Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: A Review of Current Literature
Abstract
1. Introduction
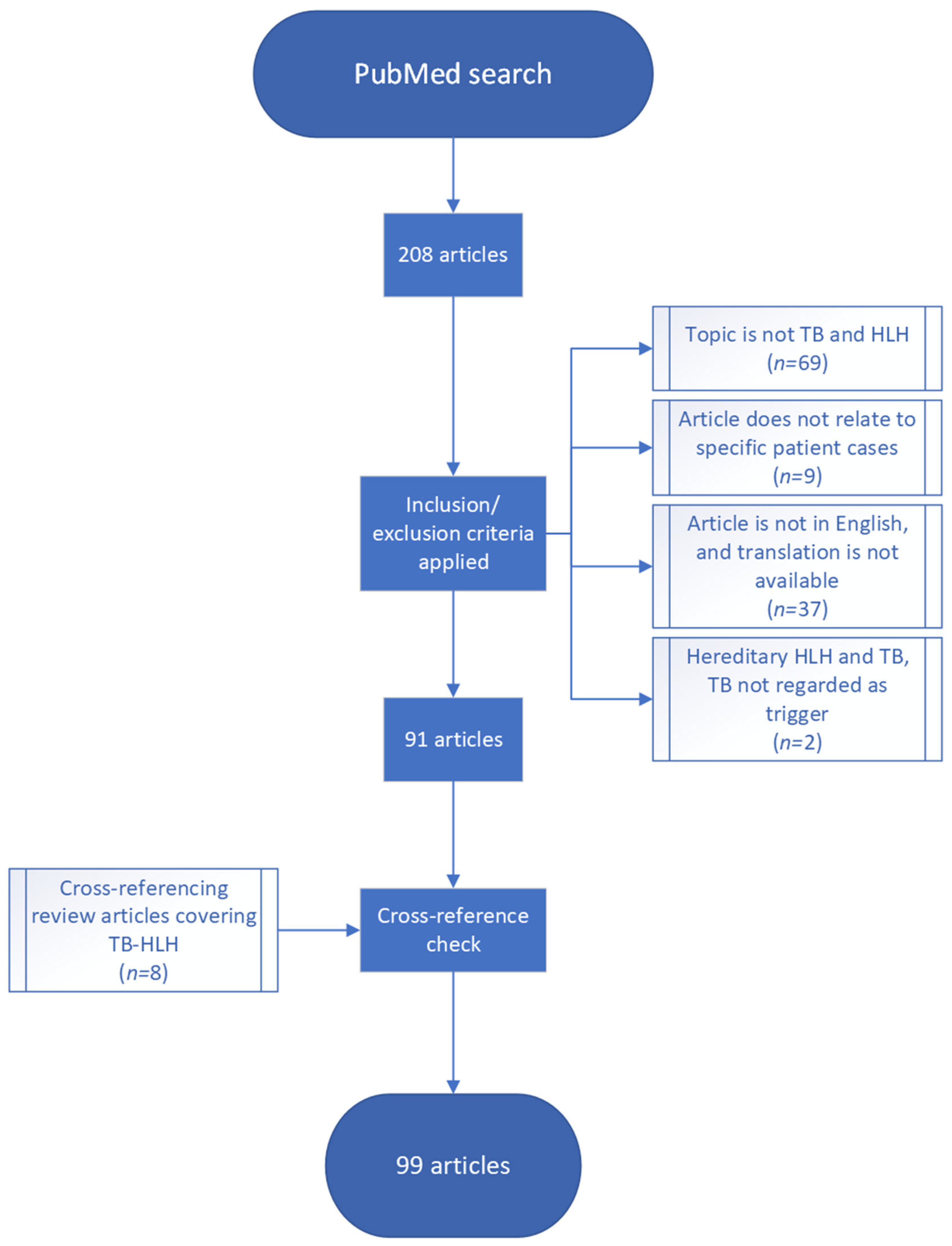
2. Materials and Methods
- Symptoms and findings supportive of a diagnosis of HLH in accordance with established diagnostic criteria (Tables S1 and S2).
- Diagnosis of MTB based on positive culture, finding of acid-fast bacilli on microscopy, or positive polymerase chain reaction (PCR).
3. Results
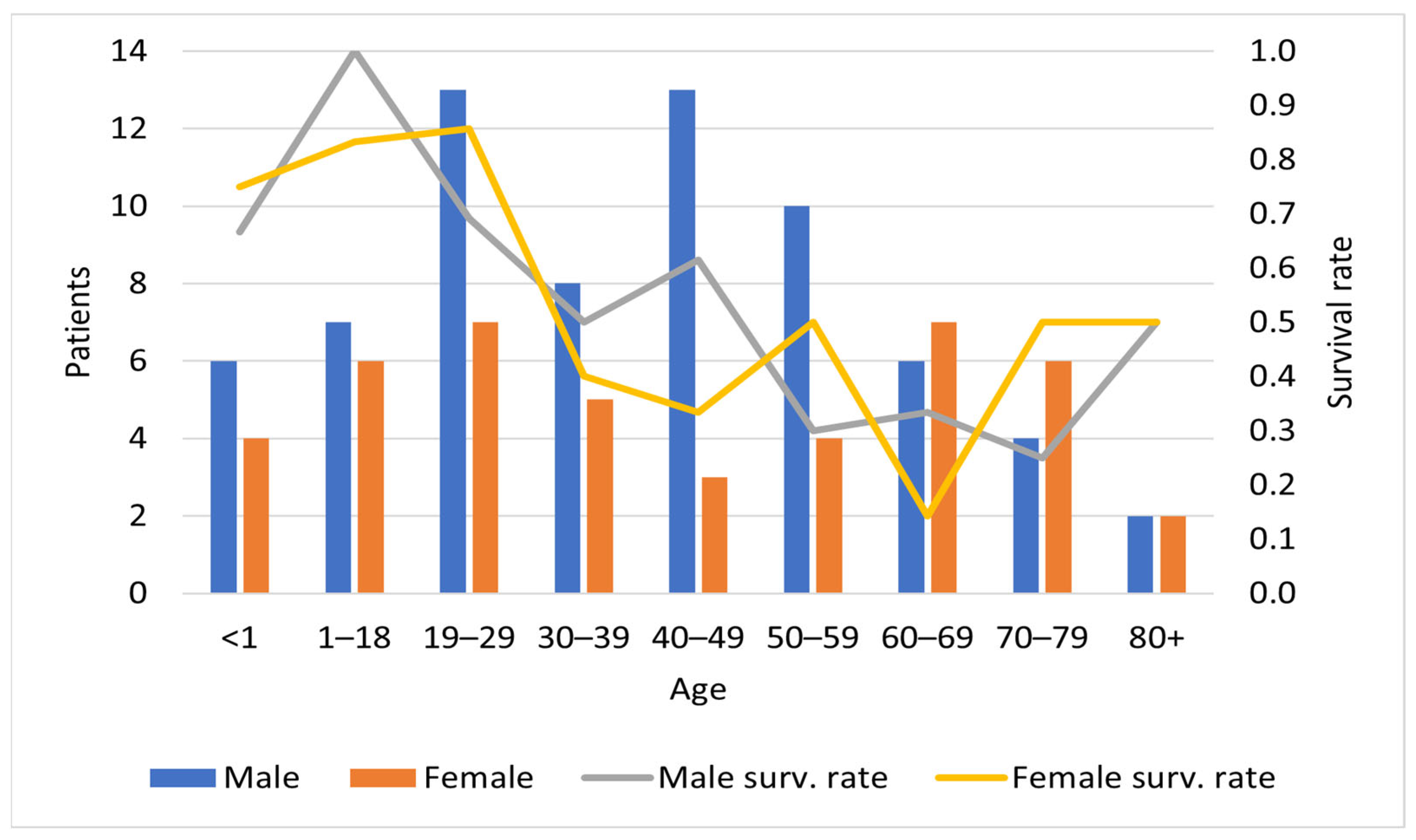
3.1. Epidemiology
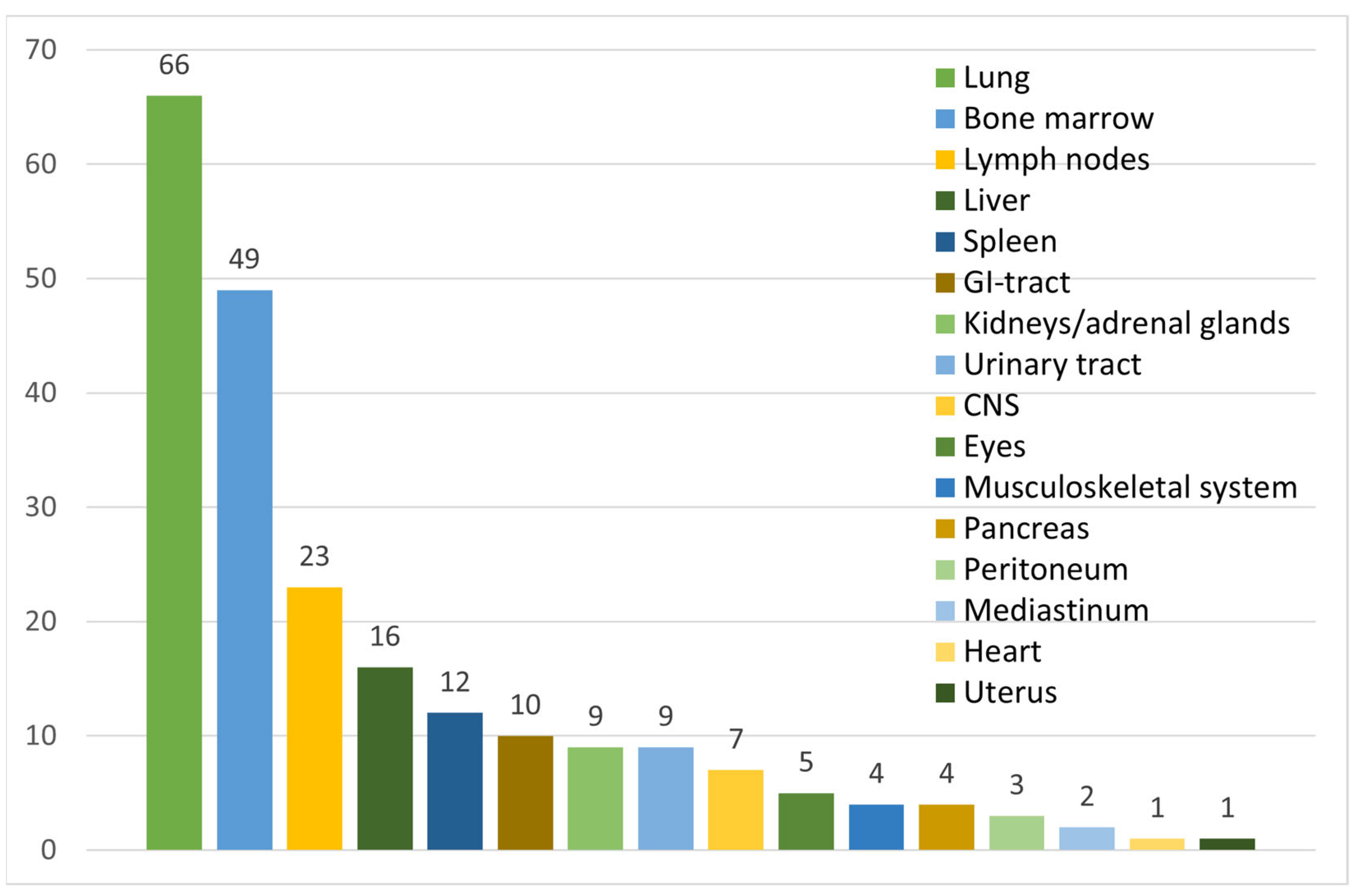
3.2. Clinical Presentation and Findings
3.3. Comorbidity
3.4. Diagnostic Clinical Criteria
3.5. Diagnosis of TB
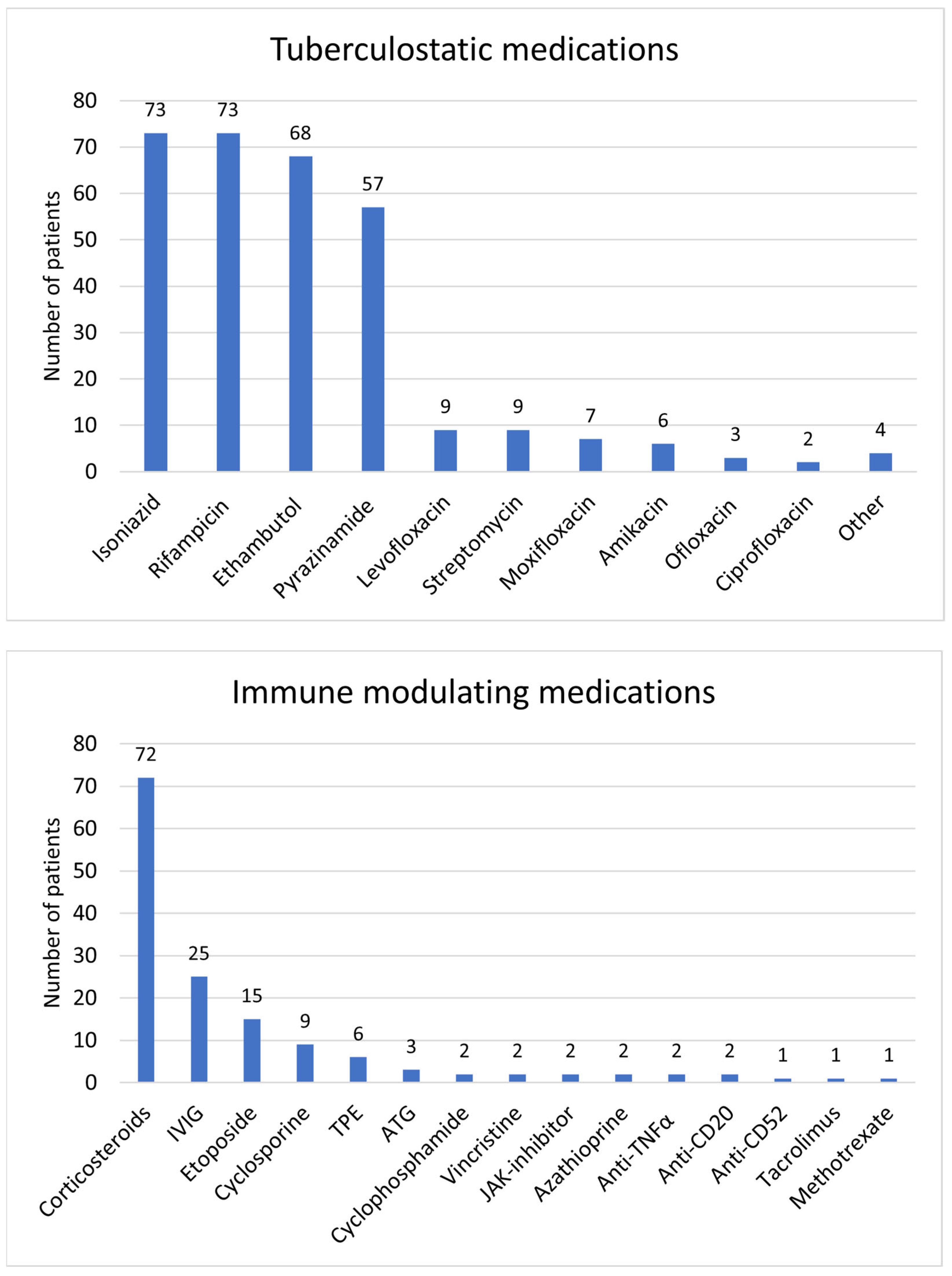
3.6. Treatment and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, M.B.; Allen, C.E.; Greenberg, J.; Henry, M.; Hermiston, M.L.; Kumar, A.; Hines, M.; Eckstein, O.; Ladisch, S.; Nichols, K.E.; et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr. Blood Cancer 2019, 66, e27929. [Google Scholar] [CrossRef]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef]
- Shiu, S.J.; Li, T.T.; Lee, B.J.; Fu, P.K.; Wang, C.Y.; Shiu, S.I. Miliary Tuberculosis-Related Acute Respiratory Distress Syndrome Complicated with Hemophagocytic Lymphohistiocytosis Syndrome. Case Rep. Infect. Dis. 2019, 2019, 9501610. [Google Scholar] [CrossRef]
- Jaiswal, A.; Mallya, V.; Singh, V.; Walia, M.; Khurana, N. Hemophagocytic lymphohistiocytosis secondary to multiple infections: Case report of a rare entity. Indian J. Pathol. Microbiol. 2017, 60, 137–138. [Google Scholar] [CrossRef]
- Shea, Y.F.; Chan, J.F.; Kwok, W.C.; Hwang, Y.Y.; Chan, T.C.; Ni, M.Y.; Li, I.W.; Chiu, P.K.; Luk, J.K.; Chu, L.W. Haemophagocytic lymphohistiocytosis: An uncommon clinical presentation of tuberculosis. Hong Kong Med. J. 2012, 18, 517–525. [Google Scholar]
- Padhi, S.; Ravichandran, K.; Sahoo, J.; Varghese, R.G.; Basheer, A. Hemophagocytic lymphohistiocytosis: An unusual complication in disseminated Mycobacterium tuberculosis. Lung India 2015, 32, 593–601. [Google Scholar] [CrossRef]
- Geerdes-Fenge, H.F.; Löbermann, M.; Hemmer, C.J.; Benedek, O.; Reisinger, E.C. Tuberculosis-associated hemophagocytic lymphohistiocytosis with subsequent unmasking cryptococcal immune reconstitution inflammatory syndrome (IRIS) in an HIV-negative man. Infection 2019, 47, 129–133. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis Fact Sheet Geneva: WHO. 2021 [Updated 14.10.21]. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 14 November 2021).
- WHO. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Brastianos, P.K.; Swanson, J.W.; Torbenson, M.; Sperati, J.; Karakousis, P.C. Tuberculosis-associated haemophagocytic syndrome. Lancet Infect. Dis. 2006, 6, 447–454. [Google Scholar] [CrossRef]
- Lerolle, N.; Laanani, M.; Rivière, S.; Galicier, L.; Coppo, P.; Meynard, J.L.; Molina, J.M.; Azoulay, E.; Aumont, C.; Marzac, C.; et al. Diversity and combinations of infectious agents in 38 adults with an infection-triggered reactive haemophagocytic syndrome: A multicenter study. Clin. Microbiol. Infect. 2016, 22, 268.e1–268.e8. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Sheng, W.-H.; Lin, B.-H.; Lin, C.-W.; Wang, J.-T.; Chen, Y.-C.; Chang, S.-C. Causes, clinical symptoms, and outcomes of infectious diseases associated with hemophagocytic lymphohistiocytosis in Taiwanese adults. J. Microbiol. Immunol. Infect. 2011, 44, 191–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, G.; Qin, H.; Li, Y.; Zeng, X. Tuberculosis-associated hemophagocytic lymphohistiocytosis with initial presentation of fever of unknown origin in a general hospital: An analysis of 8 clinical cases. Medicine 2017, 96, e6575. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Debaugnies, F.; Mahadeb, B.; Ferster, A.; Meuleman, N.; Rozen, L.; Demulder, A.; Corazza, F. Performances of the H-Score for Diagnosis of Hemophagocytic Lymphohistiocytosis in Adult and Pediatric Patients. Am. J. Clin. Pathol. 2016, 145, 862–870. [Google Scholar] [CrossRef]
- Trovik, L.H.; Sandnes, M.; Blomberg, B.; Holmaas, G.; Ahmed, A.B.; Tvedt, T.H.A.; Vintermyr, O.; Reikvam, H. Hemophagocytic lymphohistiocytosis and miliary tuberculosis in a previously healthy individual: A case report. J. Med. Case Rep. 2020, 14, 217. [Google Scholar] [CrossRef] [PubMed]
