Real-Life Performance and Clinical Outcomes of Portico Transcatheter Aortic Valve with FlexNav Delivery System: One-Year Data from a Single-Center Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Evaluation and Procedures
2.3. Endpoints
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural and 30-Day Outcomes
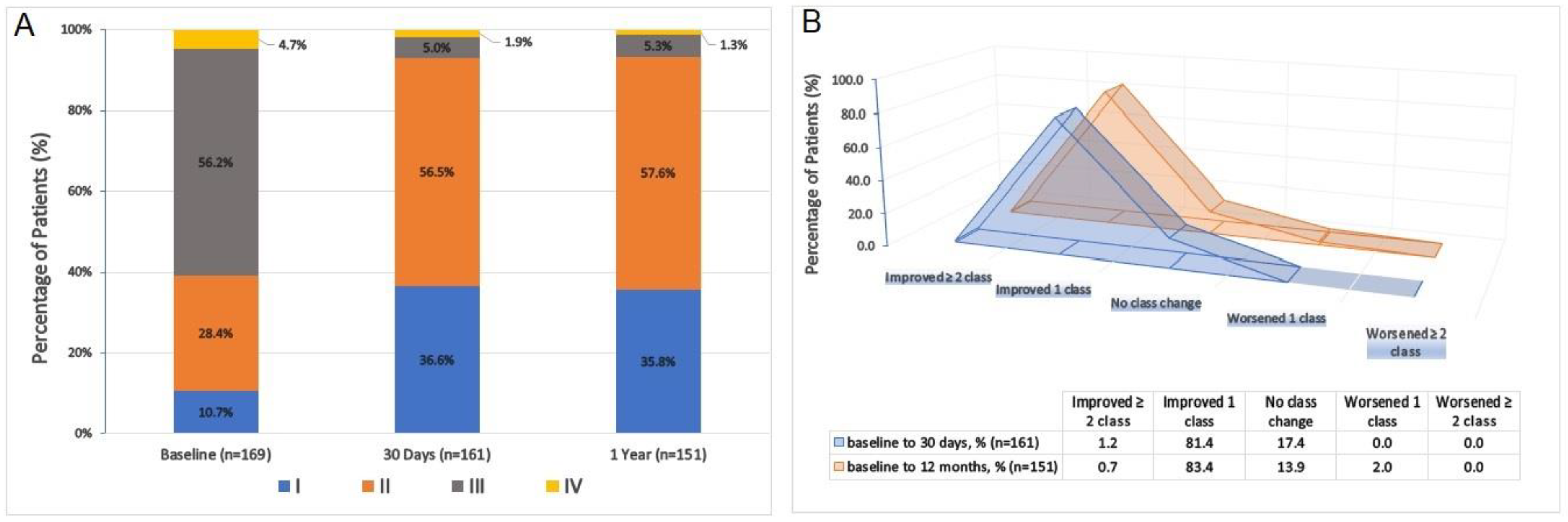
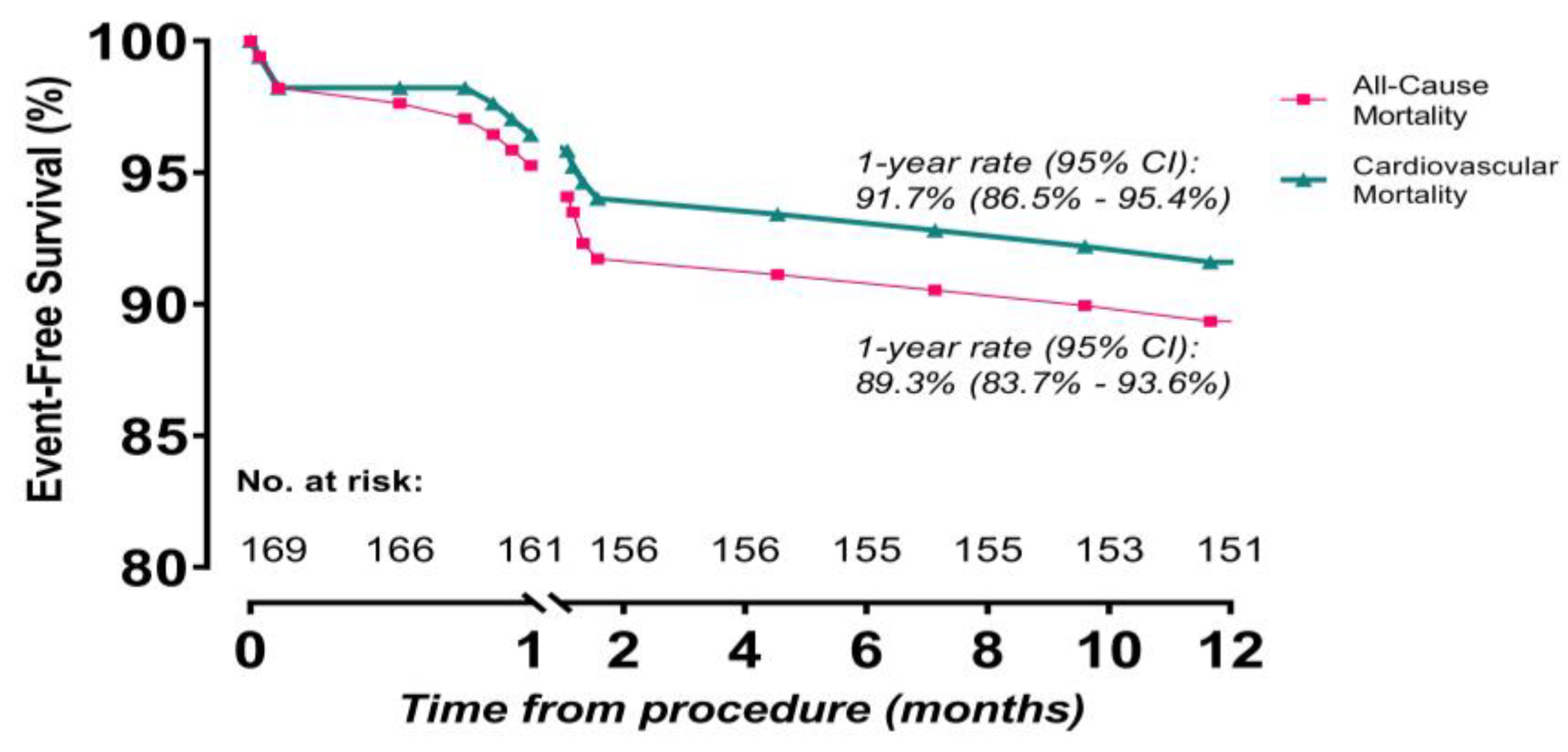
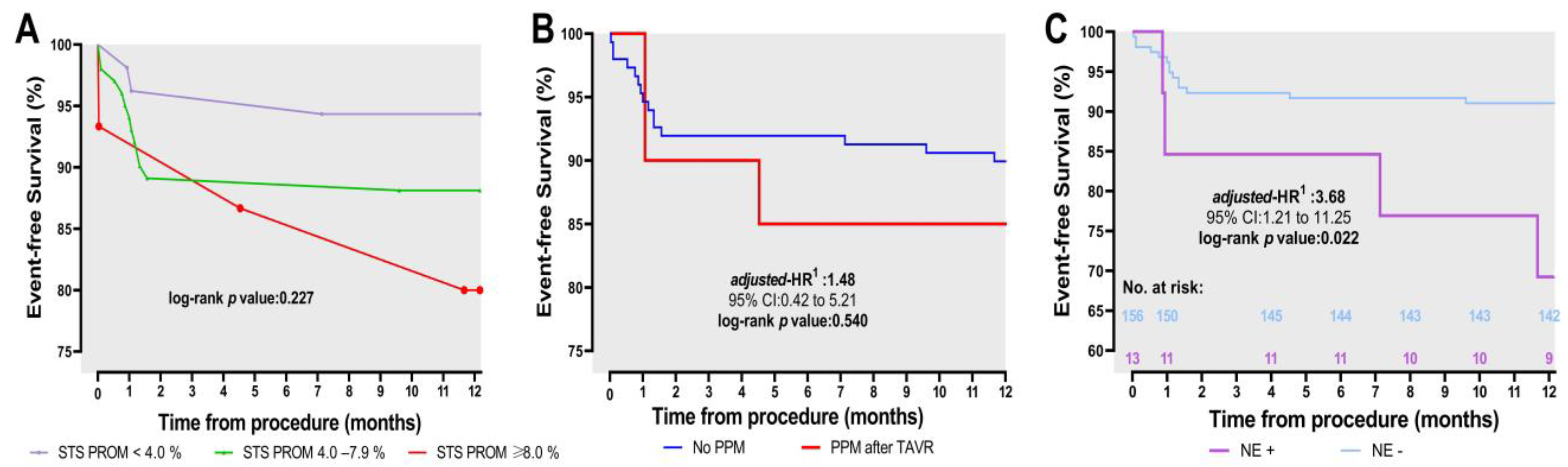
3.3. One-Year Outcomes and Timing and Causes of Mortality
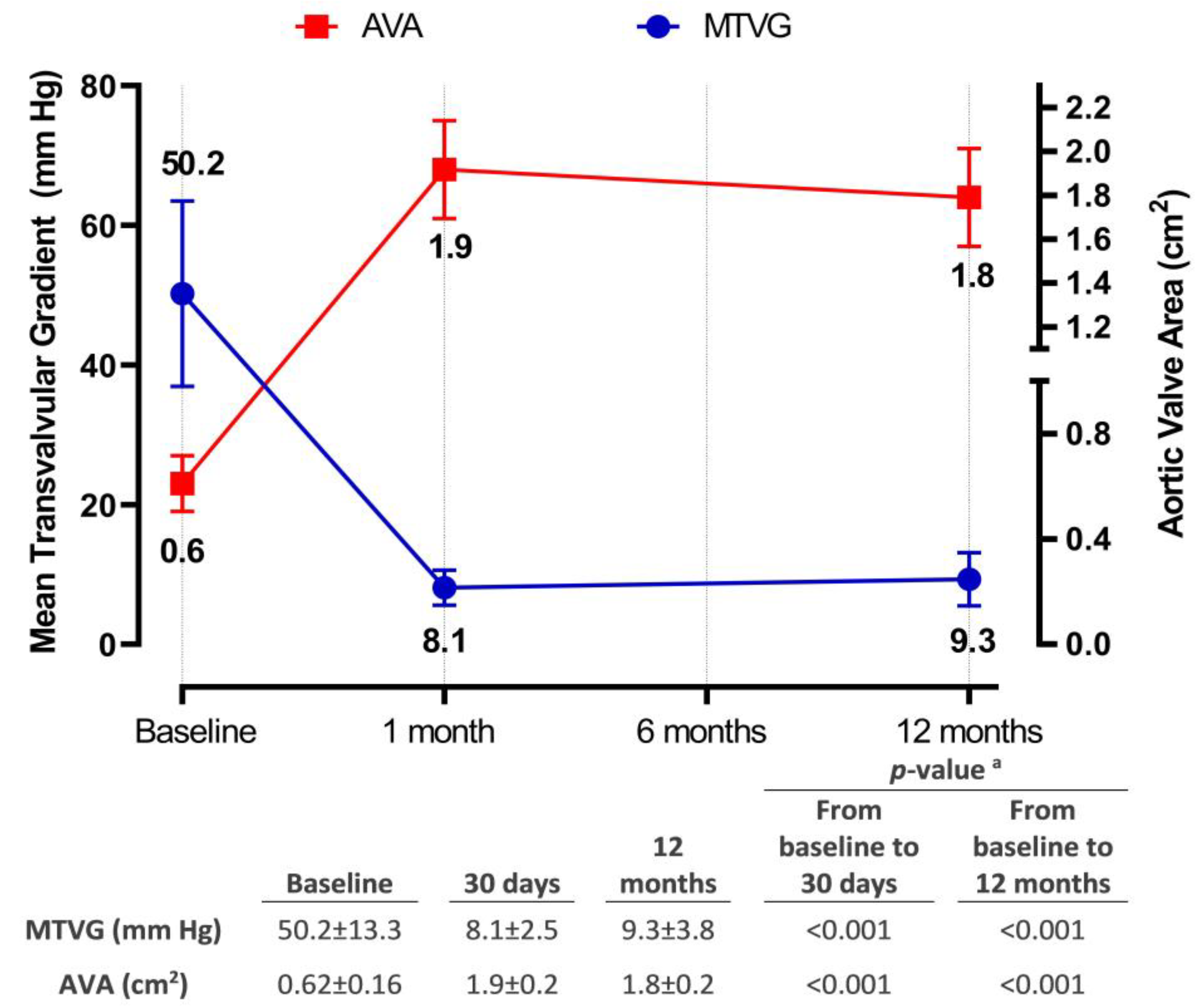
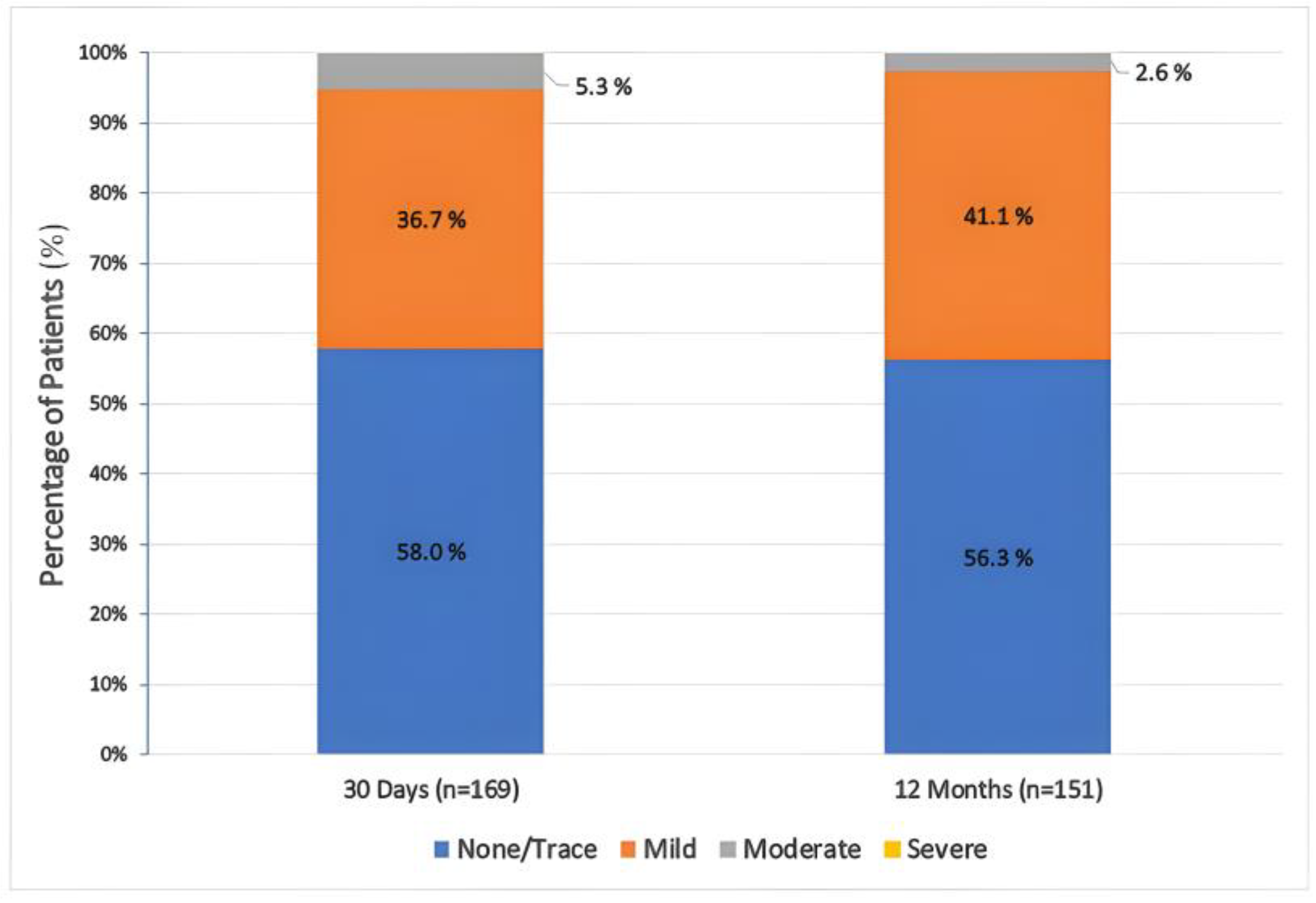
3.4. Hemodynamic and Functional Assessments
4. Discussion
4.1. Procedural and 30-Day Outcomes
4.2. Permanent Pacemaker Rates
4.3. Hemodynamic Performance
4.4. One-Year Outcomes
4.5. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results from the All-Comers NOTION Randomized Clinical Trial. JACC 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. NEJM 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. NEJM 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. NEJM 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. NEJM 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C. Five-year outcomes of transcatheter or surgical aortic-valve replacement. NEJM 2020, 382, 799–809. [Google Scholar] [CrossRef]
- Barbanti, M.; Buccheri, S.; Rodés-Cabau, J.; Gulino, S.; Généreux, P.; Pilato, G.; Dvir, D.; Picci, A.; Costa, G.; Tamburino, C. Transcatheter aortic valve replacement with new-generation devices: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 245, 83–89. [Google Scholar] [CrossRef]
- Fontana, G.P.; Bedogni, F.; Groh, M.; Smith, D.; Chehab, B.M.; Garrett, H.E., Jr.; Yong, G.; Worthley, S.; Manoharan, G.; Waksman, R. One-year results of the Portico transcatheter aortic heart valve using the next-generation FlexNav delivery system. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100562. [Google Scholar] [CrossRef]
- Leon, M.B.; Piazza, N.; Nikolsky, E.; Blackstone, E.H.; Cutlip, D.E.; Kappetein, A.P.; Krucoff, M.W.; Mack, M.; Mehran, R.; Miller, C. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: A consensus report from the Valve Academic Research Consortium. JACC 2011, 57, 253–269. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; Van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document. JACC 2012, 60, 1438–1454. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Messé, S.R.; Brickman, A.M.; Dwyer, M.; Bart Van Der Worp, H.; Lazar, R.M.; Pietras, C.G.; Abrams, K.J.; McFadden, E.; Petersen, N.H. Proposed standardized neurological endpoints for cardiovascular clinical trials: An academic research consortium initiative. Eur. Heart J. 2018, 39, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). JACC 2013, 62, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-a.; van Es, G.-A.; Zuckerman, B. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction. JACC 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Søndergaard, L.; Rodés-Cabau, J.; Hans-Peter Linke, A.; Fichtlscherer, S.; Schäfer, U.; Kuck, K.-H.; Kempfert, J.; Arzamendi, D.; Bedogni, F.; Asch, F.M. Transcatheter aortic valve replacement with a repositionable self-expanding prosthesis: The PORTICO-I trial 1-year outcomes. JACC 2018, 72, 2859–2867. [Google Scholar] [CrossRef]
- Fontana, G.P.; Bedogni, F.; Groh, M.; Smith, D.; Chehab, B.M.; Garrett, H.E.; Yong, G.; Worthley, S.; Manoharan, G.; Walton, A.; et al. Safety Profile of an Intra-Annular Self-Expanding Transcatheter Aortic Valve and Next-Generation Low-Profile Delivery System. JACC Cardiovasc. Interv. 2020, 13, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, H.; Linke, A.; Nombela-Franco, L.; Sluka, M.; Dominguez, J.F.O.; Montorfano, M.; Kim, W.-K.; Arnold, M.; Vasa-Nicotera, M.; Conradi, L. Procedural Safety and Device Performance of the Portico™ Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry. J. Clin. Med. 2022, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Gorla, R.; De Marco, F.; Garatti, A.; Bianchi, G.; Popolo Rubbio, A.; Acerbi, E.; Casenghi, M.; Spagnolo, P.; Brambilla, N.; Testa, L. Impact of aortic angle on transcatheter aortic valve implantation outcome with Evolut-R, Portico, and Acurate-NEO. Catheter. Cardiovasc. Interv. 2021, 97, E135–E145. [Google Scholar] [CrossRef]
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef]
