enhances secondary influenza vaccine responses in mice. Mol. Med. 2014, 20, 179–190. [Google Scholar] [CrossRef]
- Vitalo, A.G.; Gorantla, S.; Fricchione, J.G.; Scichilone, J.M.; Camacho, J.; Niemi, S.M.; Denninger, J.W.; Benson, H.; Yarmush, M.L.; Levine, J.B. Environmental enrichment with nesting material accelerates wound healing in isolation-reared rats. Behav. Brain Res. 2012, 226, 606–612. [Google Scholar] [CrossRef]
- Bice, B.D.; Stephens, M.R.; Georges, S.J.; Venancio, A.R.; Bermant, P.C.; Warncke, A.V.; Affolter, K.E.; Hidalgo, J.R.; Angus-Hill, M.L. Environmental Enrichment Induces Pericyte and IgA-Dependent Wound Repair and Lifespan Extension in a Colon Tumor Model. Cell Rep. 2017, 19, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.; Aranda, M.L.; González Fleitas, M.F.; Chianelli, M.S.; Fernandez, D.C.; Sande, P.; Rosenstein, R.E. Environmental enrichment protects the retina from early diabetic damage in adult rats. PLoS ONE 2014, 9, e101829. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Mahato, N.K.; Nakazawa, M.; Law, T.D.; Thomas, J.S. The power of the mind: The cortex as a critical determinant of muscle strength/weakness. J. Neurophysiol. 2014, 112, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, K.; Shen, X. Environmental enrichment attenuated sevoflurane-induced neurotoxicity through the PPAR-γ signaling pathway. Biomed. Res. Int. 2015, 2015, 107149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scarola, S.; Bardi, M. Environmental enrichment modulates inflammation during development in long-evans rats (Rattus norvegicus). Dev. Psychobiol. 2021, 63, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Matur, E.; Akyaz, İ.; Eraslan, E.; Ergul Ekiz, E.; Eseceli, H.; Keten, M.; Metiner, K.; Aktaran Bala, D. The effects of environmental enrichment and transport stress on the weights of lymphoid organs, cell-mediated immune response, heterophil functions and antibody production in laying hens. Anim. Sci. J. 2016, 87, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, F.; Unverzagt, F.W.; Smith, D.M.; Jones, R.; Wright, E.; Tennstedt, S.L. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J. Gerontol. B 2006, 61, S281–S287. [Google Scholar] [CrossRef]
- Rebok, G.W.; Ball, K.; Guey, L.T.; Jones, R.N.; Kim, H.Y.; King, J.W.; Marsiske, M.; Morris, J.N.; Tennstedt, S.L.; Unverzagt, F.W.; et al. ACTIVE Study Group. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014, 62, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M.; Karst, H.; Sarabdjitsingh, R.A. The stressed brain of humans and rodents. Acta Physiol. 2018, 223, e13066. [Google Scholar] [CrossRef]
- Korneeva, N.L. Integrated Stress Response in Neuronal Pathology and in Health. Biochemistry 2022, 87 (Suppl. S1), S111–S127. [Google Scholar] [CrossRef]
- Farley, M.M.; Watkins, T.A. Intrinsic Neuronal Stress Response Pathways in Injury and Disease. Annu. Rev. Pathol. 2018, 13, 93–116. [Google Scholar] [CrossRef]
- Kim, K.W.; Jin, Y. Neuronal responses to stress and injury in C. elegans. FEBS Lett. 2015, 589, 1644–1652. [Google Scholar] [CrossRef]
- Schulz, A.; Sekine, Y.; Oyeyemi, M.J.; Abrams, A.J.; Basavaraju, M.; Han, S.M.; Groth, M.; Morrison, H.; Strittmatter, S.M.; Hammarlund, M. The stress-responsive gene GDPGP1/mcp-1 regulates neuronal glycogen metabolism and survival. J. Cell Biol. 2020, 219, e201807127. [Google Scholar] [CrossRef] [PubMed]
- Freeland, K.; Boxer, L.M.; Latchman, D.S. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res. Mol. Brain Res. 2001, 92, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Korte, M. The impact of the digital revolution on human brain and behavior: Where do we stand? Dialogues Clin. Neurosci. 2020, 22, 101–111. [Google Scholar] [CrossRef]
- Loh, K.K.; Kanai, R. How Has the Internet Reshaped Human Cognition? Neuroscientist 2016, 22, 506–520. [Google Scholar] [CrossRef]
- Menghini, F.; van Rijsbergen, N.; Treves, A. Modelling adaptation aftereffects in associative memory. Neurocomputing 2007, 70, 2000–2004. [Google Scholar] [CrossRef]
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain health consequences of digital technology use. Dialogues Clin. Neurosci. 2020, 22, 179–187. [Google Scholar] [CrossRef]
- Advanced Cognitive Training in Vital Elderly—ACTIVE—Study. IU School of Medicine. 16 November 2017. Available online: https://medicine.iu.edu/news/2017/11/brain-exercise-dementia-prevention (accessed on 2 June 2023).