- Troncoso Mariño, A.; Campelo Sánchez, E.; Martínez López de Castro, N.; Inaraja Bobo, M.T. Haemophagocytic syndrome and paradoxical reaction to tuberculostatics after treatment with infliximab. Pharm. World Sci. 2010, 32, 117–119. [Google Scholar] [CrossRef]
- Singha, A.; Mukherjee, A.; Dasgupta, R.; Das, T. A Case of Hemophagocytic Syndrome due to Tuberculosis: Uncommon Manifestation of a Common Disease. Case Rep. Med. 2014, 2014, 613845. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Sun, H.Y.; Tsai, J.H.; Hung, P.P.; Wang, J.T. TB-IRIS presenting with haemophagocytic lymphohistiocytosis in a non-HIV-infected male. Int. J. Tuberc. Lung Dis. 2017, 21, 1183–1184. [Google Scholar] [CrossRef]
- Wong, C.K.; Wong, B.C.; Chan, K.C.; Joynt, G.M.; Yap, F.Y.; Lam, C.W.; Lee, N.; Lee, S.S.; Cockram, C.S.; Sung, J.J.; et al. Cytokine profile in fatal human immunodeficiency virus tuberculosis Epstein-Barr virus associated hemophagocytic syndrome. Arch. Intern. Med. 2007, 167, 1901–1903. [Google Scholar] [CrossRef]
- Asaji, M.; Tobino, K.; Murakami, K.; Goto, Y.; Sueyasu, T.; Nishizawa, S.; Yoshimine, K.; Munechika, M.; Ko, Y.; Yoshimatsu, Y.; et al. Miliary Tuberculosis in a Young Woman with Hemophagocytic Syndrome: A Case Report and Literature Review. Intern. Med. 2017, 56, 1591–1596. [Google Scholar] [CrossRef][Green Version]
- Balasubramanian, S.; Kaarthigeyan, K.; Aparna, V.; Srinivas, S. Tuberculosis associated hemophagocytic syndrome in infancy. Indian Pediatr. 2008, 45, 593–595. [Google Scholar] [PubMed]
- Deshpande, A.; Nayar, P.S.; Pradhan, A.M.; Manchanda, R.V. Miliary tuberculosis with hemophagocytosis in a two months old infant. Indian J. Hematol. Blood Transfus. 2010, 26, 115–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seo, J.H.; Lee, J.A.; Kim, D.H.; Cho, J.; Lim, J.S. Tuberculosis-associated hemophagocytic lymphohistiocytosis in adolescent diagnosed by polymerase chain reaction. Korean J. Pediatr. 2016, 59, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Okascharoen, C.; Nuntnarumit, P.; Sirinavin, S. Neonatal tuberculosis associated with shock, disseminated intravascular coagulation, hemophagocytic syndrome, and hypercalcemia: A case report. J. Perinatol. 2003, 23, 79–81. [Google Scholar] [CrossRef]
- Chen, C.H.; Fang, Y.H.; Chiang, P.M.; Lin, D.T.; Huang, L.M. Disseminated tuberculosis presenting as multiple hepatosplenic microabscesses and pancytopenia in a teenage boy. J. Formos. Med. Assoc. 2004, 103, 939–942. [Google Scholar]
- Gupta, A.P.; Parate, S.N.; Bobhate, S.K. Hemophagocytic syndrome: A cause for fatal outcome in tuberculosis. Indian J. Pathol. Microbiol. 2009, 52, 260–262. [Google Scholar] [CrossRef]
- Dilber, E.; Erduran, E.; Kalyoncu, M.; Aynaci, F.M.; Okten, A.; Ahmetoğlu, A. Hemophagocytic syndrome as an initial presentation of miliary tuberculosis without pulmonary findings. Scand. J. Infect. Dis. 2002, 34, 689–692. [Google Scholar] [CrossRef]
- Shaw, P.H.; Brown, D.; Shulman, S.T. Tuberculosis-associated hemophagocytic syndrome in an infant. Pediatr. Infect. Dis. J. 2000, 19, 475–477. [Google Scholar] [CrossRef]
- Balkis, M.M.; Bazzi, L.; Taher, A.; Salem, Z.; Uthman, I.; Kanj, N.; Boulos, F.I.; Kanj, S.S. Severe hemophagocytic syndrome developing after treatment initiation for disseminated Mycobacterium tuberculosis: Case report and literature review. Scand. J. Infect. Dis. 2009, 41, 535–537. [Google Scholar] [CrossRef]
- Chen, L.; Weng, H.; Li, H.; Huang, J.; Pan, J.; Huang, Y.; Ma, C. Potential killer in the ICU-severe tuberculosis combined with hemophagocytic syndrome: A case series and literature review. Medicine 2017, 96, e9142. [Google Scholar] [CrossRef]
- Erdoğan, S.; Çakır, D.; Bozkurt, T.; Karakayalı, B.; Kalın, S.; Koç, B.; Sözeri, B. Hemophagocytic Lymphohistiocytosis Related to Tuberculosis Disease. Indian J. Crit. Care Med. 2020, 24, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Haque, W.M.; Shuvo, M.E.; Rahim, M.A.; Mitra, P.; Samad, T.; Haque, J.A. Haemophagocytic syndrome in an adult suffering from pyrexia of unknown origin: An uncommon presentation of tuberculosis: A case report. BMC Res. Notes 2017, 10, 110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shoureshi, P.; Ruiz, J.; Abdulzahir, A.; Bisch, A.L.; Naddaf, N.; Gisel, J. Tuberculosis-associated HLH in a patient with chronic kidney disease on haemodialysis. Oxf. Med. Case Rep. 2020, 2020, omaa082. [Google Scholar] [CrossRef]