- Linke, A.; Holzhey, D.; Möllmann, H.; Manoharan, G.; Schäfer, U.; Frerker, C.; Worthley, S.G.; van Boven, A.; Redwood, S.; Kovac, J. Treatment of aortic stenosis with a self-expanding, resheathable transcatheter valve: One-year results of the international multicenter portico transcatheter aortic valve implantation system study. Circ. Cardiovasc. Interv. 2018, 11, e005206. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.; Almeida, J.; Bettencourt, N.; Ferreira, N.; Carvalho, M.; Ferreira, W.; Caeiro, D.; Gonçalves, H.; Ribeiro, J.; Rodrigues, A. Incidence and predictors of vascular access site complications following transfemoral transcatheter aortic valve implantation. Rev. Port. Cardiol. (Engl. Ed.) 2017, 36, 747–753. [Google Scholar] [CrossRef]
- Stangl, V.; Baldenhofer, G.; Laule, M.; Baumann, G.; Stangl, K. Influence of sex on outcome following transcatheter aortic valve implantation (TAVI): Systematic review and meta-analysis. J. Interv. Cardiol. 2014, 27, 531–539. [Google Scholar] [CrossRef]
- Genereux, P.; Kodali, S.; Leon, M.B.; Smith, C.R.; Ben-Gal, Y.; Kirtane, A.J.; Daneault, B.; Reiss, G.R.; Moses, J.W.; Williams, M.R. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc. Interv. 2011, 4, 861–867. [Google Scholar] [CrossRef]
- Toggweiler, S.; Leipsic, J.; Binder, R.K.; Freeman, M.; Barbanti, M.; Heijmen, R.H.; Wood, D.A.; Webb, J.G. Management of vascular access in transcatheter aortic valve replacement: Part 2: Vascular complications. JACC Cardiovasc. Interv. 2013, 6, 767–776. [Google Scholar] [CrossRef]
- Möllmann, H.; Linke, A.; Holzhey, D.M.; Walther, T.; Manoharan, G.; Schäfer, U.; Heinz-Kuck, K.; Van Boven, A.J.; Redwood, S.R.; Kovac, J. Implantation and 30-day follow-up on all 4 valve sizes within the portico transcatheter aortic bioprosthetic family. JACC Cardiovasc. Interv. 2017, 10, 1538–1547. [Google Scholar] [CrossRef]
- Manoharan, G.; Grube, E.; Van Mieghem, N.M.; Brecker, S.; Fiorina, C.; Kornowski, R.; Danenberg, H.; Ruge, H.; Thiele, H.; Lancellotti, P. Thirty-day clinical outcomes of the Evolut PRO self-expanding transcatheter aortic valve: The international FORWARD PRO study. EuroIntervention 2020, 16, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Schymik, G.; Lefèvre, T.; Bartorelli, A.L.; Rubino, P.; Treede, H.; Walther, T.; Baumgartner, H.; Windecker, S.; Wendler, O.; Urban, P. European experience with the second-generation Edwards SAPIEN XT transcatheter heart valve in patients with severe aortic stenosis: 1-year outcomes from the SOURCE XT Registry. JACC Cardiovasc. Interv. 2015, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, H.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schaefer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention 2018, 13, e1764–e1770. [Google Scholar] [CrossRef] [PubMed]