- Abd-Alrazaq, A.; Abuelezz, I.; AlSaad, R.; Al-Jafar, E.; Ahmed, A.; Aziz, S.; Nashwan, A.; Sheikh, J. Serious Games for Learning Among Older Adults with Cognitive Impairment: Systematic Review and Meta-analysis. J. Med. Internet Res. 2023, 25, e43607. [Google Scholar] [CrossRef]
- Abd-Alrazaq, A.; Alhuwail, D.; Ahmed, A.; Househ, M. Effectiveness of Serious Games for Improving Executive Functions Among Older Adults with Cognitive Impairment: Systematic Review and Meta-analysis. JMIR Serious Games 2022, 10, e36123. [Google Scholar] [CrossRef]
- Yang, C.; Han, X.; Jin, M.; Xu, J.; Wang, Y.; Zhang, Y.; Xu, C.; Zhang, Y.; Jin, E.; Piao, C. The Effect of Video Game-Based Interventions on Performance and Cognitive Function in Older Adults: Bayesian Network Meta-analysis. JMIR Serious Games 2021, 9, e27058. [Google Scholar] [CrossRef] [PubMed]
- Clemenson, G.D.; Stark, S.M.; Rutledge, S.M.; Stark, C.E.L. Enriching hippocampal memory function in older adults through video games. Behav. Brain Res. 2020, 390, 112667. [Google Scholar] [CrossRef]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T. Efficacy of interactive video gaming in older adults with memory complaints: A cluster-randomized exercise intervention. PLoS ONE 2021, 16, e0252016. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jolly, C.; Bublys, K.; Immler, S. Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. Proc. Natl. Acad. Sci. USA 2020, 117, 8973–8979. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.A.; Segref, A.; Dakhovnik, A. DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 2013, 501, 416–420. [Google Scholar] [CrossRef]
- Shemesh, N.; Shai, N.; Ben-Zvi, A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell 2013, 12, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.; Schumacher, B. The innate immune system as mediator of systemic DNA damage responses. Commun. Integr. Biol. 2013, 6, e26926. [Google Scholar] [CrossRef]
- Khodakarami, A.; Saez, I.; Mels, J.; Vilchez, D. Mediation of organismal aging and somatic proteostasis by the germline. Front. Mol. Biosci. 2015, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, P.; Metcalfe, N.B. The deteriorating soma and the indispensable germline: Gamete senescence and offspring fitness. Proc. Biol. Sci. 2019, 286, 20192187. [Google Scholar] [CrossRef]
- Avise, J.C. The evolutionary biology of aging, sexual reproduction and DNA repair. Evolution 1993, 47, 1293–1301. [Google Scholar] [CrossRef]
- Gracida, X.; Eckmann, C.R. Fertility and germline stem cell maintenance under different diets requires nhr-114/HNF4 in C. elegans. Curr. Biol. 2013, 23, 607–613. [Google Scholar] [CrossRef]
- Heininger, K. Aging is a deprivation syndrome driven by a germ-soma conflict. Ageing Res. Rev. 2002, 1, 481–536. [Google Scholar] [CrossRef]
- Douglas, P.M.; Dillin, A. The disposable soma theory of aging in reverse. Cell Res. 2014, 24, 7–8. [Google Scholar] [CrossRef]
- Qian, Y.; Ng, C.L.; Schulz, C. CSN maintains the germline cellular microenvironment and controls the level of stem cell genes via distinct CRLs in testes of Drosophila melanogaster. Dev. Biol. 2015, 398, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U. Paternal contributions to offspring health: Role of sperm small RNAs in intergenerational transmission of epigenetic information. Front. Cell Dev. Biol. 2019, 7, 215. [Google Scholar] [CrossRef]