- Su, N.W.; Chen, C.K.; Chen, G.S.; Hsieh, R.K.; Chang, M.C. A case of tuberculosis-induced hemophagocytic lymphohistiocytosis in a patient under hemodialysis. Int. J. Hematol. 2009, 89, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, H.R.T.; Mishra, R.; Niazi, M.; Venkatram, S.; Diaz-Fuentes, G. An Unusual Triad of Hemophagocytic Syndrome, Lymphoma and Tuberculosis in a Non-HIV Patient. Am. J. Case Rep. 2017, 18, 739–745. [Google Scholar] [CrossRef]
- Dey, A.; Shah, I.; Paikrao, P.; Iyenger, V. Tuberculosis with hemophagocytic lymphohistiocytosis in an infant. Indian J. Pediatr. 2014, 81, 214–215. [Google Scholar] [CrossRef]
- Hauch, H.; Skrzypek, S.; Woessmann, W.; Lehmberg, K.; Ehl, S.; Speckmann, C.; Schneck, E.; Koerholz, D.; Jux, C.; Neuhäuser, C. Tuberculosis-Associated HLH in an 8-Month-Old Infant: A Case Report and Review. Front. Pediatr. 2020, 8, 556155. [Google Scholar] [CrossRef]
- Koulmane Laxminarayana, S.L.; Nagaraju, S.P.; Prabhu Attur, R.; Manohar, C.; Parthasarathy, R.; Chari, B. Hemophagocytic lymphohistiocytosis: An unusual presentation of tuberculosis in hemodialysis patients. Hemodial. Int. 2015, 19, E16–E19. [Google Scholar] [CrossRef]
- Maheshwari, P.; Chhabra, R.; Yadav, P. Perinatal tuberculosis associated hemophagocytic lymphohistiocytosis. Indian J. Pediatr. 2012, 79, 1228–1229. [Google Scholar] [CrossRef]
- Martínez-Pillado, M.; Varela-Durán, M.; Said-Criado, I.; Díaz-Parada, P.; Rodríguez-Losada, M.; Mendoza-Pintos, M. Disseminated tuberculosis and hemophagocytic syndrome although TB prophylaxis in patients with inflammatory bowel disease treated with Infliximab. IDCases 2019, 16, e00518. [Google Scholar] [CrossRef]
- Mbizvo, G.K.; Lentell, I.C.; Leen, C.; Roddie, H.; Derry, C.P.; Duncan, S.E.; Rannikmäe, K. Epilepsia partialis continua complicated by disseminated tuberculosis and hemophagocytic lymphohistiocytosis: A case report. J. Med. Case Rep. 2019, 13, 191. [Google Scholar] [CrossRef]
- Ohata, S.; Hara, K.; Arai, T.; Takayoshi, T.; Nishiyama, K.; Yasutomo, Y.; Yokono, K.; Sugimoto, T. A case of pulmonary tuberculosis diagnosed in a patient with manifestations of haemophagocytic lymphohistiocytosis. Oxf. Med. Case Rep. 2019, 2019, omz013. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Varghese, R.G.; Ramdas, A.; Phansalkar, M.D.; Sarangi, R. Hemophagocytic lymphohistiocytosis: Critical reappraisal of a potentially under-recognized condition. Front. Med. 2013, 7, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Parsi, M.; Dargan, K. Hemophagocytic Lymphohistiocytosis Induced Cytokine Storm Secondary to Human Immunodeficiency Virus Associated Miliary Tuberculosis. Cureus 2020, 12, e6589. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, P.V.; Kularathne, W.K.; De Silva, G.C.; Athauda, B.M.; Nanayakkara, S.N.; Siribaddana, A.; Baminiwatte, D. Disseminated tuberculosis presenting as hemophagocytic lymphohistiocytosis in an immunocompetent adult patient: A case report. J. Med. Case Rep. 2015, 9, 294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seminari, E.; Contardi, G.; Rubert, L.; Fronti, E.; Comoli, P.; Minoli, L.; Zecca, M. Tuberculosis-induced haemophagocytic syndrome in a patient on haemodialysis treated with anti-thymocyte globulin. Int. J. Tuberc. Lung Dis. 2014, 18, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Kwong, Y.L.; Yuen, K.Y. Hemophagocytosis in the peripheral blood due to tuberculosis mycobacteremia. Am. J. Med. 2005, 118, 1298–1299. [Google Scholar] [CrossRef]
- Campo, E.; Condom, E.; Miro, M.J.; Cid, M.C.; Romagosa, V. Tuberculosis-associated hemophagocytic syndrome. A systemic process. Cancer 1986, 58, 2640–2645. [Google Scholar] [CrossRef]
- Chien, C.C.; Chiou, T.J.; Lee, M.Y.; Hsiao, L.T.; Kwang, W.K. Tuberculosis-associated hemophagocytic syndrome in a hemodialysis patient with protracted fever. Int. J. Hematol. 2004, 79, 334–336. [Google Scholar] [CrossRef]