- Grube, E.; Van Mieghem, N.M.; Bleiziffer, S.; Modine, T.; Bosmans, J.; Manoharan, G.; Linke, A.; Scholtz, W.; Tchétché, D.; Finkelstein, A. Clinical outcomes with a repositionable self-expanding transcatheter aortic valve prosthesis: The international FORWARD study. JACC 2017, 70, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Hengstenberg, C.; Hilker, M.; Kerber, S.; Schäfer, U.; Rudolph, T.; Linke, A.; Franz, N.; Kuntze, T.; Nef, H. The SAVI-TF registry: 1-year outcomes of the European post-market registry using the ACURATE neo transcatheter heart valve under real-world conditions in 1000 patients. JACC Cardiovasc. Interv. 2018, 11, 1368–1374. [Google Scholar] [CrossRef]
- Santos-Martinez, S.; Halim, J.; Castro-Mejía, A.; De Marco, F.; Trani, C.; Martin, P.; Infusino, F.; Ancona, M.; Moreno, R.; den Heijer, P. Myval versus alternative balloon-and self-expandable transcatheter heart valves: A central core lab analysis of conduction disturbances. Int. J. Cardiol. 2022, 351, 25–31. [Google Scholar] [CrossRef]
- Makkar, R.R.; Groh, M.; Bedogni, F.; Worthley, S.G.; Smith, D.; Chehab, B.M.; Waksman, R.; Monoharan, G.; Asch, F.M.; Ramana, R.K. CRT-700.32 One-Year Outcomes for an Intra-Annular Self-Expanding Transcatheter Aortic Valve and Next-Generation Low-Profile Delivery System. JACC Cardiovasc. Interv. 2022, 15, S64. [Google Scholar] [CrossRef]
- Brott, T.; Marler, J.R.; Olinger, C.P.; Adams, H.P., Jr.; Tomsick, T.; Barsan, W.G.; Biller, J.; Eberle, R.; Hertzberg, V.; Walker, M. Measurements of acute cerebral infarction: Lesion size by computed tomography. Stroke 1989, 20, 871–875. [Google Scholar] [CrossRef]
- Van Nieuwkerk, A.C.; Alfonso, F.; Tchetche, D.; De Brito, F.S., Jr.; Barbanti, M.; Latib, A.; Kornowski, R.; D’Onofrio, A.; Ribichini, F.; Mehran, R.; et al. Predictors and outcomes of acute, sub-acute and early stroke following transcatheter aortic valve implantation. Eur. Heart J. 2022, 43, ehac544-2105. [Google Scholar] [CrossRef]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchétché, D.; Chandrasekhar, J.; Brito, F.S.d.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F.; et al. Predictors, Incidence, and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation Complicated by Stroke. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef]