- Eaton, S.A.; Jayasooriah, N.; Buckland, M.E.; Martin, D.I.K.; Cropley, J.E.; Suter, C.M. Roll over Weismann: Extracellular vesicles in the transgenerational transmission of environmental effects. Epigenomics 2015, 7, 1165–1171. [Google Scholar] [CrossRef]
- Marchal, I.; Tursun, B. Induced Neurons from Germ Cells in Caenorhabditis elegans. Front. Neurosci. 2021, 15, 771687. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Opitz, T.; Kischlat, T. Adult germ line stem cells as a source of functional neurons and glia. Stem Cells 2008, 26, 2434–2443. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.A.; Kim, K.J. Effects of paracrine factors on CD24 expression and neural differentiation of male germline stem cells. Int. J. Mol. Med. 2015, 36, 255–262. [Google Scholar] [CrossRef]
- Streckfuss-Bömeke, K.; Vlasov, A.; Hülsmann, S. Generation of functional neurons and glia from multipotent adult mouse germ-line stem cells. Stem Cell Res. 2009, 2, 139–154. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Hai, Y. Efficient Conversion of Spermatogonial Stem Cells to Phenotypic and Functional Dopaminergic Neurons via the PI3K/Akt and P21/Smurf2/Nolz1 Pathway. Mol. Neurobiol. 2015, 52, 1654–1669. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Zhang, Y.; Li, B.; Xu, Q.; Song, C. Isolation and Culture of Pig Spermatogonial Stem Cells and Their in Vitro Differentiation into Neuron-Like Cells and Adipocytes. Int. J. Mol. Sci. 2015, 16, 26333–26346. [Google Scholar] [CrossRef]
- Teichert, A.M.; Pereira, S.; Coles, B. The neural stem cell lineage reveals novel relationships among spermatogonial germ stem cells and other pluripotent stem cells. Stem Cells Dev. 2014, 23, 767–778. [Google Scholar] [CrossRef]
- Levi-Ferber, M.; Salzberg, Y.; Safra, M.; Haviv-Chesner, A.; Bülow, H.E.; Henis-Korenblit, S. It’s all in your mind: Determining germ cell fate by neuronal IRE-1 in C. elegans. PLoS Genet. 2014, 10, e1004747. [Google Scholar] [CrossRef]
- Bonefas, K.M.; Iwase, S. Soma-to-germline transformation in chromatin-linked neurodevelopmental disorders? FEBS J. 2022, 289, 2301–2317. [Google Scholar] [CrossRef]
- Calogero, A.E.; Burrello, N.; Barone, N.; Palermo, I.; Grasso, U.; D’Agata, R. Effects of progesterone on sperm function: Mechanisms of action. Hum. Reprod. 2000, 15 (Suppl. S1), 28–45. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T.; Min, K.J.; D’Alterio, C.; Villa-Cuesta, E.; Cumbers, J.; Lehmann, R.; Jones, D.L.; Tatar, M. Drosophila germ-line modulation of insulin signaling and lifespan. Proc. Natl. Acad. Sci. USA 2008, 105, 6368–6373. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Inoue, Y.U.; Kikkawa, T.; Tatehana, M.; Morimoto, Y.; Inada, H.; Oki, S.; Inoue, T.; Osumi, N. Detection of REST expression in the testis using epitope-tag knock-in mice generated by genome editing. Dev. Dyn. 2022, 251, 525–535. [Google Scholar] [CrossRef]
- Cheng, Y.; Yin, Y.; Zhang, A.; Bernstein, A.M.; Kawaguchi, R.; Gao, K.; Potter, K.; Gilbert, H.Y.; Ao, Y.; Ou, J.; et al. Transcription factor network analysis identifies REST/NRSF as an intrinsic regulator of CNS regeneration in mice. Nat. Commun. 2022, 13, 4418. [Google Scholar] [CrossRef]