- Shin, B.C.; Kim, S.W.; Ha, S.W.; Sohn, J.W.; Lee, J.M.; Kim, N.S. Hemophagocytic syndrome associated with bilateral adrenal gland tuberculosis. Korean J. Intern. Med. 2004, 19, 70–73. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ba, J.H.; Shi, X.W.; Wu, B.Q. Successful treatment of mycobacterial infection associated hemophagocytic lymphohistiocytosis with etoposide and anti-tuberculous therapy: A case report. BMC Infect. Dis. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Elhence, A.; Aggarwal, A.; Goel, A.; Aggarwal, M.; Das, P.; Shalimar. Granulomatous Tubercular Hepatitis Presenting as Secondary Hemophagocytic Lymphohistiocytosis: A Case Report and Systematic Review of the Literature. J. Clin. Exp. Hepatol. 2021, 11, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Shi, X.H.; Zhang, Y.; Chen, J.X.; Lai, W.X.; Luo, J.M.; Ba, J.H.; Wang, Y.H.; Chen, J.N.; Wu, B.Q. Disseminated Tuberculosis Associated Hemophagocytic Lymphohistiocytosis in a Pregnant Woman With Evans syndrome: A Case Report and Literature Review. Front. Immunol. 2021, 12, 676132. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Jat, K.R.; Singh, A.; Mridha, A.R.; Kabra, S.K. Autoimmune haemolytic anaemia and haemophagocytic lymphohistiocytosis in an adolescent boy with tuberculosis: An unusual association. Trop. Doct. 2017, 47, 249–253. [Google Scholar] [CrossRef]
- Eliopoulos, G.; Vaiopoulos, G.; Kittas, C.; Fessas, P. Tuberculosis associated hemophagocytic syndrome complicated with severe bone marrow failure and disseminated intravascular coagulation. Nouv. Rev. Fr. Hematol. 1992, 34, 273–276. [Google Scholar]
- Koduri, P.R.; Carandang, G.; DeMarais, P.; Patel, A.R. Hyperferritinemia in reactive hemophagocytic syndrome report of four adult cases. Am. J. Hematol. 1995, 49, 247–249. [Google Scholar] [CrossRef]
- Avasthi, R.; Mohanty, D.; Chaudhary, S.C.; Mishra, K. Disseminated tuberculosis: Interesting hematological observations. J. Assoc. Physicians India 2010, 58, 243–244. [Google Scholar]
- Basu, S.; Mohan, H.; Malhotra, H. Pancytopenia due to hemophagocytic syndrome as the presenting manifestation of tuberculosis. J. Assoc. Physicians India 2000, 48, 845–846. [Google Scholar]
- Browett, P.J.; Varcoe, A.R.; Fraser, A.G.; Ellis-Pegler, R.B. Disseminated tuberculosis complicated by the hemophagocytic syndrome. Aust. N. Z. J. Med. 1988, 18, 79–80. [Google Scholar] [CrossRef]
- Aggarwal, P.; Kumar, G.; Dev, N.; Kumari, P. Haemophagocytic lymphohistiocytosis: A cause for rare but fatal outcome in tuberculosis. BMJ Case Rep. 2012, 2012, bcr2012006982. [Google Scholar] [CrossRef]
- Barnes, N.; Bellamy, D.; Ireland, R.; Parsons, V.; Costello, J. Pulmonary tuberculosis complicated by haemophagocytic syndrome and rifampicin-induced tubulointerstitial nephritis. Br. J. Dis. Chest 1984, 78, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.P.; van Burgel, N.D.; Marijt, W.A.; van Dissel, J.T.; von dem Borne, P.A. Fever, shock, and pancytopenia in a patient treated with alemtuzumab. Clin. Infect. Dis. 2009, 49, 1540. [Google Scholar] [CrossRef][Green Version]
- Castellano, I.; Gómez-Martino, J.R.; Hernández, T.; Mateos, L.; Argüello, C. Hemophagocytic syndrome as an unusual form of presentation of tuberculosis in a hemodialysis patient: Case report and review of the literature. Am. J. Nephrol. 2000, 20, 214–216. [Google Scholar] [CrossRef]
- Cassim, K.M.; Gathiram, V.; Jogessar, V.B. Pancytopaenia associated with disseminated tuberculosis, reactive histiocytic haemophagocytic syndrome and tuberculous hypersplenism. Tuber. Lung Dis. 1993, 74, 208–210. [Google Scholar] [CrossRef]
- Cherif, E.; Bel Feki, N.; Ben Hassine, L.; Khalfallah, N. Haemophagocytic syndrome with disseminated intravascular coagulation associated with tuberculosis. BMJ Case Rep. 2013, 2013, bcr2013008743. [Google Scholar] [CrossRef]