- Reid, C.; Gwivedi, G.; Yong, G. 827 Factors Associated With Stroke Post TAVI. Heart Lung Circ. 2020, 29, S409. [Google Scholar] [CrossRef]
- Ricco, J.-B.; Castagnet, H.; Christiaens, L.; Palazzo, P.; Lamy, M.; Mergy, J.; Corbi, P.; Neau, J.-P. Predictors of Early Stroke or Death in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Stroke Cerebrovasc. Dis. 2021, 30, 105912. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, P.D.; Muluk, S.; Udesh, R.; Mehta, A.; Schindler, J.; Mulukutla, S.; Jeevanantham, V.; Wechsler, L.; Gleason, T. Carotid artery disease and periprocedural stroke risk after transcatheter aortic valve implantation. Ann. Card. Anaesth. 2017, 20, 145–151. [Google Scholar] [CrossRef]
- van Wiechen, M.P.; Faure, M.E.; Hokken, T.W.; Ooms, J.F.; de Ronde-Tillmans, M.J.; Hirsch, A.; Daemen, J.; de Jaegere, P.P.; Budde, R.P.J.; Van Mieghem, N.M. Left atrial appendage thrombus and cerebrovascular events post-transcatheter aortic valve implantation. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Denegri, A.; Nietlispach, F.; Kottwitz, J.; Suetsch, G.; Haager, P.; Rodriguez, H.; Taramasso, M.; Obeid, S.; Maisano, F. Real-world procedural and 30-day outcome using the Portico transcatheter aortic valve prosthesis: A large single center cohort. Int. J. Cardiol. 2018, 253, 40–44. [Google Scholar] [CrossRef]
| Characteristic | |
|---|---|
| Age, years | 75.8 ± 7.7 |
| Gender, female, n (%) | 115 (68.0) |
| Coronary artery disease, n (%) | 68 (40.2) |
| Previous CABG, n (%) | 45 (26.6) |
| Previous coronary stenting, n (%) | 39 (23.1) |
| Prior atrial fibrillation, n (%) | 30 (17.8) |
| Previous mitral valve prosthesis, n (%) | 3 (1.8) |
| NYHA class, n (%) | |
| I | 18 (10.7) |
| II | 48 (28.4) |
| III | 95 (56.2) |
| IV | 8 (4.7) |
| Diabetes mellitus, n (%) | 48 (28.4) |
| Hypertension, n (%) | 91 (53.8) |
| Prior neurological events, n (%) | 11 (6.5) |
| Peripheral artery disease, n (%) | 30 (17.8) |
| Permanent pacemaker, n (%) | 6 (3.6) |
| Smoking, n (%) | 33 (19.5) |
| Chronic lung disease, n (%) | 23 (13.6) |
| Anticoagulant use, n (%) | 19 (11.2) |
| Chronic kidney disease, n (%) | 25 (14.8) |
| Chronic liver disease or cirrhosis, n (%) | 3 (1.8) |