- Zullo, J.M.; Drake, D.; Aron, L.; O’Hern, P.; Dhamne, S.C.; Davidsohn, N.; Mao, C.A.; Klein, W.H.; Rotenberg, A.; Bennett, D.A.; et al. Regulation of lifespan by neural excitation and REST. Nature 2019, 574, 359–364. [Google Scholar] [CrossRef]
- Calculli, G.; Lee, H.J.; Shen, K.; Pham, U.; Herholz, M.; Trifunovic, A.; Dillin, A.; Vilchez, D. Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans. Sci. Adv. 2021, 7, eabg3012. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.H.; Yan, J.L.; Wang, Q.J.; Chen, H.C.; Ma, X.Z.; Yin, J.; Gao, L.P. Spermidine ameliorates the neuronal aging by improving the mitochondrial function in vitro. Exp. Gerontol. 2018, 108, 77–86. [Google Scholar] [CrossRef]
- Ghosh, I.; Sankhe, R.; Mudgal, J.; Arora, D.; Nampoothiri, M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides 2020, 83, 102083. [Google Scholar] [CrossRef]
- Yang, N.; Liu, X.; Niu, X.; Wang, X.; Jiang, R.; Yuan, N.; Wang, J.; Zhang, C.; Lim, K.L.; Lu, L. Activation of Autophagy Ameliorates Age-Related Neurogenesis Decline and Neurodysfunction in Adult Mice. Stem Cell Rev. Rep. 2022, 18, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tian, G.G.; Pei, X.; Hu, X.; Wu, J. Spermidine induces cytoprotective autophagy of female germline stem cells in vitro and ameliorates aging caused by oxidative stress through upregulated sequestosome-1/p62 expression. Cell Biosci. 2021, 11, 107. [Google Scholar] [CrossRef]
- Bhukel, A.; Madeo, F.; Sigrist, S. Spermidine boosts autophagy to protect against synapse ageing. Autophagy 2017, 13, 444–445. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Deshmukh, R. Neuroprotective potential of spermidine against rotenone induced Parkinson’s disease in rats. Neurochem. Int. 2018, 116, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Müller, M.; et al. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef]
- Babajanyan, S.G.; Koonin, E.V.; Allahverdyan, A.E. Thermodynamic selection: Mechanisms and scenarios. New J. Phys. 2022, 24, 053006. [Google Scholar] [CrossRef] [PubMed]
- Teulière, J.; Bhattacharya, D.; Bapteste, E. Ancestral germen/soma distinction in microbes: Expanding the disposable soma theory of aging to all unicellular lineages. Ageing Res. Rev. 2020, 60, 101064. [Google Scholar] [CrossRef]
- Lorenzini, A.; Stamato, T.; Sell, C. The disposable soma theory revisited: Time as a resource in the theories of aging. Cell Cycle 2011, 10, 3853–3856. [Google Scholar] [CrossRef][Green Version]
- Drenos, F.; Kirkwood, T.B. Modelling the disposable soma theory of ageing. Mech. Ageing Dev. 2005, 126, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Neurons vs. Germline: A War of Hormetic Tradeoffs. Curr. Aging Sci. 2017, 10, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, M. Reversal of informational entropy and the acquisition of germ-like immortality by somatic cells. Curr. Aging Sci. 2014, 7, 9–16. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P. The expensive-tissue hypothesis. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Roff, D.A.; Mostowy, S.; Fairbairn, D.J. The evolution of trade-offs: Testing predictions on response to selection and environmental variation. Evolution 2002, c56, 84–95. [Google Scholar]