- Claessens, Y.E.; Pene, F.; Tulliez, M.; Cariou, A.; Chiche, J.D. Life-threatening hemophagocytic syndrome related to mycobacterium tuberculosis. Eur. J. Emerg. Med. 2006, 13, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Aoike, I.; Shibasaki, Y.; Morita, T.; Miyazaki, S.; Shimizu, T.; Suzuki, M. A successfully treated case of disseminated tuberculosis-associated hemophagocytic syndrome and multiple organ dysfunction syndrome. Am. J. Kidney Dis. 2001, 38, E19.1–E19.4. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.M.; Pillinger, T.; Luqmani, A.; Cooper, N. Haemophagocytic lymphohistiocytosis associated with Mycobacterium tuberculosis infection. BMJ Case Rep. 2015, 2015, bcr2014208220. [Google Scholar] [CrossRef]
- Kaur, N.; Britton, P.N.; Isaacs, D.; Ging, J.; Campbell, D.E. Haemophagocytic lymphohistiocytosis secondary to presumed congenital tuberculosis in a neonate. J. Paediatr. Child. Health 2019, 55, 988–992. [Google Scholar] [CrossRef]
- Ko, Y.C.; Lee, C.T.; Cheng, Y.F.; Hung, K.H.; Kuo, C.Y.; Huang, C.C.; Chen, J.B. Hypercalcaemia and haemophagocytic syndrome: Rare concurrent presentations of disseminated tuberculosis in a dialysis patient. Int. J. Clin. Pract. 2004, 58, 723–725. [Google Scholar] [CrossRef]
- Lee, S.W.; Wang, C.Y.; Lee, B.J.; Kuo, C.Y.; Kuo, C.L. Hemophagocytic syndrome in miliary tuberculosis presenting with noncaseating granulomas in bone marrow and liver. J. Formos. Med. Assoc. 2008, 107, 495–499. [Google Scholar] [CrossRef]
- Long, B.; Cheng, L.; Lai, S.P.; Zhang, J.W.; Sun, Y.L.; Lai, W.X.; Zhang, H.Y.; Lu, Y.; Lin, D.J.; Li, X.D. Tuberculosis-associated hemophagocytic lymphohistiocytosis in an umbilical cord blood transplant recipient. Clin. Chim. Acta 2017, 468, 111–113. [Google Scholar] [CrossRef]
- Tesi, B.; Sieni, E.; Neves, C.; Romano, F.; Cetica, V.; Cordeiro, A.I.; Chiang, S.; Schlums, H.; Galli, L.; Avenali, S.; et al. Hemophagocytic lymphohistiocytosis in 2 patients with underlying IFN-γ receptor deficiency. J. Allergy Clin. Immunol. 2015, 135, 1638–1641. [Google Scholar] [CrossRef]
- Verma, T.; Aggarwal, S. Childhood tuberculosis presenting with haemophagocytic syndrome. Indian J. Hematol. Blood Transfus. 2012, 28, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Mancebo, E.; Allende, L.M.; Guzmán, M.; Paz-Artal, E.; Gil, J.; Urrea-Moreno, R.; Fernández-Cruz, E.; Gayà, A.; Calvo, J.; Arbós, A.; et al. Familial hemophagocytic lymphohistiocytosis in an adult patient homozygous for A91V in the perforin gene, with tuberculosis infection. Haematologica 2006, 91, 1257–1260. [Google Scholar] [PubMed]
- Naha, K.; Dasari, S.; Vivek, G.; Prabhu, M. Disseminated tuberculosis presenting with secondary haemophagocytic lymphohistiocytosis and Poncet’s disease in an immunocompetent individual. BMJ Case Rep. 2013, 2013, bcr2012008265. [Google Scholar] [CrossRef] [PubMed]
- Osowicki, J.; Wang, S.; McKenzie, C.; Marshall, C.; Gard, J.; Ke Juin, W.; Steer, A.C.; Connell, T.G. Congenital Tuberculosis Complicated by Hemophagocytic Lymphohistiocytosis. Pediatr. Infect. Dis. J. 2016, 35, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, B.; Blanes, M.; Vinaixa, C.; Aguilera, V.; Rubín, A.; Prieto, M.; Berenguer, M. Haemophagocytic syndrome in a liver transplant patient during treatment with Telaprevir. Ann. Hepatol. 2013, 12, 974–978. [Google Scholar] [CrossRef]
- Ruiz-Argüelles, G.J.; Arizpe-Bravo, D.; Garcés-Eisele, J.; Sánchez-Sosa, S.; Ruiz-Argüelles, A.; Ponce-de-León, S. Tuberculosis-associated fatal hemophagocytic syndrome in a patient with lymphoma treated with fludarabine. Leuk. Lymphoma 1998, 28, 599–602. [Google Scholar] [CrossRef]
- Sáez-González, E.; Salavert, M.; Cerrillo, E.; Moret, I.; Iborra, M.; Nos, P.; Beltrán, B. Secondary Haemophagocytic Syndrome and Overlapping Immune Reconstitution Syndrome: Life-Threatening Complications of Anti-TNF-α Treatment for Crohn’s Disease. Am. J. Gastroenterol. 2019, 114, 177–179. [Google Scholar] [CrossRef]
- Satomi, A.; Nagai, S.; Nagai, T.; Niikura, K.; Ideura, T.; Ogata, H.; Akizawa, T. Effect of plasma exchange on refractory hemophagocytic syndrome complicated with myelodysplastic syndrome. Ther. Apher. 1999, 3, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Baraldès, M.A.; Domingo, P.; González, M.J.; Aventin, A.; Coll, P. Tuberculosis-associated hemophagocytic syndrome in patients with acquired immunodeficiency syndrome. Arch. Intern. Med. 1998, 158, 194–195. [Google Scholar] [CrossRef]