| History of malignancy, n (%) | 5 (3.0) |
| STS PROM score 1, % | 4.63 ± 1.73 |
| Low risk (<4.0%) | 53 (31.4) |
| Moderate risk (4.0–7.9%) | 101 (59.8) |
| High risk (≥8.0%) | 15 (8.9) |
| Laboratory Parameters | |
| Creatinine, mg/dL | 0.96 ± 0.48 |
| e-GFR 2, mL/min/1.73 m2 | 68 ± 22 |
| LDL-C, mg/dL | 118 ± 51 |
| HDL-C, mg/dL | 48 ± 15 |
| Total cholesterol, mg/dL | 187 ± 31 |
| Glucose, mg/dL | 124 ± 42 |
| NT-proBNP, pg/mL | 1110 (374–3925) |
| WBC, 103/µL | 7.5 ± 3.1 |
| Hemoglobin, mg/dL | 11.5 ± 1.8 |
| Platelet count, 103/µL | 224 ± 73 |
| hs-cTnI, pg/mL | 16 (3–32) |
| Echocardiographic Parameters | |
| Left ventricular ejection fraction, % | 53.9 ± 10.2 |
| Left ventricular systolic dysfunction 3, n (%) | 38 (22.5) |
| Mean transvalvular aortic gradient, mm Hg | 50.2 ± 13.3 |
| Maximum transvalvular aortic gradient, mm Hg | 74.8 ± 19.2 |
| Maximum aortic valve velocity, m/s | 4.33 ± 0.57 |
| Aortic valve area, cm2 | 0.62 ± 0.16 |
| Aortic valve area/BSA, cm2/m2 | 0.34 ± 0.10 |
| Bicuspid aortic valve, n (%) | 29 (17.2) |
| Mitral insufficiency (moderate-to-severe), n (%) | 22 (13.0) |
| Mitral stenosis (moderate-to-severe), n (%) | 15 (8.8) |
| Aortic insufficiency (moderate-to-severe), n (%) | 54 (32.0) |
| Tricuspid insufficiency (moderate-to-severe), n (%) | 32 (18.9) |
| e-PASP on tricuspid regurgitation, mm Hg | 33 ± 11 |
| Characteristic | |
|---|---|
| Anesthesia, n (%) | |
| General | 5 (3.0) |
| Conscious sedation/local anesthesia | 164 (97.0) |
| Portico THV size, n (%) | |
| 23 mm | 6 (3.5) |
| 25 mm | 24 (14.2) |
| 27 mm | 38 (22.5) |
| 29 mm | 101 (59.8) |
| Pre-dilatation, n (%) | 135 (79.8) |
| Post-dilatation, n (%) | 89 (52.6) |
| Total procedure time 1 (min) | 58.8 ± 12.8 |
| Fluoroscopy time (min) | 25.8 ± 6.1 |
| Contrast volume (mL) | 232 ± 58 |
| Need for extra-stiff wire 2, n (%) | 2 (1.2) |
| Access route, n (%) | |
| Right transfemoral | 142 (94.1) |
| Left transfemoral | 7 (4.1) |
| Left subclavian/axillary | 3 (1.8) |
| Any resheathing performed, n (%) | 142 (84.0) |
| One resheath | 55/142 (38.7) |
| Two resheaths | 48/142 (33.8) |
| Three or more resheaths | 39/142 (27.5) |
| Intraprocedural mortality, n (%) | 0 (0.0) |
| Conversion to open-heart surgery, n (%) | 0 (0.0) |
| Annular rupture, n (%) | 0 (0.0) |
| Correct positioning of single Portico THV, n (%) | 162 (95.9) |