- Lesch, R.; Kotrschal, K.; Kitchener, A.C.; Fitch, W.T.; Kotrschal, A. The expensive-tissue hypothesis may help explain brain-size reduction during domestication. Commun. Integr. Biol. 2022, 15, 190–192. [Google Scholar] [CrossRef]
- Huang, C.H.; Yu, X.; Liao, W.B. The Expensive-Tissue Hypothesis in Vertebrates: Gut Microbiota Effect, a Review. Int. J. Mol. Sci. 2018, 19, 1792. [Google Scholar] [CrossRef]
- Nengovhela, A.; Ivy, C.M.; Scott, G.; Denys, C.; Taylor, P.J. Counter-gradient variation and the expensive tissue hypothesis explain parallel brain size reductions at high elevation in cricetid and murid rodents. Sci. Rep. 2023, 13, 5617. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Immler, S. The Expensive Germline and the Evolution of Ageing. Curr. Biol. 2016, 26, R577–R586. [Google Scholar] [CrossRef]
- Teplyuk, N.M. Near-to-perfect homeostasis: Examples of universal aging rule which germline evades. J. Cell Biochem. 2012, 113, 388–396. [Google Scholar] [CrossRef]
- Kyriazis, M. The Indispensable Soma Hypothesis. Available online: www.indispensablesoma.info (accessed on 5 August 2023).
- Calabrese, E.J.; Calabrese, V. Enhancing health span: Muscle stem cells and hormesis. Biogerontology 2022, 23, 151–167. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis Mediates Acquired Resilience: Using Plant-Derived Chemicals to Enhance Health. Annu. Rev. Food Sci. Technol. 2021, 12, 355–381. [Google Scholar] [CrossRef]
- Patrick, R.P.; Johnson, T.L. Sauna use as a lifestyle practice to extend healthspan. Exp. Gerontol. 2021, 154, 111509. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free. Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef]
- Cook, R.; Calabrese, E.J. The importance of hormesis to public health. Cien. Saude. Colet. 2007, 12, 955–963. [Google Scholar] [CrossRef][Green Version]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008, 7, 43–48. [Google Scholar] [CrossRef]
- Bukowski, J.A.; Lewis, R.J. Hormesis and health: A little of what you fancy may be good for you. South Med. J. 2000, 93, 371–374. [Google Scholar] [CrossRef]
- Poumadère, M. Hormesis: Public health policy, organizational safety and risk communication. Hum. Exp. Toxicol. 2003, 22, 39–41; discussion 43–49. [Google Scholar] [CrossRef]
- Rattan, S.I. Ageing, gerontogenes, and hormesis. Indian J. Exp. Biol. 2000, 38, 1–5. [Google Scholar]
- Forcina, L.; Franceschi, C.; Musarò, A. The hormetic and hermetic role of IL-6. Ageing Res. Rev. 2022, 80, 101697. [Google Scholar] [CrossRef]
- Lajqi, T.; Stojiljkovic, M.; Wetzker, R. Toxin-induced hormesis may restrain aging. Biogerontology 2019, 20, 571–581. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.H.; Wu, C.; Peng, H.H.; Hwang, T.L.; Ko, Y.F.; Young, J.D.; Ojcius, D.M. Recent advances in the field of caloric restriction mimetics and anti-aging molecules. Ageing Res. Rev. 2021, 66, 101240. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Iavicoli, I.; Calabrese, V. Hormesis: Why it is important to biogerontologists. Biogerontology 2012, 13, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Possible role of NF-κB in hormesis during ageing. Biogerontology 2012, 13, 637–646. [Google Scholar] [CrossRef]
- Jacome Burbano, M.S.; Gilson, E. The Power of Stress: The Telo-Hormesis Hypothesis. Cells 2021, 10, 1156. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).