- Brito-Zerón, P.; Bosch, X.; Pérez-de-Lis, M.; Pérez-Álvarez, R.; Fraile, G.; Gheitasi, H.; Retamozo, S.; Bové, A.; Monclús, E.; Escoda, O.; et al. Infection is the major trigger of hemophagocytic syndrome in adult patients treated with biological therapies. Semin. Arthritis Rheum. 2016, 45, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Alduaij, W.; Biggs, C.M.; Belga, S.; Luecke, K.; Merkeley, H.; Chen, L.Y.C. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: A case series. Eur. J. Haematol. 2021, 106, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.; Siegman-Igra, Y.; Josiphov, J.; Rahmani, R.; Liron, M. Histiocytic hemophagocytosis in miliary tuberculosis. Arch. Intern. Med. 1984, 144, 2055–2056. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Lee, J.H.; Kim, Y.G.; Kim, Y.O.; Lee, S.H.; Kim, B.K.; Bang, B.K. Tuberculosis-associated hemophagocytic syndrome in a hemodialysis patient: Case report and review of the literature. Nephron 1996, 72, 690–692. [Google Scholar] [CrossRef]
- Quiquandon, I.; Plantier, I.; Hatron, P.Y.; Chassaing, O.; Bauters, F.; Desablens, B.; Devulder, B. Tuberculosis associated haemophagocytic syndrome: Two cases with a favourable outcome. Nouv. Rev. Fr. Hematol. 1995, 37, 149–152. [Google Scholar]
- Rosales-Castillo, A.; López-Ruz, M. Miliary tuberculosis complicated with acute respiratory distress syndrome and hemophagocytic lymphohistiocytosis syndrome in an immunocompetent patient. Med. Clin. 2020, 157, 454–455. [Google Scholar] [CrossRef]
- Subhash, H.S.; Sowmya, S.; Sitaram, U.; Cherian, A.M. Tuberculosis associated haemophagocytic syndrome. J. Postgrad. Med. 2001, 47, 220. [Google Scholar] [PubMed]
- Fernández, A.A.; de Velasco Pérez, D.F.; Fournier, M.C.; Moreno Del Prado, J.C.; Torras, B.P.; Cañete Palomo, M.L. Hemophagocytic syndrome secondary to tuberculosis at 24-week gestation. Int. J. Mycobacteriol. 2017, 6, 108–110. [Google Scholar] [CrossRef]
- Undar, L.; Karpuzoğlu, G.; Karadoğan, I.; Gelen, T.; Artvinli, M. Tuberculosis-associated haemophagocytic syndrome: A report of two cases and a review of the literature. Acta Haematol. 1996, 96, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ng, W.F.; Chan, A.C. Miliary tuberculosis with splenic rupture: A fatal case with hemophagocytic syndrome and possible association with long standing sarcoidosis. Pathology 1994, 26, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Ng, C.S.; Law, C.K.; Ng, W.F.; Wong, K.F. Reactive hemophagocytic syndrome: A study of 7 fatal cases. Pathology 1987, 19, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Pal, R.S.; Kumar, A.; Chandra Ojha, U. Haemophagocytic lymphohistiocytosis (HLH) secondary to miliary tuberculosis. Indian J. Tuberc. 2020, 67, 366–370. [Google Scholar] [CrossRef]
- Vaiphei, K.; Ahuja, V.; Sinha, S.K.; Bhasin, D.K. Prolonged fever with lymph nodal and liver involvement in a chronic alcoholic man. Indian J. Gastroenterol. 2008, 27, 123–129. [Google Scholar] [PubMed]
- Talluri, M.R.; Uppin, S.; Paritala, V.; Kumar, N.; Challa, S.; Rao, N.; Kakarla, B.; Gk, P. Miliary Tuberculosis: A Rare Cause of Hemophagocytic Lymphohistiocytosis. Chest 2013, 144, 211A. [Google Scholar] [CrossRef]
- Halabi, H.; Hafiz, W.; Bawayan, M.; Maulawi, A.; Almoallim, H. Mycobacterium Tuberculosis-Associated Hemophagocytic Syndrome in Systemic Lupus Erythematosus: A Case Report/Sistemik Lupus Eritematözde Mycobacterium Tuberculosis ile Iliskili Hemofagositik Sendrom: Olgu Sunumu. Turk. J. Rheumatol. 2012, 27, 267–270. [Google Scholar] [CrossRef]
- Jain, D.; Dash, S. Pancytopenia due to extensive hemophagocytosis following anti-tubercular treatment. Am. J. Hematol. 2004, 75, 118–119. [Google Scholar] [CrossRef]
- Khan, F.; Fawzy, Z.; Siddiqui, I.; Yassin, M. Hemophagocytosis and miliary tuberculosis in a patient in the intensive care unit. Indian J. Crit. Care Med. 2006, 10, 112–114. [Google Scholar] [CrossRef]
- Monier, B.; Fauroux, B.; Chevalier, J.Y.; Leverger, G.; Nathanson, M.; Costil, J.; Tournier, G. Miliary tuberculosis with acute respiratory failure and histiocytic hemophagocytosis. Successful treatment with extracorporeal lung support and epipodophyllotoxin VP 16-213. Acta Paediatr. 1992, 81, 725–727. [Google Scholar] [CrossRef]