| Need for second Portico THV, n (%) | 7 (4.1) |
| 25 mm | 1/7 (14.3) |
| 29 mm | 6/7 (85.7) |
| Coronary obstruction 3, n (%) | 1 (0.6) |
| >2 units ES replacement 4, n (%) | 20 (11.8) |
| Length of hospital stay, days | 4 (2–5) |
| Technical outcomes, n (%) | |
| Concomitant coronary intervention before TAVR | 11 (6.5) |
| Successful access, delivery, and implantation of the Portico THV with FlexNav DS | 169 (100.0) |
| Procedural success 5 | 162 (95.9) |
| Technical success 6 | 150 (88.8) |
| Outcomes | |
|---|---|
| All-cause mortality | 8 (4.7) |
| Cardiovascular mortality | 6 (3.6) |
| Neurological events 1 | 5 (3.0) |
| Myocardial infarction | 3 (1.8) |
| Periprocedural (≤48 h after TAVR procedure) 2 | 2 (1.2) |
| Spontaneous (>48 h after TAVR procedure) 3 | 1 (0.6) |
| Acute kidney injury | 25 (14.8) |
| Stage 1 | 20 (11.8) |
| Stage 2 or 3 | 3 (1.8) |
| Stage 4 | 2 (1.2) |
| Bleeding 4 | 21 (12.4) |
| Type 2 | 13 (7.7) |
| Type 3 | 8 (4.7) |
| Type 4 | 0 (0.0) |
| Overall pacemaker implantation 5 | 18 (10.7) |
| New pacemaker implantation 6 | 18 (11.0) |
| New-onset atrial fibrillation | 12 (7.1) |
| New-onset LBBB | 5 (3.0) |
| Pericardial tamponade | 0 (0.0) |
| Vascular and access site-related outcomes, n (%) | |
| No peripheral or access site-related complications | 147 (87.0) |
| Overall vascular complications | 22 (13.0) |
| Major vascular complications | 19 (11.2) |
| Major non-FlexNav DS site complications | 6 (3.6) |
| Iliofemoral dissection | 4 (2.4) |
| Inguinal hematoma (moderate-to-severe) | 12 (7.1) |
| Pseudoaneurysm (moderate-to-severe) | 6 (3.6) |
| Major percutaneous closure device failure | 2 (1.2) |
| Percutaneous vascular-covered stent implantation | 5 (3.0) |
| İliofemoral arteries | 4/5 (80.0) |
| Left subclavian/axillary | 1/5 (20.0) |
| Requiring unplanned peripheric surgery | 1 (0.6) |
| 31 Days to 12 Months | Total (12 Months) | |
|---|---|---|
| Primary Endpoints, n (%) | ||
| All-cause mortality | 10 (5.9) | 18 (10.7) |
| Cardiovascular mortality | 8 (4.7) | 14 (8.3) |
| Neurological events 1 | 8 (4.7) | 13 (7.7) |
| Secondary Endpoints, n (%) | ||
| Myocardial infarction | 0 (0.0) | 3 (1.8) |
| Acute kidney injury 2 | 4 (2.4) | 29 (17.2) |
| Bleeding 3 | 5 (3.0) | 26 (15.4) |
| Overall pacemaker implantation 4 | 2 (1.1) | 20 (11.8) |