- Karras, A.; Thervet, E.; Legendre, C. Hemophagocytic syndrome in renal transplant recipients: Report of 17 cases and review of literature. Transplantation 2004, 77, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kürşat, S.; Cağirgan, S.; Ok, E.; Unsal, A.; Tokat, Y.; Saydam, G.; Akçiçk, F.; Başçi, A. Haemophagocytic-histiocytic syndrome in renal transplantation. Nephrol. Dial. Transplant. 1997, 12, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Phatarpekar, A.; Currimbhoy, Z.; Desai, M. Haemophagocytic lymphohistiocytosis: A case series from Mumbai. Ann. Trop. Paediatr. 2011, 31, 135–140. [Google Scholar] [CrossRef] [PubMed]
- McCall, C.M.; Mudali, S.; Arceci, R.J.; Small, D.; Fuller, S.; Gocke, C.D.; Vuica-Ross, M.; Burns, K.H.; Borowitz, M.J.; Duffield, A.S. Flow cytometric findings in hemophagocytic lymphohistiocytosis. Am. J. Clin. Pathol. 2012, 137, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Modi, C.; Dhamne, A.; Rege, J.D. Haemophagocytosis in tuberculosis—A case report. Indian J. Pathol. Microbiol. 2003, 46, 463–465. [Google Scholar] [PubMed]
- Akinbami, L.J.; Selby, D.M.; Slonim, A.D. Hepatosplenomegaly and pulmonary infiltrates in an infant. J. Pediatr. 2001, 139, 124–129. [Google Scholar] [CrossRef]
- Rolsdorph, L.Å.; Mosevoll, K.A.; Helgeland, L.; Reikvam, H. Concomitant Hemophagocytic Lymphohistiocytosis and Cytomegalovirus Disease: A Case Based Systemic Review. Front. Med. 2022, 9, 819465. [Google Scholar] [CrossRef]
- Yao, S.; Wang, Y.; Sun, Y.; Liu, L.; Zhang, R.; Fang, J.; Jin, R.; Yu, J.; Li, F.; Bai, J.; et al. Epidemiological investigation of hemophagocytic lymphohistiocytosis in China. Orphanet J. Rare Dis. 2021, 16, 342. [Google Scholar] [CrossRef]
- McCain, K.L.; Eckstein, O. Clinical Features and Diagnosis of Hemophagocytic Lymphohistiocytosis; UpToDate: Waltham, MA, USA, 2022; Available online: https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-hemophagocytic-lymphohistiocytosis?search=hlh%20criteria&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H2524981 (accessed on 18 March 2023).
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.-C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef]
- Lin, H.; Scull, B.P.; Goldberg, B.R.; Abhyankar, H.A.; Eckstein, O.E.; Zinn, D.J.; Lubega, J.; Agrusa, J.; El Mallawaney, N.; Gulati, N.; et al. IFN-γ signature in the plasma proteome distinguishes pediatric hemophagocytic lymphohistiocytosis from sepsis and SIRS. Blood Adv. 2021, 5, 3457–3467. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Nancy, A.; Viswanathan, V.; Shruthi, B.S.; Sivakumar, S.; Natarajan, M.; Kornfeld, H.; Babu, S. Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis. Sci. Rep. 2019, 9, 18217. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Berliner, N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 2015, 125, 2908–2914. [Google Scholar] [CrossRef]
- Schram, A.M.; Campigotto, F.; Mullally, A.; Fogerty, A.; Massarotti, E.; Neuberg, D.; Berliner, N. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015, 125, 1548–1552. [Google Scholar] [CrossRef]
- Allen, C.E.; Yu, X.; Kozinetz, C.A.; McClain, K.L. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Brisse, E.; Matthys, P.; Wouters, C.H. Understanding the spectrum of haemophagocytic lymphohistiocytosis: Update on diagnostic challenges and therapeutic options. Br. J. Haematol. 2016, 174, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Hejblum, G.; Lambotte, O.; Galicier, L.; Coppo, P.; Marzac, C.; Aumont, C.; Fardet, L. A web-based delphi study for eliciting helpful criteria in the positive diagnosis of hemophagocytic syndrome in adult patients. PLoS ONE 2014, 9, e94024. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Operational Handbook on Tuberculosis. Module 3: Diagnosis—Rapid Diagnostics for Tuberculosis Detention; 2021 Update; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- NIPH. Tuberkuloseveilederen Oslo: Norwegian Institute of Public Health. 2021. Available online: https://www.fhi.no/nettpub/tuberkuloseveilederen/ (accessed on 18 March 2023).
- European Centre for Disease Prevention and Controll. Handbook on Tuberculosis Laboratory Diagnostic Methods in the European Union—Updated 2018; ECDC: Stockholm, Sweden, 2018.
- Kaito, K.; Kobayashi, M.; Katayama, T.; Otsubo, H.; Ogasawara, Y.; Sekita, T.; Saeki, A.; Sakamoto, M.; Nishiwaki, K.; Masuoka, H.; et al. Prognostic factors of hemophagocytic syndrome in adults: Analysis of 34 cases. Eur. J. Haematol. 1997, 59, 247–253. [Google Scholar] [CrossRef]
- Arca, M.; Fardet, L.; Galicier, L.; Rivière, S.; Marzac, C.; Aumont, C.; Lambotte, O.; Coppo, P. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: Impact of triggering disease and early treatment with etoposide. Br. J. Haematol. 2015, 168, 63–68. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Zheng, W.; Ma, J.; Zhang, W.; Wang, W.; Tian, X. Hemophagocytic Lymphohistiocytosis: Clinical Analysis of 103 Adult Patients. Medicine 2014, 93, 100–105. [Google Scholar] [CrossRef]
- Buyse, S.; Teixeira, L.; Galicier, L.; Mariotte, E.; Lemiale, V.; Seguin, A.; Bertheau, P.; Canet, E.; de Labarthe, A.; Darmon, M.; et al. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010, 36, 1695–1702. [Google Scholar] [CrossRef]
- Parikh, S.A.M.; Kapoor, P.M.D.; Letendre, L.M.D.; Kumar, S.M.D.; Wolanskyj, A.P.M.D. Prognostic Factors and Outcomes of Adults With Hemophagocytic Lymphohistiocytosis. Mayo Clin. Proc. 2014, 89, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Dhote, R.; Simon, J.; Papo, T.; Detournay, B.; Sailler, L.; Andre, M.H.; Dupond, J.L.; Larroche, C.; Piette, A.M.; Mechenstock, D.; et al. Reactive hemophagocytic syndrome in adult systemic disease: Report of twenty-six cases and literature review. Arthritis Rheum. 2003, 49, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Imashuku, S. Differential diagnosis of hemophagocytic syndrome: Underlying disorders and selection of the most effective treatment. Int. J. Hematol. 1997, 66, 135–151. [Google Scholar] [CrossRef]
- Apodaca, E.; Rodríguez-Rodríguez, S.; Tuna-Aguilar, E.J.; Demichelis-Gómez, R. Prognostic Factors and Outcomes in Adults With Secondary Hemophagocytic Lymphohistiocytosis: A Single-center Experience. Clin. Lymphoma Myeloma Leuk. 2018, 18, e373–e380. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
| Synonyms for tuberculosis | TB |
| TBC | |
| mycobacterium tuberculosis | |
| mycobacterial infection | |
| miliary tb/tbc | |
| secondary tb/tbc | |
| Koch(’s) disease | |
| Synonyms for HLH | h(a)emophagocytic lymphohistiocytosis |
| h(a)emophagocytic syndrome | |
| h(a)emophagocytic histiocytosis | |
| erythrophagocytic lymphohistiocytosis | |
| h(a)emophagocytic lymphocytosis | |
| hps |
| Median | Range | Number * (Percentage) | |
|---|---|---|---|
| Sex | |||
| Male | 69/116 (59%) | ||
| Female | 45/116 (39%) | ||
| Unspecified | 2/116 (2%) | ||
| Age, years | 40 | 0–83 | |
| HLH-04 criteria | |||
| Fever, °C | 39 | 37.3–41.0 | 114/116 (98%) |
| Hepatosplenomegaly | 57/96 (59%) | ||
| Isolated splenomegaly | 22/96 (23%) | ||
| Isolated hepatomegaly | 6/96 (6%) | ||
| Bi-/pancytopenia | 103/116 (89%) | ||
| Hemoglobin, g/dL | 7.8 | 2.4–15.5 | 70/90 (78%) |
| 37 | 2.5–545 | 77/92 (84%) | |
| 2.0 | 0.0–59.5 | 68/85 (80%) | |
| Ferritin, μg/L | 5000 | 370–395,644 | 79/83 (95%) |
| Triglycerides, mg/dL | 292 | 88–777 | 38/58 (66%) |
| Fibrinogen, g/L | 1.2 | 0.15–9.9 | 29/43 (67%) |
| Hemophagocytosis | 106/116 (91%) | ||
| Soluble IL-2r, U/mL | 2500–30,247 | 20/21 (95%) | |
| Low NK-cell activity | 13/16 (81%) | ||
| Other findings | |||
| CRP, mg/L | 107 | 0.9–462 | 36/38 (95%) |
| ESR, mm/hour | 57 | 4–150 | 28/29 (97%) |
| LDH, U/L | 1144 | 247–10,646 | 46/53 (87%) |
| Hyponatremia, mmol/L | 130 | 123–143 | 17/32 (53%) |
| Creatinine, μmol/L | 184 | 26.5–910 | 15/22 (68%) |
| AST, U/L | 141 | 21–1787 | 51/56 (91%) |
| ALT, U/L | 97 | 10–600 | 35/55 (64%) |
| Bilirubin (total), μmol/L | 55 | 6–444 | 33/43 (77%) |
| INR | 1.6 | 0.87–10 | 16/22 (73%) |
| Albumin, g/L | 22 | 11–37 | 28/31 (90%) |
| Comorbidity | Number | Percentage | |
|---|---|---|---|
| No comorbidity | 61 | 53% | |
| Malignancy | 10 | 9% | |
| Hematologic malignancy | 8 * | 7% | |
| Other malignancy | 2 | 2% | |
| Transplant recipient | 5 | 4% | |
| Kidney | 4 | 3% | |
| Liver | 1 | 1% | |
| Autoimmune disease | 10 † | 9% | |
| Concomitant infection | 9 | 8% | |
| HIV/AIDS | 3 | 3% | |
| Other | 6 ‡ | 5% | |
| Lifestyle-associated conditions | 21 | 18% | |
| Diabetes mellitus | 9 | 8% | |
| Hypertension | 7 | 6% | |
| Coronary artery disease | 2 | 2% | |
| Aortoiliac bypass | 1 | 1% | |
| Active smoker | 2 | 2% | |
| Pregnancy | 2 | 2% | |
| Renal failure | 11 | 9% | |
| Other | PCOS, ADS, CVA, AF, mitral insufficiency, cervical disc prolapse, hip fracture, adrenal insufficiency, alcoholism | 15 | 13% |
| Treatment | Number | Percentage | |
|---|---|---|---|
| Tuberculostatic | |||
| Rifampicin | 73 | 63% | |
| Isoniazid | 73 | 63% | |
| Pyrazinamide | 57 | 49% | |
| Ethambutol | 68 | 59% | |
| Fluoroquinolones | 21 | 18% | |
| Streptomycin | 9 | 8% | |
| Amikacin | 6 | 5% | |
| Other * | 4 | 3% | |
| Unspecified | 24 | 20% | |
| Cytostatic | |||
| Etoposide | 15 | 13% | |
| Cyclophosphamide | 2 | 2% | |
| Vincristine | 2 | 2% | |
| Methotrexate | 1 | 1% | |
| Immune modulating | |||
| Corticosteroids | 71 | 61% | |
| IVIG | 25 | 22% | |
| Cyclosporine | 9 | 8% | |
| Tacrolimus | 1 | 1% | |
| ATG | 3 | 3% | |
| JAK-inhibitor | 2 | 2% | |
| TPE | 6 | 5% | |
| Azathioprine | 2 | 2% | |
| Anti-TNFα | 2 | 2% | |
| Anti-CD20 | 2 | 2% | |
| Anti-CD52 | 1 | 1% | |
| Supportive treatment | |||
| ECMO | 2 | 2% | |
| Invasive respiratory support | 28 | 24% | |
| NIV | 7 | 6% | |
| Hemodynamic support | 13 | 11% | |
| Renal replacement therapy | 10 | 9% | |
| Transfusion | 28 | 24% | |
| G-CSF | 3 | 3% | |
| Antimicrobial therapy | 7 | 6% | |
| Splenectomy | 4 | 3% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauchald, T.; Blomberg, B.; Reikvam, H. Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: A Review of Current Literature. J. Clin. Med. 2023, 12, 5366. https://doi.org/10.3390/jcm12165366
Fauchald T, Blomberg B, Reikvam H. Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: A Review of Current Literature. Journal of Clinical Medicine. 2023; 12(16):5366. https://doi.org/10.3390/jcm12165366
Chicago/Turabian StyleFauchald, Trym, Bjørn Blomberg, and Håkon Reikvam. 2023. "Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: A Review of Current Literature" Journal of Clinical Medicine 12, no. 16: 5366. https://doi.org/10.3390/jcm12165366
APA StyleFauchald, T., Blomberg, B., & Reikvam, H. (2023). Tuberculosis-Associated Hemophagocytic Lymphohistiocytosis: A Review of Current Literature. Journal of Clinical Medicine, 12(16), 5366. https://doi.org/10.3390/jcm12165366