| New pacemaker implantation 5 | 2 (1.2) | 20 (12.2) |
| New-onset atrial fibrillation | 3 (1.8) | 15 (8.9) |
| Overall vascular complications | 0 (0.0) | 22 (13.0) |
| Major vascular complications 6 | 0 (0.0) | 13 (7.7) |
| Pericardial tamponade | 0 (0.0) | 0 (0.0) |
| 30 Days | 12 Months | |
|---|---|---|
| Main Causes of Cardiovascular Death, n (%) | ||
| Intraprocedural mortality | 0 (0.0) | 0 (0.0) |
| Heart failure | 1 (0.6) | 3 (1.8) |
| Myocardial infarction | 0 (0.0) | 0 (0.0) |
| Stroke | 1 (0.6) | 2 (1.2) |
| Vascular access site-related complication | 1 (0.6) | 1 (0.6) |
| Bleeding | 2 (1.2) | 3 (1.8) |
| Cardiac tamponade | 0 (0.0) | 0 (0.0) |
| Sudden cardiac death | 1 (0.6) | 3 (1.8) |
| Death of unknown cause | 0 (0.0) | 2 (1.2) |
| Valve-related mortality, n (%) | 0 (0.0) | 0 (0.0) |
| Timing of All-Cause Mortality, n (%) | ||
| Periprocedural mortality | 9 (5.3) | |
| ≤30 days after the TAVR procedure | 8 (4.7) | |
| >30 days but during hospitalization after TAVR 1 | 1 (0.6) | |
| Early mortality 2 | 10 (5.9) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, A.; Genc, O.; Pacaci, E.; Sen, O.; Kurt, I.H. Real-Life Performance and Clinical Outcomes of Portico Transcatheter Aortic Valve with FlexNav Delivery System: One-Year Data from a Single-Center Experience. J. Clin. Med. 2023, 12, 5373. https://doi.org/10.3390/jcm12165373
Yildirim A, Genc O, Pacaci E, Sen O, Kurt IH. Real-Life Performance and Clinical Outcomes of Portico Transcatheter Aortic Valve with FlexNav Delivery System: One-Year Data from a Single-Center Experience. Journal of Clinical Medicine. 2023; 12(16):5373. https://doi.org/10.3390/jcm12165373
Chicago/Turabian StyleYildirim, Abdullah, Omer Genc, Emre Pacaci, Omer Sen, and Ibrahim Halil Kurt. 2023. "Real-Life Performance and Clinical Outcomes of Portico Transcatheter Aortic Valve with FlexNav Delivery System: One-Year Data from a Single-Center Experience" Journal of Clinical Medicine 12, no. 16: 5373. https://doi.org/10.3390/jcm12165373
APA StyleYildirim, A., Genc, O., Pacaci, E., Sen, O., & Kurt, I. H. (2023). Real-Life Performance and Clinical Outcomes of Portico Transcatheter Aortic Valve with FlexNav Delivery System: One-Year Data from a Single-Center Experience. Journal of Clinical Medicine, 12(16), 5373. https://doi.org/10.3390/jcm12165373





